Open Access | Research
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Endoscopic lithotripsy in patients with asymptomatic bacteriuria
* Corresponding author: Sergey O. Sukhikh
Mailing address: Botkin City Clinical Hospital, Moscow, Russian Federation.
Email: docsukhikh@gmail.com
Received: 12 February 2025 / Revised: 05 March 2025 / Accepted: 25 April 2025 / Published: Published: 26 June 2025
DOI: 10.31491/UTJ.2025.06.036
Abstract
Introduction: Endoscopic surgery is a highly effective method for
treating urolithiasis; however, it carries a risk of postoperative infectious
complications. One of the main risk factors for these complications is a positive
urine culture. The persistence of asymptomatic bacteriuria (ASB) in patients,
combined with the absence of standardized guidelines for its managing prior to
endoscopic procedures, highlights the need for further investigation. Thus,
conducting a comparative analysis of infectious complications in patients with
negative urine culture versus those with persistent ASB undergoing endoscopic
surgery for renal and ureteral stones using different regimes of antibiotic
prophylaxis seems clinically relevant issue.
Objective: To assess the safety of endoscopic stone surgery in
patients with persistent asymptomatic bacteriuria and patients’ negative urine
culture undergoing endoscopic surgery for renal and ureteral stones using different
regimes of antibiotic prophylaxis.
Materials and Methods: We conducted a retrospective study analyzing
data from patients who underwent endoscopic removal of renal and ureteral stones
between January 2023 and July 2023. Of the 449 patient records reviewed,
211 patients meeting the inclusion and exclusion criteria were selected for further
analysis. Antibacterial prophylaxis was administered as follows: a few hours prior
to surgery for patients with an initially sterile urine culture, three days prior
to surgery for those with clinically insignificant ASB, and seven days prior to
surgery for patients with clinically significant ASB.
Results: A preoperative sterile urine culture was identified in 152
patients (72.0%), while 59 patients (28.0%) [95% CI: 22.0%; 34.5%] were diagnosed with
bacteriuria. Among these, 28 patients (13.3%) [95% CI: 9.0%; 18.6%] had clinically significant
bacteriuria, defined as a bacterial count of ≥105 CFU/mL. Despite culture based antibiotic
therapy prior to surgery persistent ASB was observed in six patients (22.0%). Consequently,
37 patients (17.5%) [95% CI: 12.79%; 23.4%] with clinically significant and insignificant
ASB along with patients with sterile urine underwent endoscopic surgery. In the postoperative
period, leukocytosis alone was observed in 54 patients (25.6%), fever in 17 patients (8.1%),
and fever accompanied by leukocytosis in 11 patients (5.2%). Logistic regression analysis
demonstrated a statistically significant association between bacteriuria and postoperative
fever. A positive urine culture increased the odds of hyperthermia by 4,75 times
(OR = 4,75, 95% CI: 1.256; 21.123, P = 0.022). Additional factors influencing leukocytosis
included maximum stone size (P = 0.013), stone volume, and dwelling ureteral stent (P = 0.006).
Specifically, an increase in stone volume by 1.0 cc raised the odds of leukocytosis by 1.54 times
(OR = 1.543, 95% CI: 1.128; 2.158, P = 0.008).
Conclusion: Our study highlights that a positive urine culture
is a significant risk factor for infectious complications following endoscopic surgery.
Prolonged antibiotic prophylaxis for patients with clinically significant ASB appears
to be an effective strategy to minimize the risk of postoperative infectious complications.
Keywords
Kidney stones, ureteral stones, asymptomatic bacteriuria, infection, complications, endoscopy
Introduction
Urolithiasis is one of the common urological diseases affecting the
adult population [1], accounting for approximately 50.0–60.0% of inpatients [2].
Endourological procedures are the primary treatment for patients with urolithiasis.
Currently, ureteroscopy (URS), retrograde intrarenal surgery (RIRS), and percutaneous
nephrolithotomy (PCNL) are the most common methods used for treating upper urinary tract
stones. The widespread use of these surgical interventions is attributed to their
high efficacy and safety. However, the most significant complications are infectious
complications, which include fever, pyelonephritis, systemic inflammatory response
syndrome (SIRS), and sepsis.
According to current data, the probability of infectious complications after
stone surgery might reach up to 19% (up to 13.4% after RIRS and up to 18.9% after PCNL) [3].
Fever is the most frequently observed postoperative complication, typically not
necessitating adjustments to the treatment regimen or additional therapeutic interventions.
In certain cases, it may reflect reactive or resorptive inflammatory responses rather
than an underlying infectious process [4-6]. The incidence of postoperative hyperthermia
after endourological procedures ranges from 2.8% to 17.5% [7]. Along with this the risk
of systemic inflammatory response syndrome (SIRS) can reach up to 9.7%, while the
incidence of urosepsis can be observed in up to 4.7% of cases [2, 8-11]. One of the
known modifiable risk factors for infectious complications is bacteriuria [12].
The recommendations of the European Association of Urology (EAU) mandate
bacterial urine culture for patients scheduled for endourological procedures.
For patients with asymptomatic bacteriuria, antibiotic therapy is recommended
to prevent infectious complications during endourological procedures breaching
the mucosa [13].
The guidelines of the American Urological Association also emphasize
the necessity of performing bacterial urine culture to determine the
appropriateness of further antibiotic prophylaxis [14]. Preoperative
antibiotic prophylaxis is known to be an effective measure in reducing
the incidence of infectious complications in patients with bacteriuria,
increasing the rate of sterile cultures of renal pelvis urine and stones
obtained intraoperatively [15]. For patients with bacteriuria undergoing
endourological procedures, it is recommended to prescribe specific antibiotic
prophylaxis based on the results of antibiotic sensitivity testing [16].
As part of antibiotic prophylaxis prior to performing URS or RIRS, a single
dose of an antibacterial drug is recommended for patients with sterile urine
culture. For planned PCNL, standard antibiotic prophylaxis with continued
antibiotic therapy is recommended by many author [17].
Unfortunately, clinical guidelines lack specific algorithms and treatment
protocols for managing asymptomatic bacteriuria prior to endoscopic surgery.
Current research evidence suggests that extended courses of antibiotic
prophylaxis are more effective in preventing systemic inflammatory response
and sepsis in patients with high-risk factors for infectious complications,
such as a positive urine culture [18-20].
Although it is well known that patients with a positive urine culture
have an increased risk of infectious complications, approximately 17.0% of
patients with a persistent positive urine culture after antibiotic prophylaxis
still undergo PCNL [21]. It is also worth noting that repeated courses of
antibiotic therapy, administered in an attempt to sterilize the urine culture
before surgery, do not always achieve the desired outcome and may contribute
to the development of multidrug-resistant bacterial strains.
Objective of the study
Primary objective: To assess the incidence of postoperative infectious
complications—specifically fever and leukocytosis—in patients with
persistent asymptomatic bacteriuria compared to those with sterile
urine cultures undergoing endoscopic surgery for renal and ureteral stones.
Secondary objective: To evaluate the influence of different preoperative
antibiotic prophylaxis regimens (single-dose, 3-day, and 7-day protocols)
on the risk of these complications.
Methods
Inclusion and exclusion criteria
The study included patients who underwent endoscopic treatment of
upper urinary tract stones and completed a full standard preoperative
examination at our clinic. Eligible patients had either positive or
negative preoperative urine cultures and received antibiotic prophylaxis
strictly in accordance with the standardized protocol adopted in our institution.
Patients were excluded from the study if their medical records lacked
complete information regarding the preoperative assessment or if the
evaluation was conducted partially or entirely at an external facility.
Additional exclusion criteria were deviations from the clinic’s standard
antibiotic prophylaxis protocols, including cases involving multidrug-resistant
bacterial strains, the presence of leukocyturia, congenital or acquired anomalies
of the urinary system, HIV infection, ongoing immunosuppressive therapy, and the
presence of permanent urethral catheters or cystostomy tubes.
Grouping
A total of 449 patient records were selected for the analysis.
Subsequently, records of 211 patients aged 18 to 75 years,
who met the inclusion and exclusion criteria, were analyzed.
All patients underwent urine culture with antibiotic sensitivity testing
including blood tests, uranalysis, and CT scan before the surgical treatment.
Patients with sterile urine received antimicrobial prophylaxis according
to the standard protocol approved by the hospital [ceftriaxone 1000 mg (IM)
2-3 hours before the surgery] (Figure 1). In cases of bacteriuria < 105 CFU/mL,
a 3-day course of antimicrobial prophylaxis was prescribed, tailored to the
sensitivity of the identified pathogens. When one or more bacterial species
grew in the urine greater than or equal to ≥ 105 CFU/mL, a 7-day course of
antibiotic therapy was prescribed, adjusted according to the antibiotic
sensitivity. After 7 days of treatment, a repeat urine culture was performed.
In the absence of microbial growth, antimicrobial prophylaxis was conducted as
per the standard protocol for patients with sterile urine. If pathogenic flora
was detected, patients were minutely informed about the high risk of infectious
complications before surgery. Patients with bacteriuria less than 105 CFU/mL
were given a 3-day course of antibiotic therapy based on the antibiotic
susceptibility test before surgery, while patients with bacteriuria greater
than 105 CFU/mL received a 7-day course of antibiotics, also tailored to
the antibiotic sensitivity test results. In the postoperative period, the
incidence of infectious complications was evaluated, including episodes of
fever (body temperature > 37.6°C), an elevated white blood cell count > 12 × 109 /L,
acute purulent pyelonephritis confirmed by contrast CT, and sepsis, following the
criteria of the Quick Sequential Organ Failure Assessment (qSOFA).
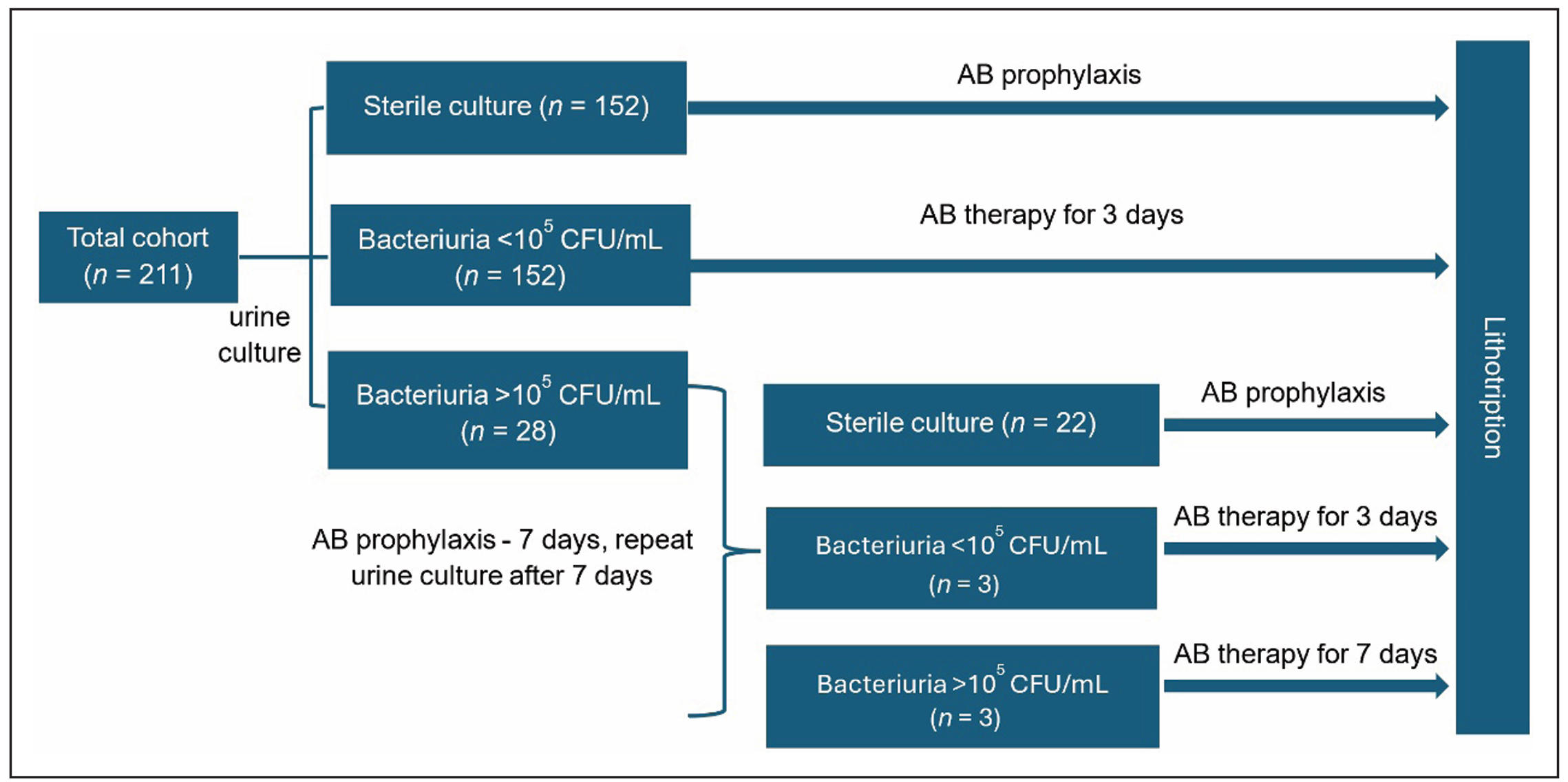
Figure 1. Study design.
Statistical analysis
Quantitative data were first tested for normal distribution using the Shapiro-Wilk test. If the data were normally distributed, the values were presented as the mean (M) ± standard deviation (SD). For data that deviated from normal distribution, values were presented as the median (Me) and the lower and upper quartiles ([Q1; Q3]). Categorical data are presented with absolute values (n) and proportions (%). For evaluating proportions and constructing 95% confidence intervals (CIs), the binomial test was used. A multivariate logistic regression (MLR) model was employed to analyze the likelihood of hyperthermia and leukocytosis, as well as factors influencing these probabilities. ROC analysis was performed to assess the quality of the model. Statistical analysis was performed using R version 4.1.3 (‘The R Foundation for Statistical Computing’, Vienna, Austria).
Results
The patient’s age was 57 [44; 66] years, with 93 patients (44.1%) being
female and 118 patients (55.9%) male. The body mass index (BMI) was
28.7 [25.08; 32.4] kg/m². The comorbid background was complicated by type 2
diabetes in 26 patients (12.3%). 144 patients were primary stone formers (68.2%),
while 67 patients (31.8%) were treated due to recurrent (secondary) stones.
The maximum stone size was 12 (9; 17) cm³. The stone volume was 0.44 (0.15; 1.11) cm³.
The stone density was 1100 (803.5; 1337) HU (Hounsfield units). A single stone was
diagnosed in 89 patients (42.2%), two stones in 48 cases (22.7%), three stones in 33
cases (15.6%), and four or more stones were treated in 41 patients (19.4%), while 122
patients had multiple stones.Before the surgical treatment, 43 patients (20%) had
drained upper urinary tract. In 31 patients (14.7%), had dwelling ureteral stent,
while in 12 patients (5.7%), had nephrostomy tube.
Semiregid URS (7 Fr) was performed in 26 patients (12.3%), retrograde intrarenal
surgery (RIRS) in 16 patients (7.6%), and percutaneous nephrolithotomy (PCNL) in 169
patients (80.1%).
In 149 cases (70.6%), the surgical procedure was performed under general anesthesia,
in 55 cases (26.1%) under spinal anesthesia, and in 7 cases (3.3%) intravenous anesthesia
was used.
A total of 155 patients (73.5%) had ASA score II, while 56 patients (26.5%)
had a III ASA score. The median operation time was 40 (30; 50) minutes.
All patients underwent a bacteriological urine analysis prior to surgery.
Primary sterile urine was diagnosed in 152 patients (72%), while 59 patients (28%)
[22%; 34.5%] were found to have bacteriuria on preoperative work out. Among these,
31 patients (14.7%) had clinically insignificant less than 105 CFU/mL bacteriuria,
and 28 patients (13.3%) [9%; 18.6%] had clinically significantly greater than or
equal to ≥ 105 CFU/mL bacteriuria. The proportion of patients with positive urine
culture among those with drained urinary tract (ureteral stent or nephrostomy
drainage) was 34.9% [21%; 50.6%], compared to 26.2% [19.7%; 33.5%] in patients
without any urinary tract drainage. However, this difference was not statistically
significant (P-value of Fisher’s exact test = 0.427). Dwelling ureteral stent or
nephrostomic tube, Positive urine culture was found in 4 (33%) patients with a
ureteral stent and in 11 (92%) patients with nephrostomy tube.
The most common pathogens were Enterococcus faecalis in 18 (8.5%) patients,
Escherichia coli in 15 (7.1%), and Klebsiella pneumoniae in 8 (3.8%) patients (Table 1).
Table 1.
Clinical and microbiological characteristics.
| Parameter | n (%) or Value [95% CI] |
|---|---|
| ASA Score II | 155 (73.5%) |
| ASA Score III | 56 (26.5%) |
| Median operation time, min [Q1; Q3] | 40 [30; 50] |
| Sterile urine | 152 (72.0%) |
| Bacteriuria (total) | 59 (28.0%) [22%; 34.5%] |
| < 105 CFU/mL | 31 (14.7%) |
| ≥ 105 CFU/mL | 28 (13.3%) [9%; 18.6%] |
| With urinary drainage (stent or nephrostomy) | n = 43 |
| Positive culture (with drainage) | 15 (34.9%) [21%; 50.6%] |
| - Ureteral stent | 4 (33%) |
| - Nephrostomy tube | 11 (92%) |
| No urinary drainage | n = 168 |
| Positive culture (without drainage) | 26.2% [19.7%; 33.5%] |
| Most common pathogens | |
| Enterococcus faecalis | 18 (8.5%) |
| Escherichia coli | 15 (7.1%) |
| Klebsiella pneumoniae | 8 (3.8%) |
After a course of primary antibiotic therapy in patients with significant
bacteriuria (≥ 105 CFU/mL), urine sterility was achieved in 22 patients (78.6%)
[59%; 91.7%]. In 6 patients (22%), bacteriuria persisted with 3 patients (11%)
showing persistent significant bacterial counts, and another 3 patients (11%)
showing bacterial counts reduction. Eventually, 37 patients (17.5%) [12.8%; 23.4%]
with bacteriuria underwent surgery Among them 3 patients (8%) with clinically
significant bacteriuria and 34 (92%) with clinically insignificant bacteriuria.
In the postoperative period, leukocytosis greater than 12 × 109 /L was observed
in 54 patients (25.6%), 17 patients (8.1%) had fever, in 11 patients (5.2%)
fever was accompanied by leukocytosis.
Fever 6 (16.2%) was significantly more common in patients with bacteriuria
compared to 11 (6.3%) in patients with sterile urine [95% CI] 2.85 [0.8; 9.18],
(P-value for Fisher's exact test = 0.087).
Fever was observed only in 2 patients with clinically significant bacteriuria.
No cases of purulent pyelonephritis or sepsis were registered.
According to the results of the multivariate logistic regression analysis,
statistically significant associations were found between the probability of
developing fever and bacteriuria before surgery (Figure 2). A positive urine
culture increases the risk of fever by 4.75 times (OR = 4.75, 95% CI [1.222; 18.803],
P = 0.023). No statistical significance was found between fever and other model
parameters such as gender, age, BMI, presence of diabetes, stone volume and size,
types of urinary tract drainage, and duration of the surgery (P > 0.05). The AUC
for the ROC curve was 0.78, indicating satisfactory model quality.
There was no statistically significant association between bacteriuria and urinary
tract drainage in this model (P = 0.427).
Based on the results of the multivariate logistic regression analysis, the factors
statistically significantly influencing leukocytosis were stone volume (P = 0.008)
and dwelling ureteral stent (P = 0.006). Ureteral stent (OR = 0.154, 95% CI [0.033;
0.512], P = 0.006) reduces the chance of leukocytosis, while greater stone volume
increases the likelihood of leukocytosis by 1.54 times (OR = 1.543, 95% CI [1.128;
2.158], P = 0.008). There was no statistically significant correlation between
leukocytosis and other parameters in the model, such as gender, age, BMI, diabetes,
nephrostomy tube, positive urine culture, operation time, or type of operation
(P > 0.05). The AUC for the ROC curve is 0.75, indicating an acceptable model
quality (Figure 3). Additionally, the analysis revealed that the development of
leukocytosis > 12 × 109 /L increases the risk of fever by 8.57 times
(OR = 8.57, 95% CI [2.63; 32.85], P < 0.001).
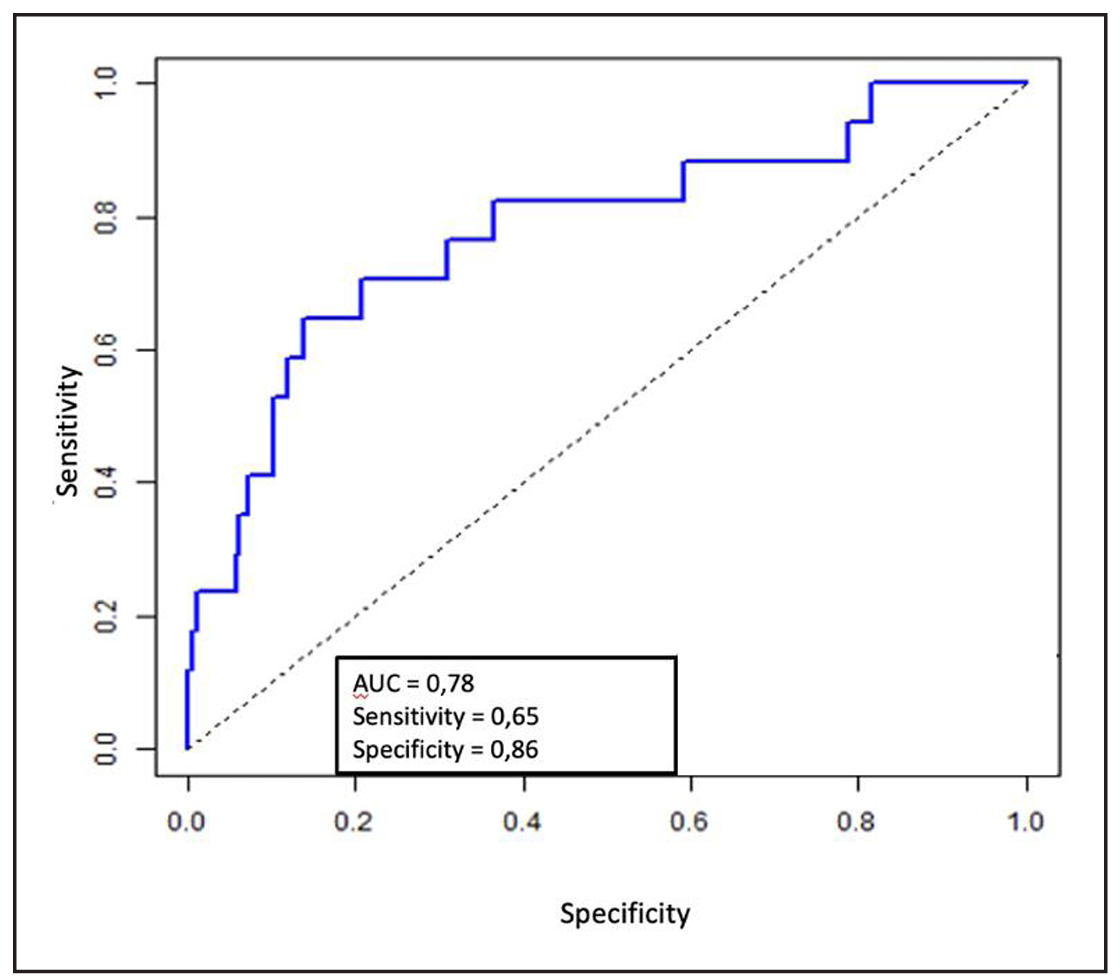
Figure 2. ROC curve of multivariate logistic regression for hyperthermia.
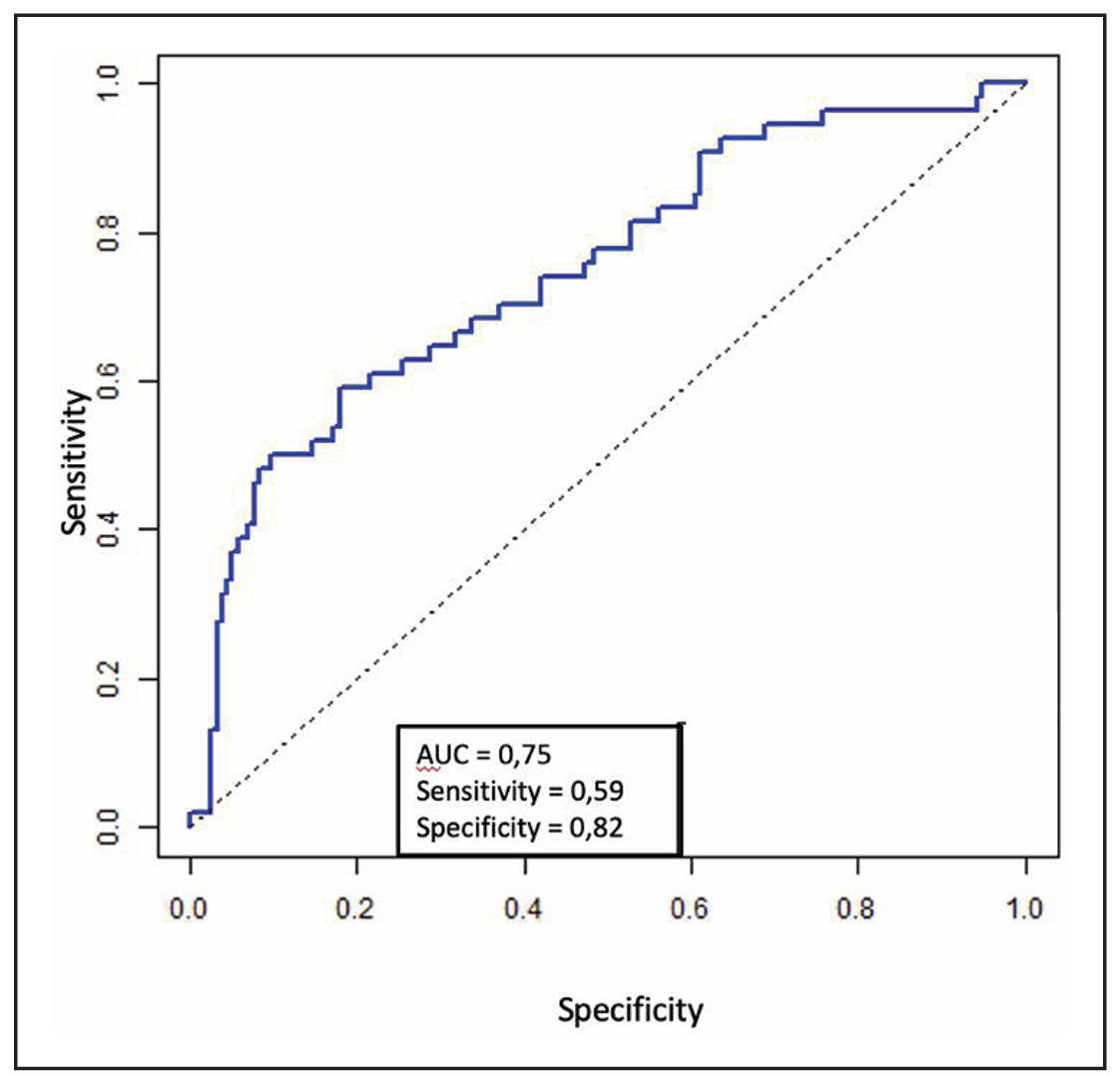
Figure 3. ROC curve of multivariate logistic regression for leukocytosis.
Discussion
Infectious complications remain one of the most significant
problems of endourology. According to the results of a meta-analysis
by R. Bapir et al. (2022), incidence of infectious complications does
not depend on type of endourological procedure [3]. The study demonstrated
that the risk of infectious complications remains similar for various procedures,
such as retrograde intrarenal surgery (RIRS), standard PCNL, mini-PCNL,
and tubless PCNL. The only advantage observed was the lower odds for fever
after PCNL with suctioning sheath, which is likely related to the decreased
intrarenal pressure. On the other hand, several authors have identified key
risk factors for infectious complications [10, 21, 22], including female gender,
leukocyturia, leukocytosis, high neutrophil-to-lymphocyte ratio, ureteral stents,
diabetes mellitus, stone size, multiple accesses to the kidney, operation time,
residual fragments, infectious stones, positive urine culture, positive pelvic
urine culture, and positive stone culture. Although bacteriuria is a well-recognized
risk factor for the development of infectious complications, current data indicate
that a significant portion of patients with positive urine cultures still undergo
surgical treatment. In a multicenter, retrospective study by J. Gutierrez et al. (2013),
an analysis of 5,354 patients treated with PCNL was conducted [20]. Preoperative
diagnostics revealed bacteriuria in 865 patients (16.2%), with the most common pathogen
being E. coli — 350 patients (6.5%). In the postoperative period, hyperthermia was
observed in 8.8% of patients with negative urine cultures and in 18.2% of those with
positive cultures. Unfortunately, to date, no clinical guideline includes recommendations
on the algorithm for the preoperative management of patients with bacteriuria; therefore,
the decision regarding the possibility of performing endoscopic surgery and decision on
the use of antibacterial prophylaxis regimen is left to the doctor. The current
literature contains data challenging the paradigm of the sufficiency of a single dose
of antibacterial medication in preventing infectious complications [23] and
demonstrating higher efficacy of extended (up to 7 days) courses of antibacterial
prophylaxis in preventing systemic inflammatory response and sepsis in patients
with high-risk factors.
Our study cohort, the proportion of patients with bacteriuria seeking surgical
treatment was 28.0%, with clinically significant bacteriuria identified in almost
half of the cases. The preliminary course of antibacterial therapy based on urine
culture achieved urine sterility in 79.0% of treated patients, with 11.0% still
having persistent clinically significant bacteriuria after treatment. Our study
also revealed a statistically significant association between bacteriuria and the
development of infectious complications. No statistically significant association
was found between infectious complications and other known risk factors, nor between
bacteriuria and urinary tract drainage, which was present in 35.0% of patients
compared to 26.0% in patients without stents or nephrostomy tubes.
Moderate fever rates in our study, that are consistent with global averages (8.0%),
along with the absence of complications such as purulent pyelonephritis, systemic inflammatory
response, and sepsis, suggest that endourologic procedures can be performed safely in patients
with positive urine culture provided adequate antimicrobial prophylaxis is given.
Limitations of the study include its retrospective design, as well as the non-inclusion
of patients with multidrug-resistant bacteriuria, which limits the results obtained to
the population of patients contaminated with non-hospital strains of bacteria.
Conclusions
Our study highlights that a positive urine culture is a significant risk factor for infectious complications following endoscopic surgery. Prolonged antibiotic prophylaxis for patients with clinically significant ASB appears to be an effective strategy to minimize the risk of postoperative infectious complications.
Declaration
Authors' contribution
V. Malkhasyan—study design development, data analysis, drafting the manuscript. N. Gadzhiev—study concept, scientific editing. S. Sukhikh—statistical data processing, drafting the manuscript. E. Maltsev—literature review; I. Kindarov—data acquisition, data analysis; D. Pushkar—supervision, study design development, critical review.
Financial support and sponsorship
None.
Conflicts of interest
Not applicable.
Ethical approval and informed consent
The study was designed according to the prescriptions of the Declaration of Helsinki (revised in Fortaleza, Brazil, October 2013). The study was approved by the Intercollegiate Ethics Committee (Protocol No 02-24 dated February 15, 2023).
References
1. Gadzhiev N, Prosyannikov M, Malkhasyan V, Akopyan G, Somani B, Sivkov A, et al. Urolithiasis prevalence in the Russian Federation: analysis of trends over a 15-year period. World J Urol, 2021, 39(10): 3939-3944. [Crossref]
2. Ghani KR, Roghmann F, Sammon JD, Trudeau V, Sukumar S, Rahbar H, et al. Emergency department visits in the United States for upper urinary tract stones: trends in hospitalization and charges. J Urol, 2014, 191(1): 90-96. [Crossref]
3. Bapir R, Bhatti KH, Eliwa A, García-Perdomo HA, Gherabi N, Hennessey D, et al. Infectious complications of endourological treatment of kidney stones: a meta-analysis of randomized clinical trials. Arch Ital Urol Androl, 2022, 94(1): 97-106. [Crossref]
4. Seitz C, Desai M, Häcker A, Hakenberg OW, Liatsikos E, Nagele U, et al. Incidence, prevention, and management of complications following percutaneous nephrolitholapaxy. Eur Urol, 2012, 61(1): 146-158. [Crossref]
5. Dybowski B, Bres-Niewada E, Rzeszutko M, Tkaczyk A, Woźniak B, Wójcik M, et al. Risk factors for infectious complications after retrograde intrarenal surgery - a systematic review and narrative synthesis. Cent European J Urol, 2021, 74(3): 437-445. [Crossref]
6. Senocak C, Ozcan C, Sahin T, Yilmaz G, Ozyuvali E, Sarikaya S, et al. Risk factors of infectious complications after flexible uretero-renoscopy with laser lithotripsy. Urol J, 2018, 15(4): 158-163. [Crossref]
7. Kreydin EI, & Eisner BH. Risk factors for sepsis after percutaneous renal stone surgery. Nat Rev Urol, 2013, 10(10): 598-605. [Crossref]
8. Li T, Sun XZ, Lai DH, Li X, & He YZ. Fever and systemic inflammatory response syndrome after retrograde intrarenal surgery: risk factors and predictive model. Kaohsiung J Med Sci, 2018, 34(7): 400-408. [Crossref]
9. Mi Q, Meng X, Meng L, Chen D, & Fang S. Risk factors for systemic inflammatory response syndrome induced by flexible ureteroscope combined with holmium laser lithotripsy. Biomed Res Int, 2020, 2020: 6842479. [Crossref]
10. Chen Y, Wen Y, Yu Q, Duan X, Wu W, & Zeng G. Percutaneous nephrolithotomy versus flexible ureteroscopic lithotripsy in the treatment of upper urinary tract stones: a meta-analysis comparing clinical efficacy and safety. BMC Urol, 2020, 20(1): 109-119. [Crossref]
11. Zhou G, Zhou Y, Chen R, Wang D, Zhou S, Zhong J, et al. The influencing factors of infectious complications after percutaneous nephrolithotomy: a systematic review and meta-analysis. Urolithiasis, 2022, 51(1): 17-27. [Crossref]
12. "Russian society of urologists" clinical recommendations "urolithiasis". Ministry of Health of the Russian Federation (2020). [Crossref]
13. Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American urological association/endourological society guideline, part I. J Urol, 2016, 196(4): 1153-1160. [Crossref]
14. Wolf JS, Jr., Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, & Schaeffer AJ. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol, 2008, 179(4): 1379-1390. [Crossref]
15. Sur RL, Krambeck AE, Large T, Bechis SK, Friedlander DF, Monga M, et al. A randomized controlled trial of preoperative prophylactic antibiotics for percutaneous nephrolithotomy in moderate to high infectious risk population: a report from the edge consortium. J Urol, 2021, 205(5): 1379-1386. [Crossref]
16. Yu J, Guo B, Yu J, Chen T, Han X, Niu Q, et al. Antibiotic prophylaxis in perioperative period of percutaneous nephrolithotomy: a systematic review and meta-analysis of comparative studies. World J Urol, 2020, 38(7): 1685-1700. [Crossref]
17. European association of urology. Guidelines on urolithiasis (2023). [Crossref]
18. Jung HD, Cho KS, Moon YJ, Chung DY, Kang DH, & Lee JY. Antibiotic prophylaxis for percutaneous nephrolithotomy: an updated systematic review and meta-analysis. PLoS One, 2022, 17(4): e0267233. [Crossref]
19. Xu P, Zhang S, Zhang Y, Zeng T, Chen D, Wu W, et al. Preoperative antibiotic therapy exceeding 7 days can minimize infectious complications after percutaneous nephrolithotomy in patients with positive urine culture. World J Urol, 2022, 40(1): 193-199. [Crossref]
20. Gutierrez J, Smith A, Geavlete P, Shah H, Kural AR, de Sio M, et al. Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol, 2013, 31(5): 1135-1140. [Crossref]
21. Bhojani N, Miller LE, Bhattacharyya S, Cutone B, & Chew BH. Risk factors for urosepsis after ureteroscopy for stone disease: a systematic review with meta-analysis. J Endourol, 2021, 35(7): 991-1000. [Crossref]
22. Wang F, Hong Y, Yang Z, & Ye L. Comparison of retrograde intrarenal surgery and standard percutaneous nephrolithotomy for management of stones at ureteropelvic junction with high-grade hydronephrosis. Sci Rep, 2021, 11(1): 14050. [Crossref]
23. Chew BH, Miller NL, Abbott JE, Lange D, Humphreys MR, Pais VM, Jr., et al. A randomized controlled trial of preoperative prophylactic antibiotics prior to percutaneous nephrolithotomy in a low infectious risk population: a report from the edge consortium. J Urol, 2018, 200(4): 801-808. [Crossref]