Open Access | Brief
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Early detection of non-muscle invasive bladder cancer with photodynamic diagnosis based on an advanced technology and a new imaging approach
* Corresponding author: Haefner Monika
Mailing address: Richard Wolf GmbH, Clinical Affairs Dept., Knittlingen, Germany.
Email: Monika.Haefner@richard-wolf.com
Received: 18 December 2024 / Revised: 14 January 2025 / Accepted: 20 January 2025 / Published: 26 March 2025
DOI: 10.31491/UTJ.2025.03.031
Abstract
Bladder cancer has a high incidence worldwide. Its early diagnosis
is crucial for the long-term course of the disease. Photodynamic diagnosis
(PDD) in the bladder, also referred to as blue light cystoscopy (BLC),
has been able to enhance cancer detection as an adjunct to white light
cystoscopy (WLC). The aim of this paper is to update information on the
technological advancements of a PDD medical device and the resulting
benefits in terms of improvement of detection of non-muscle-invasive
bladder cancer (NMIBC) in general and the aggressive carcinoma in situ
(CIS) in particular.
Patient summary: An advanced device technology combined with a new
imaging approach allows further enhanced bladder cancer detection
at an early stage, which is assumed to further reduce recurrence
and progression, and consequently minimize long-term treatment.
A major benefit is the improvement in quality of life.
Keywords
Photodynamic diagnosis, PDD, bladder cancer, carcinoma in situ, imaging technology
Worldwide, bladder cancer (BC) is the ninth most frequently
diagnosed cancer, with approximately 614,000 new cases and 220,000
deaths occurring in 2022 [1]. At diagnosis, about 75% of patients
have non-muscle invasive bladder cancer (NMIBC), whose early detection
should lead to a good long-term outcome. However, BC is also characterized
by a recurrence rate of up to 78% within 5 years and a possible progression
rate to muscle invasive disease in up to 17% at 1 year, and up to 45% at 5
years [2]. There are essentially three causes to which the high recurrence
rate is attributed: BC is often multifocal, spreading over large areas of
the bladder wall with some lesions being overlooked, sometimes tumor margins
are vague and hard to identify, and finally, early malignant lesions often
hardly stand out from the healthy tissue and therefore remain practically
invisible [3]. In particular, the high grade and in that sense aggressive
CIS belong to the group of barely visible early malignant lesions thus
specifically contributing to the high progression rate [4].
BLC has been established as an adjunct to WLC increasing the
diagnostic efficiency of NMIBC significantly [5]. Especially the
CIS detection rates were considerably increased when additionally
using BLC. However, still existing non-neglectable recurrence and
progression rates indicate that there is still potential for
technical improvement [6].
The principle of PDD/BLC is the selective accumulation of a
photosensitizer (PS) in cancerous tissue. The PS commonly
used in the bladder is protoporphyrin IX (PPIX). If bladder
tissue with accumulated PPIX is irradiated with short-wave
blue light, so-called excitation light, the PPIX emits red
fluorescence light thus highlighting the malignant lesions.
At the same time, endogenous fluorochromes in the mucosa and
submucosa of the healthy tissue, are also stimulated to emit
fluorescence light, a known phenomenon called autofluorescence.
In contrast to the red PPIX fluorescence light, this autofluorescence
light comes essentially from the long-wave blue and green and makes
the healthy tissue appear cyan or greenish [7].
With previous PDD devices, the autofluorescence intensity
has been very low and therefore has practically played no role for
the visualization of healthy tissue in BLC. In order to make the
healthy tissue still visible as background image, previous devices
have specifically detected a small amount of backscattered blue
excitation light in addition to the red PPIX fluorescence light.
Unfortunately, this additionally detected small amount of backscattered
blue light not only marks the healthy tissue, but also superimposes the
red PPIX fluorescence light originating from the lesions thus generating
a kind of blue offset affecting the entire endoscopic image including
the appearance of the lesions. Depending on the spectral range the
detected backscattered blue light originates from, and consequently
depending on the extent of the blue overlap at the lesion sites,
this approach of detecting a small amount of backscattered blue
excitation light can lead to a significant impairment of BLC in
terms of distinguishing between lesion and healthy tissue. If this blue
offset is strong enough at the lesion sites, it can adversely dominate
at least those malignant sites, which are characterized by an only
relatively weak red PPIX fluorescence, to such an extent that these
malignant sites are no longer visually distinguishable from the healthy
tissue. Such a reduced PPIX fluorescence light can originate from known
effects such as bleaching of the PS ( e.g. due to prolonged examination time),
a reduced number of cancer cells or a decreased thickness of the malignant
tissue layer (e.g. occurring at the tumor margins) or a tumor-tissue-specific
reduced PPIX accumulation (different tumors exhibit different metabolism of the PS).
Since these effects play a non-neglectable role in BLC, they contribute in a
corresponding way to the suboptimal tissue differentiation and thus to the
still noteworthy recurrence and progression rate with BLC.
From this perspective, a background image is required that is
based on a selectively and at the same time greatly reduced
light intensity at the lesion sites compared to the light
intensity of the surrounding healthy tissue. That means a
background image is needed, which maintains a sufficiently
good brightness of the healthy tissue, but also impairs as
little as possible the visualization of the red PPIX fluorescence
of the cancer cells and in this respect the optical highlighting of the tumor.
Chang et al. observed that with a PDD device based on the detection of
a small amount of backscattered blue excitation light, the blue channel
of the imaging system shows a smaller decrease in intensity at the
lesion sites than the green channel compared to the intensity of the
surrounding healthy tissue [8]. In this context, it is important to
realize that with such a PDD device the blue channel of the imaging
system is essentially supplied with detected backscattered blue
excitation light whereas the green channel is mainly supplied
with the (weak) autofluorescence light of the healthy tissue [8].
Pursuing the aforementioned idea of selectively and greatly
reducing the intensity of the background light at the lesion
sites, this observation suggests the use of autofluorescence
light instead of backscattered blue light as background image
for BLC. Kriegmair et al. quantified the decrease of blue
light-stimulated autofluorescence of lesions compared to the
autofluorescence of the surrounding healthy bladder tissue.
They found a strong difference in light intensity between malignant
and healthy tissue with a weak intensity at the lesion sites.
They concluded that the use of pure autofluorescence imaging has
the potential to increase the detection rates of bladder tumors [9].
In conclusion, when aiming for an optimum differentiation between
lesions and healthy tissue in terms of color-contrast these results
suggest to optimize and combine these two imaging techniques as complementary
approaches in order to further improve BLC: The pure PPIX fluorescence imaging
(BLC without the additional detection of backscattered blue excitation light)
optimized in terms of brightness provides a strong red signal from the lesions
without an additional blue offset, on the one side, and an autofluorescence
imaging, which equally optimized in terms of brightness , provides a strong
cyan or greenish signal exclusively from the surrounding healthy tissue avoiding
thereby any impairing color-offset, on the other side.
The biggest challenges with this approach are both a sufficiently strong
fluorescence intensity of PPIX accumulated in cancerous tissue and a
sufficiently strong autofluorescence intensity of the fluorochromes
of the healthy tissue.
With System blue (R. Wolf, Germany) these problems have been solved
by using a selected LED and an optimized illumination path for BLC.
The emission spectrum of the special blue light emitting LED is matched
to the absorption spectrum of PPIX. Light cable and endoscopes used are
equipped with special fibers characterized by a superior transmission
in the blue spectral range. All together result in an optimized fluorescence
excitation in both, lesions and healthy tissue. The detection of a small
amount of backscattered blue light, performed with former equipment, causing
a blue color offset in suspicious tissue and thus resulting in a hampered
tissue differentiation, can be avoided with such an enhanced PDD equipment
whose background image is now solely generated by the autofluorescence of
the healthy tissue. A special image processing in combination with 4K HD
technology helps to improve the differentiation between lesions and healthy
tissue even further. Finally, when performing BLC with System blue, the
healthy tissue appears in an inconspicuous cyan-/greenish-like pastel based
on the autofluorescence of the healthy urothelium, whereas tumor lesions
appear in a bright and striking red based on the fluorescence of PPIX
(Figure 1B shows a CIS with BLC which is practically invisible with WLC, Figure 1A).
Even tumor margins with a strongly reduced PPIX fluorescence can still be clearly
differentiated from the surrounding healthy tissue (Figure 2B shows the conspicuous
margin of a papillary tumor with BLC, Figure 2A shows the corresponding inconspicuous
site with WLC).
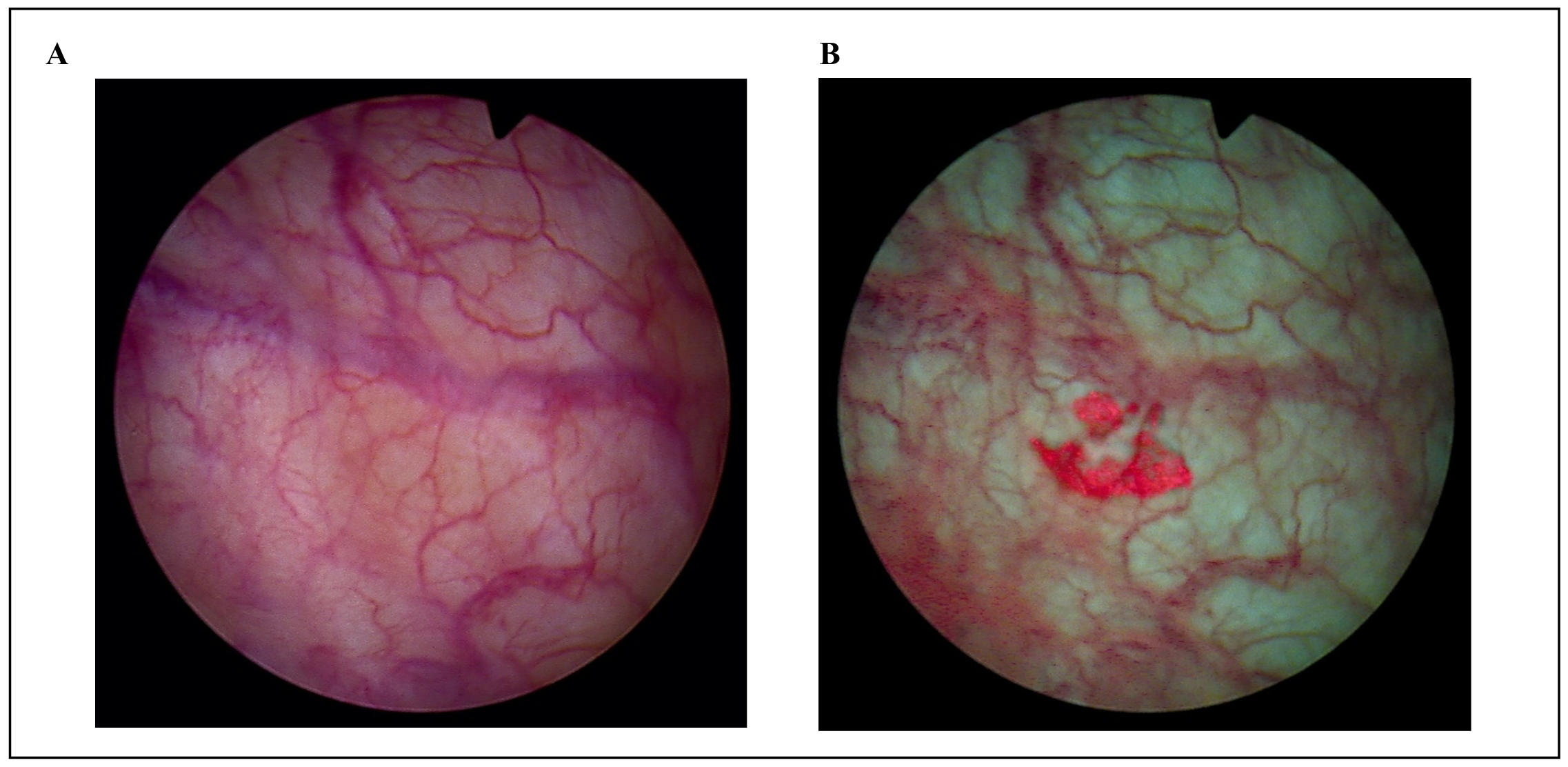
Figure 1. (A) CIS with WLC. (B) CIS with BLC (red fluorescing site).
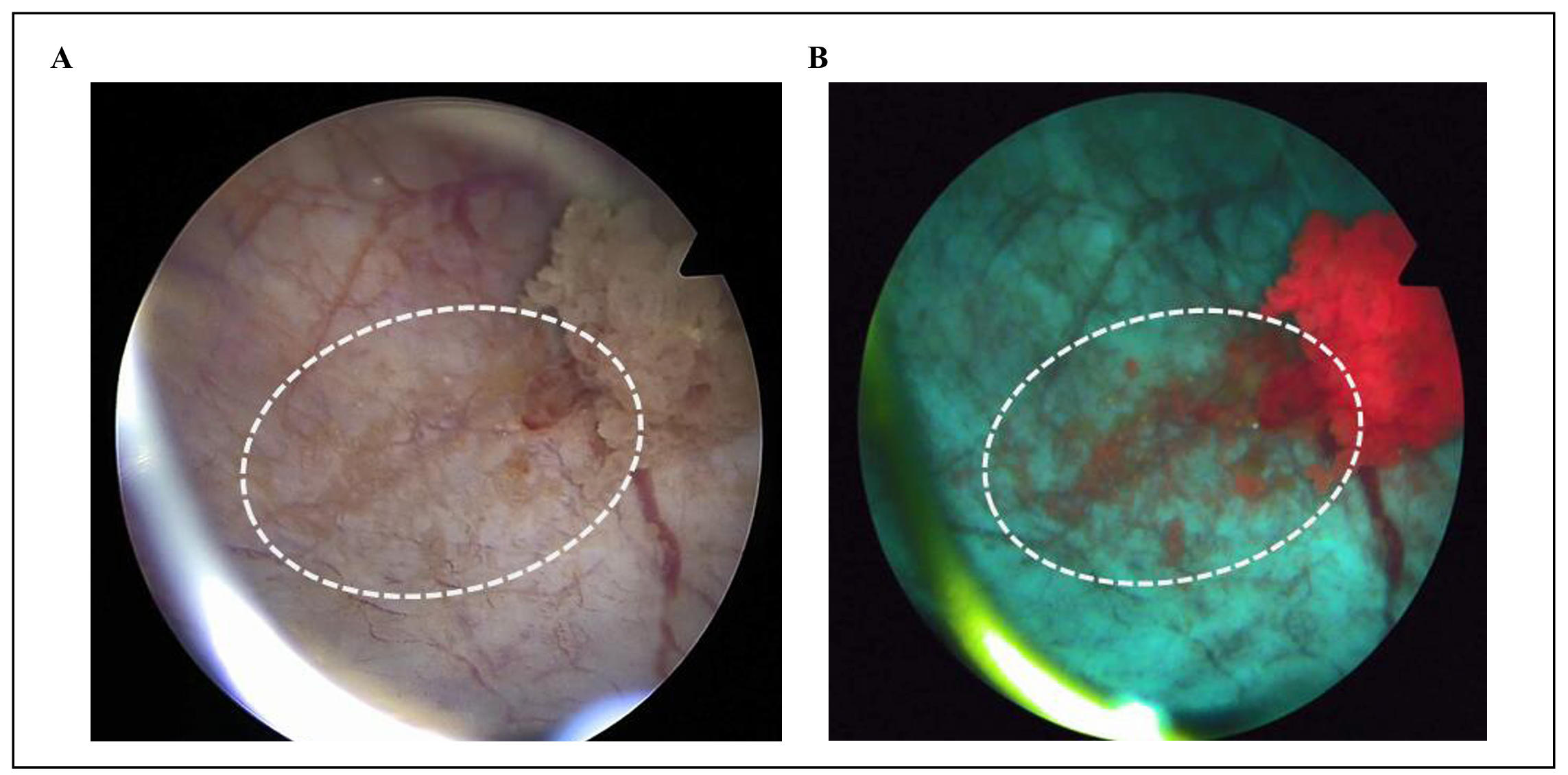
Figure 2. (A) Inconspicuous margin (dashed white ellipse) of a papillary tumor with WLC. (B) Conspicuous margin (dashed white ellipse) with BLC; clearly visible despite weak PPIX fluorescence.
In a multi-center trial the detection rate of NMIBC with BLC was compared
to WLC alone (NCT05600322) [10]. The improvement of the overall NMIBC
detection rate with BLC compared to WLC in this trial was 43.3% and was
therefore better than the improvement in previous trials conducted with
devices based on earlier technologies with values between 12% and 32% [3].
Particularly striking is the number of additionally detected CIS, which is
plus 200% in this trial with System blue compared to plus 24%–93.9% in
former trials with previous equipment [3]. In this context it should be
mentioned that System blue operates with an improved image resolution, namely
High Definition (HD), compared to the image resolution of the devices in former
studies [Standard Definition (SD)]. This means already when performing WLC, an
improved detection rate can be expected with the new device compared to the
previous PDD devices.
The significantly improved detection rate of NMIBC lesions in general and
CIS lesions in particular with BLC is mainly attributed to the combination
of the advanced technology with the new imaging approach, which is
implemented in the new PDD device, although additional factors such as
a different ethnicity, different experience of the physicians, and the
comparatively small number of patients in this trial must be taken into
account. Knowing that CIS lesions significantly contribute to the progression
rate and play a key role for the treatment plan, the clearly increased CIS
detection rate gains a special meaning, even today with other enhanced
imaging technologies available ( e.g. HD-WLC, NBI).
Conclusions
Enhanced imaging is crucial for improved bladder cancer detection. New technologies allow significant advancements in fluorescence excitation and at the same time form the prerequisite for a new imaging approach in BLC. In combination with an improved image resolution and an improved image processing, an increased detection rate of NMIBC lesions and especially CIS lesions could be achieved. Since above all the latter is a decisive factor in terms of progression rate, an improved long-term outcome should be expected with the new approach. Further studies are needed to reconfirm these findings.
Declarations
Ethical statement
None.
Funding
None.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
None.
Competing interests
All authors declare that they have no competing interests.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2024, 74(3): 229-263. [Crossref]
2. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol, 2006, 49(3): 466-465; discussion 475-467. [Crossref]
3. Di Stasi SM, De Carlo F, Pagliarulo V, Masedu F, Verri C, Celestino F, et al. Hexaminolevulinate hydrochloride in the detection of nonmuscle invasive cancer of the bladder. Ther Adv Urol, 2015, 7(6): 339-350. [Crossref]
4. Drejer D, Béji S, Oezeke R, Nielsen AM, Høyer S, Bjerklund Johansen TE, et al. Comparison of white light, photodynamic diagnosis, and narrow-band imaging in detection of carcinoma in situ or flat dysplasia at transurethral resection of the bladder: the DaBlaCa-8 study. Urology, 2017, 102: 138-142. [Crossref]
5. Konecki T, Kutwin P, Łowicki R, Juszczak AB, & Jabłonowski Z. Hexaminolevulinate in the management of nonmuscle invasive bladder cancer: a meta-analysis. Photobiomodul Photomed Laser Surg, 2019, 37(9): 551-558. [Crossref]
6. Das SK, Nasrallah A, Gu L, Eve CT, Parrish J, A. DH, et al. PD48-05Use of blue light cystoscopy among non-muscle invasive bladder cancer patients and outcomes in an equal access setting: a propensity scored matched analysis. Journal of Urology, 2024, 211(5S): e989. [Crossref]
7. Lange N, Jichlinski P, Zellweger M, Forrer M, Marti A, Guillou L, et al. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: a pilot study. Br J Cancer, 1999, 80(1-2): 185-193. [Crossref]
8. Chang S, Bermoy ME, Chang SS, Scarpato KR, Luckenbaugh AN, Kolouri S, et al. Enhancing the image quality of blue light cystoscopy through green-hue correction and fogginess removal. Sci Rep, 2023, 13(1): 21484. [Crossref]
9. Kriegmair MC, Honeck P, Theuring M, Bolenz C, & Ritter M. Wide-field autofluorescence-guided TUR-B for the detection of bladder cancer: a pilot study. World Journal of Urology, 2018, 36(5): 745-751. [Crossref]
10. Hanzhong Li, Hailong Hu, Jian Huang, Lulin Ma, Shudong Zhang, Jianming Guo, et al. MP71-18 Blue light cystoscopy versus white light cystoscopy for the detection of bladder cancer using modern HD 4K equipment: an analysis of pivotal and real-wrold data in China. Journal of Urology 2024, 211(5S): e1168. [Crossref]