Open Access | Technique
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Comparison between convective radiofrequency water vapor thermal ablation (Rezum) versus tamsulosin in management of lower urinary tract symptoms in patients with benign prostatic enlargement
* Corresponding author: Khaled Abdelsattar Gad Ibrahim
Mailing address: Ass lecturer of urology, military medical academy, Egypt.
Email: khaledagaduro@gmail.com
Received: 10 October 2025 / Revised: 28 October 2025 / Accepted: 07 November 2025 / Published: 30 December 2025
DOI: 10.31491/UTJ.2025.12.050
Abstract
Objectives:
To assess the efficacy of REZUM water vapor thermal ablation therapy against the conventional medical treatment for benign prostatic hyperplasia using tamsulosin.
Patients and methods:
A total of 94 patients with lower urinary tract symptoms due to benign prostatic hyperplasia were enrolled in the study. They were divided into 2 equal groups, Rezūm group and tamsulosin group. Both groups were assessed preoperatively and at 3, 6, 12 months post-procedure for International Prostate Symptom Score (IPSS), maximum urinary flow rate (Qmax), quality of life (QoL), post-void residual (PVR) and complication.
Results:
At 3 months, Rezūm was associated with significantly greater improvements in IPSS, QoL, Qmax, prostate volume, and PVR compared with tamsulosin (all P < 0.01). At 6 months, Qmax, QoL, and PVR remained significantly better in the Rezūm group (P < 0.001), while IPSS and prostate volume showed no significant difference. At 12 months, the Rezūm group continued to demonstrate significantly greater improvements in Qmax, QoL, prostate volume, and PVR (all P < 0.01), whereas IPSS was similar between groups (P = 0.792). The overall complication rate was significantly lower in the Rezūm group compared with the tamsulosin group (55.3% vs. 85.1%, P = 0.0017).
Conclusion:
Compared with tamsulosin, Rezūm therapy achieved more durable improvements in urinary symptoms, flow rate, prostate size, and residual urine, with benefits maintained throughout follow-up. Importantly, it was also associated with a lower overall rate of complications and fewer sexual adverse effects.
Keywords
Rezūm, BPH, tamsulosin
Introduction
Benign prostatic hyperplasia (BPH) is the most common benign neoplasm of aging men and is present in approximately 8% of men in the fourth decade of life but up to 90% of men in the ninth decade [1]. Men with moderate to severe LUTS from BPH (AUASI score of 8 or higher) or mild LUTS that are deemed bothersome by the patient may be offered pharmacologic treatment. The 2 major classes of medications for BPH are alpha-adrenergic blockers and 5-alpha reductase inhibitors. The PDE-5 inhibitor tadalafil is also approved by the Food and Drug Administration (FDA) for the treatment of BPH. Many men also are interested in the use of alternative therapy to treat LUTS [2].
Rezūm therapy utilizes thermal energy generated by water vapor to ablate prostatic tissues [3]. In 2015, it gained approval from the U.S. Food and Drug Administration (FDA) based on results from a pivotal study (NCT01912339) [4]. The system comprises a radiofrequency (RF) generator and a single-use transurethral delivery device, which incorporates a standard 4 mm 30-degree cystoscopy lens. With the patient in a lithotomy position, an RF current is applied to an inductive coil heater, producing thermal energy in the form of water vapor. Water vapor is delivered through a retractable vapor needle via emitter holes in the transurethral device [5].
So far, data from available studies point towards good clinical outcomes with a short-term risk of self-limiting minor complications. Its application has demonstrated clinical effectiveness and possesses specific benefits that distinguish it among other treatments. It is applicable to outpatient setting, is effective in preserving sexual function and is versatile in its ability to treat a variety of prostate gland morphologies [6].
Despite increasing global adoption, comparative data between Rezūm and standard pharmacological therapy in real-world patient populations remain limited. Clarifying the relative efficacy and safety of Rezūm versus tamsulosin is essential for guiding clinical decision-making, particularly in patients seeking alternatives to chronic medication or more invasive surgery. This study was therefore designed to evaluate the outcomes of Rezūm therapy compared with tamsulosin in men with symptomatic BPH [7, 8].
Patients and methods
Study design and study population
This was a prospective randomized controlled trial, conducted in our tertiary care center between May 2022 and May 2023. Patients aged 50–80 years with prostate volumes of 30– 80 mL, and have mild to mod LUTS (maximum urinary flow rate [Qmax] of < 15 mL/s and International Prostate Symptom Score [IPSS] of > 13) and PVR urine < 250 mL were included in our study. Patients known to have prostate cancer, neurogenic bladder, urethral stricture, urinary bladder stone or previous prostatic surgery were excluded.
The primary outcomes were the mean and SD of IPSS of Rezūm versus Tamsulosin arm in the study population. G power 3.1.9.4 was used to calculate the sample size of this comparative study. Assuming 95% power, 0.05 level of significance, therefore, the minimum required sample size to detect statistical significance difference was 39 patients in each group. After adding 20 % drop out rate so the finalestimated sample size was 47 per group.
Technique
Rezūm therapy was carried out as a day-case procedure, using either general anesthesia or a combination of sedation with local anesthesia, and was performed by the same experienced urology team. A disposable transurethral needle was deployed into the prostatic tissue, with each treatment cycle lasting approximately nine seconds.
A total of 168 cases with BPH were examined for eligibility to be included in the study. Seventy-four cases were excluded for these reasons: 49 were excluded for not fulfilling inclusion criteria, while 25 were rejected to share in the study. The remaining 94 cases using computer-based software were randomly divided into two equal groups; group A underwent Rezum procedure and group B on tamsulosin were shown in Figure 1.
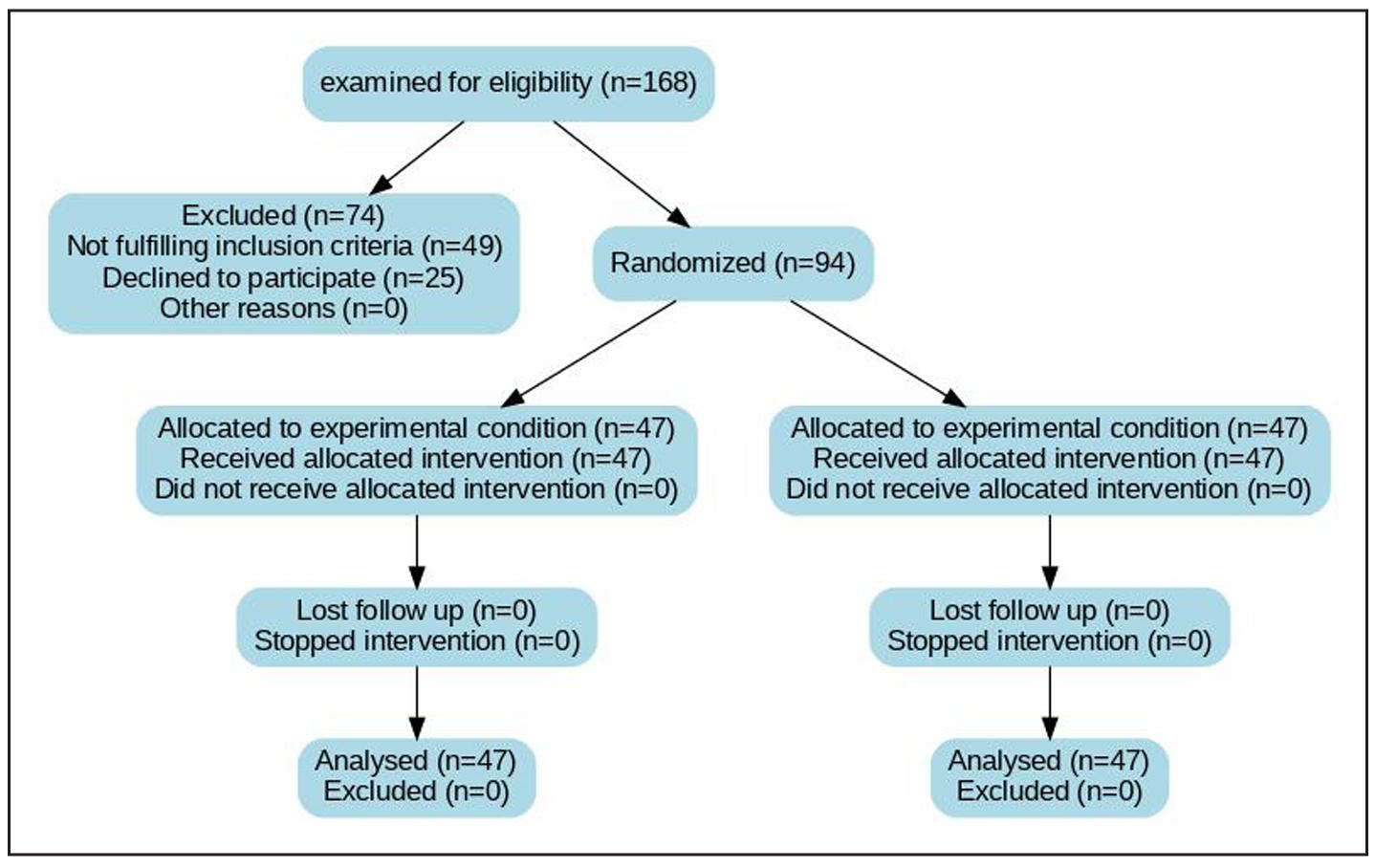
Figure 1. Consort flow chart. A total of 168 patients with BPH were examined for eligibility to be included in the study. Seventy-four patients were excluded for these re- asons: 49 were excluded for not fulfilling inclusion criteria, while 25 were rejected to share in the study, as shown in. Figure 1. The remaining 100 patients using computer-based software were randomly divided into two equal groups; group A underwent Rezūm procedure and group B underwent B-TURP.
Outcome
The primary outcome of this study was to assess the therapeutic effectiveness of both interventions, while the secondary outcome focused on their safety profile. Effectiveness was evaluated through changes in IPSS, QoL, Qmax, PVR, and residual prostate volume. Safety was determined by the incidence of postoperative complications.
Statistical methods
Changes from baseline were reported as mean values with standard deviations and percentage change. For comparisons between the two groups, Student’s t-test was applied to normally distributed variables, whereas the Mann–Whitney U test was used for non-parametric data. Associations between categorical variables were assessed using the Chi-squared test, and Fisher’s exact test was applied when more than 20% of cells had an expected frequency of fewer than five. Data analysis was performed with SPSS software (version 25, IBM Corp., Armonk, NY, USA). A P-value of < 0.05 was considered statistically significant.
Results
At baseline, there were no significant differences between the Rezūm and tamsulosin groups regarding age, body mass index, symptom severity, quality-of-life score, prostate volume, or urinary flow rate. The only notable differences were observed in post-void residual urine, which was significantly lower in the Rezūm group, and PSA level, which was slightly higher compared with the tamsulosin group were shown in Table 1.
Table 1.
Baseline parameters of the patients.
| Group | P-value | ||
|---|---|---|---|
| Rezūm | Tamsulosin | ||
| Mean ± SD | Mean ± SD | ||
| Age (year) | 70.6 ± 4.9 | 69.5 ± 3.8 | 0.266 |
| BMI | 26.1 ± 2.8 | 26 ± 1.2 | 0.793 |
| IPSS score | 25.5 ± 4.6 | 25.9 ± 4.5 | 0.737 |
| QoL | 4.7 ± 1.05 | 4.9 ± 1.09 | 0.389 |
| Prostate volume (mL) | 55.7 ± 12.3 | 54.6 ± 12.5 | 0.654 |
| PVR (mL) | 108.7 ± 34.8 | 174.8 ± 24.6 | < 0.001 |
| PSA (ng/mL) | 6.1 ± 1.8 | 5.4 ± 1.5 | 0.045 |
| Qmax (mL/s) | 9.1 ± 1.8 | 9.7 ± 1.6 | 0.089 |
At 12 months, patients in the Rezūm group demonstrated significantly greater improvement compared with the Tamsulosin group across multiple outcome domains, including IPSS, QoL, Qmax, PVR, and prostate size (P < 0.001). The degree of symptom relief was evident as early as 3 months, with Rezūm achieving more rapid and sustained reductions in IPSS and QoL scores. Functional outcomes also favored Rezūm, with a marked increase in Qmax and a pronounced reduction in PVR over follow-up. Prostate size showed a progressive decline in the Rezūm group, while changes in the Tamsulosin group were less pronounced. When assessing response magnitude, Rezūm produced a higher percentage reduction in symptom scores and prostate volume compared to Tamsulosin. By 12 months, clinically meaningful improvement in IPSS (≥ 8-point reduction) was achieved in the majority of Rezūmtreated patients, whereas fewer patients in the Tamsulosin group reached this threshold. Safety analysis showed a lower overall complication rate in the Rezūm arm, with most adverse events being mild and self-limiting; while Tamsulosin was more frequently associated with sexual side effects, particularly retrograde ejaculation were shown in Table 2.
Table 2.
carcinoma.
| Group | P-value | ||||
|---|---|---|---|---|---|
| Rezūm | Tamsulosin | ||||
| Mean ± SD | mean % of change | Mean ± SD | mean % of change | ||
| Qmax (mL/s) | |||||
| Baseline | 9.1 ± 1.8 | 9.7 ± 1.6 | 0.089 | ||
| 3 months | 13.9 ± 2.4 | 52.7% | 10.4 ± 1.1 | 7.2% | < 0.001 |
| 6 months | 16.5 ± 2.9 | 81.3% | 13 ± 1.4 | 34.0% | < 0.001 |
| 12 months | 17.3 ± 3.1 | 90.1% | 15.2 ± 1.9 | 56.7% | < 0.001 |
| IPSS | |||||
| Baseline | 25.5 ± 4.6 | 25.9 ± 4.5 | 0.737 | ||
| 3 months | 10.2 ± 4.3 | 60.0% | 15.6 ± 5.7 | 39.8% | < 0.001 |
| 6 months | 11.3 ± 5 | 55.7% | 9.4 ± 4.5 | 63.7% | 0.055 |
| 12 months | 9.6 ± 1.8 | 62.4% | 9.4 ± 4.6 | 63.7% | 0.792 |
| PVR (mL) | |||||
| Baseline | 108.7 ± 34.8 | 102.67 ± 23.1 | < 0.001 | ||
| 3 months | 82.4 ± 29.4 | −24.2% | 147 ± 26.9 | −15.9% | < 0.001 |
| 6 months | 47.7 ± 14.6 | 56.1% | 127.7 ± 30 | −26.9% | < 0.001 |
| 12 months | 48.4 ± 13.7 | 55.5% | 92.4 ± 25 | −47.1% | < 0.001 |
| QoL | |||||
| Baseline | 4.7 ± 1.05 | 4.9 ± 1.09 | 0.389 | ||
| 3 months | 2.2 ± 1.1 | −53.2% | 3.8 ± 1.1 | −22.4% | < 0.001 |
| 6 months | 1.9 ± 0.8 | −59.6% | 3.6 ± 1.1 | −26.5% | < 0.001 |
| 12 months | 1.9 ± 0.8 | −59.6% | 2.6 ± 1.1 | −46.9% | < 0.001 |
| Prostate size (mL) | |||||
| Baseline | 55.7 ± 12.3 | 54.6 ± 12.5 | 0.654 | ||
| 3 months | 45.6 ± 9.9 | −18.1% | 51.4 ± 11.8 | −5.9% | 0.011 |
| 6 months | 43 ± 8.9 | −22.8% | 42.2 ± 12.1 | −22.7% | 0.147 |
| 12 months | 36.3 ± 7 | −34.8% | 42.6 ± 12.5 | −22.0% | < 0.001 |
The overall complication rate was significantly higher in the Tamsulosin group compared with the Rezūm group (85.1% vs. 55.3%, P = 0.0034). Specific adverse events such as dizziness, retrograde ejaculation, urgency, and urinary tract infection showed statistically significant differences between the two groups. Dizziness, retrograde ejaculation, and urgency were more frequent in the Tamsulosin group, while urinary tract infections occurred exclusively in the Rezūm group. Other complications, including dysuria, hematuria, orthostatic hypotension, and urinary retention, did not differ significantly between groups were shown in Table 3.
Table 3.
Perioperative complications.
| Complication | Rezūm (n = 47) | Tamsulosin (n = 47) | P-value |
|---|---|---|---|
| Overall complications | 26 | 40 | 0.0034 |
| Dizziness | 0 | 6 | 0.0264 |
| Dysuria | 9 | 5 | 0.7554 |
| Foreign body floating | 1 | 0 | 1.0 |
| Hematuria | 5 | 2 | 0.4349 |
| Orthostatic hypotension | 0 | 4 | 0.117 |
| Retrograde ejaculation | 1 | 14 | 0.0004 |
| Urgency | 0 | 9 | 0.0026 |
| Urinary retention | 2 | 0 | 0.4946 |
| Urinary tract infection | 8 | 0 | 0.0056 |
| Retrograde ejaculation | 1 | 14 | 0.0004 |
| Urgency | 0 | 9 | 0.0026 |
| Urinary retention | 2 | 0 | 0.4946 |
| Urinary tract infection | 8 | 0 | 0.0056 |
Discussion
This was a randomized controlled trial conducted on 168 patients with benign prostatic hyperplasia (BPH); to assess the efficacy of Rezūm water vapor thermal ablation therapy against medical treatment for benign prostatic hyperplasia using tamsulosin. In 2015, the Rezūm therapy was approved by the Food and Drug Administration (FDA) according to the results of the important study (NCT01912339). Also, in June 2020 its use was accepted by the National Institute for Health and Care Excellence (NICE) [9]
Rezūm represents a minimally invasive option for managing BPH, requiring no more than two minutes to perform and typically carried out under sedation. This contrasts with procedures such as Photoselective vaporization of the prostate (PVP) or TURP, which are more time-consuming and invasive. The technique is relatively straightforward to master, especially when compared with the steeper learning curve of holmium laser enucleation of the prostate (HoLEP) [10]. Additional benefits of Rezūm include a lower risk of retrograde ejaculation and preservation of erectile function [7, 11].
Regarding efficacy, our study demonstrated that the Rezūm group achieved significantly greater improvement than the Tamsulosin group in multiple domains, particularly Qmax, PVR, QoL, and prostate volume across all follow-up points (P < 0.001). Qmax in the Rezūm group increased by over 90% at 12 months compared to baseline, versus a 56.7% improvement in the Tamsulosin group. This outcome agreed with the results of Bilhim et al., who reported significant improvements three months after Rezūm therapy and remained stable at the four-year follow-up control [12].
Prostate volume reduction was also more pronounced after Rezūm (−34.8% at 12 months) than with Tamsulosin (−22.0%), in concordance with our findings, Sterling et al. concluded that, MRI studies have confirmed whole prostate volume decreases of 29% and transition zone volume decreases of 38% 3 months after treatment with Rezūm. In contrast, IPSS scores improved significantly from baseline in both groups, but without a statistically significant difference between them at 6 and 12 months [13].
With regards to complication of Rezūm therapy and tamsulosin, Rezūm demonstrated a significantly lower complication rate compared with Tamsulosin (55.3% vs. 85.1%, P = 0.0017). Reported adverse events with Rezūm were mostly mild and transient, such as dysuria, UTI, hematuria, and limited cases of urinary retention or retrograde ejaculation, consistent with prior studies [6, 12, 14-16]. In contrast, Tamsulosin was associated with higher rates of functional side effects including retrograde ejaculation, urgency, dizziness, and orthostatic hypotension, in line with its known pharmacologic profile [17]. Although some reports describe complication rates with Rezūm up to 30% at early follow-up, these events were generally mild and self-limiting [13]. Overall, our findings support Rezūm’s favorable safety profile compared to medical therapy.
The limitations of our study were the relatively small number of patients and short follow-up duration. However, to the best of our knowledge, it is the first study to compare the efficacy and safety of Rezūm with medical treatment using alpha blockers only.
Conclusions
Rezūm therapy demonstrates high efficacy in alleviating lower urinary tract symptoms (LUTS) in patients with benign prostatic hyperplasia (BPH) when compared to conventional medical treatment with tamsulosin, and it is associated with fewer complications.
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
None.
Ethical approval and informed consent
Approval of the research protocol by an institutional Reviewer Board MD-177-2022. All eligible patients signed written informed consent form matching with good clinical practice.
Consent for publication
Not applicable.
Registry and the Registration of the study /trial
NCT07169773
References
1. Aaron L, Franco O, & Hayward S. Review of prostate anatomy and embryology and the etiology of benign prostatic hyperplasia. Urol Clin North Am, 2016, 43(3): 279-288. [Crossref]
2. Langan R. Benign prostatic hyperplasia. Prim Care, 2019, 46(2): 223-232. [Crossref]
3. Samir M, Elaal A, Gad K, & Basyony M. Two-year followup comparing Rezūm therapy versus bipolar transurethral resection of the prostate for treating benign prostatic hyperplasia. A prospective randomized study. Int J Urol, 2024, 31(5): 545-550. [Crossref]
4. McVary K, Gange S, Gittelman M, Goldberg K, Patel K, Shore N, et al. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol, 2016, 195(5): 1529-1538. [Crossref]
5. Mynderse L, Hanson D, Robb R, Pacik D, Vit V, Varga G, et al. Rezūm system water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: validation of convective thermal energy transfer and characterization with magnetic resonance imaging and 3-dimensional renderings. Urology, 2015, 86(1): 122-127. [Crossref]
6. Westwood J, Geraghty R, Jones P, Rai B, & Somani B. Rezum: a new transurethral water vapour therapy for benign prostatic hyperplasia. Ther Adv Urol, 2018, 10(11):327-333. [Crossref]
7. McVary K, & Roehrborn C. Three-year outcomes of the prospective, randomized controlled Rezūm system study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology, 2018, 111: 1-9. [Crossref]
8. Dixon C, Cedano E, Pacik D, Vit V, Varga G, Wagrell L, et al. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Res Rep Urol, 2016, 8: 207-216. [Crossref]
9. Elterman D, Bhojani N, Vannabouathong C, Chughtai B, & Zorn K. Rezūm therapy for ≥ 80-mL benign prostatic enlargement: a large, multicentre cohort study. BJU Int, 2022, 130(4): 522-527. [Crossref]
10. Bole R, Gopalakrishna A, Kuang R, Alamiri J, Yang D, Helo S, et al. Comparative postoperative outcomes of Rezūm prostate ablation in patients with large versus small glands. J Endourol, 2020, 34(7): 778-781. [Crossref]
11. McVary KT, Roehrborn CG. Three-year outcomes of the prospective, randomized controlled Rezum system study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2018;111:1–9. [Crossref]
12. McVary K, Gange S, Gittelman M, Goldberg K, Patel K, Shore N, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med, 2016, 13(6): 924-933. [Crossref]
13. Bilhim T, Betschart P, Lyatoshinsky P, Müllhaupt G, & Abt D. Minimally invasive therapies for benign prostatic obstruction: a review of currently available techniques including prostatic artery embolization, water vapor thermal therapy, prostatic urethral lift, temporary implantable nitinol device and aquablation. Cardiovasc Intervent Radiol, 2022, 45(4): 415-424. [Crossref]
14. Sterling J, Farber N, & Gupta N. Comparing outcomes of medical management and minimally invasive surgical techniques for lower urinary tract symptoms due to BPH. Curr Urol Rep, 2019, 20(6): 29-40. [Crossref]
15. Barbosa J, & Antunes A. Minimally invasive techniques for the treatment of benign prostatic hyperplasia. Revista de Medicina, 2018, 97: 314-325. [Crossref]
16. Madersbacher S, Roehrborn C, & Oelke M. The role of novel minimally invasive treatments for lower urinary tract symptoms associated with benign prostatic hyperplasia. BJU Int, 2020, 126(3): 317-326. [Crossref]
17. Malde S, Lam W, Adwin Z, & Hashim H. Pharmacological and interventional treatment of benign prostatic obstruction: an evidence-based comparative review. BJUI Compass, 2021, 2(4): 238-259. [Crossref]
18. Pramana IBP, Oka AAG, Duarsa GWK, Santosa KB, Yudiana IW, Tirtayasa PMW, et al. The effectiveness of tamsulosin in benign prostate hyperplasia (BPH) patients with lower urinary tract symptoms (LUTS): a multi-centre cohort retrospective study. Indonesia Journal of Biomedical Science, 2019, 14(1): 17-20. [Crossref]