Open Access | Mini-review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Factors influencing urinary incontinence after robot-assisted radical prostatectomy
* Corresponding author: Marco Rinaldi
Mailing address: Urology Unit, Santa Maria della Misericordia University Hospital, Udine, Italy.
Email: marrin91@yahoo.it
This article belongs to the Special Issue: Technologic advances on robot-assisted prostatectomy
Received: 25 November 2024 / Revised: 11 December 2024 / Accepted: 15 January 2025 / Published: 26 March 2025
DOI: 10.31491/UTJ.2024.12.026
Abstract
Regarding the management of prostate cancer, radical prostatectomy with subsequent urethral bladder anastomosis is one of the most effective options in terms of results according to the type and stage of the tumor. It has potential short- and long-term adverse events and some of them can have important consequences on a man’s quality of life, such as urinary incontinence and erectile dysfunction. The advent of new mini-invasive techniques, and in particular robotic surgery, has made it possible to reduce these side effects. In this mini-narrative review we discuss some of the most important factors that influence the urinary continence functional results and that Robot-assisted Radical prostatectomy has improved directly or indirectly by allowing to perform techniques with great finesse and precision.
Keywords
Robot-assisted radical prostatectomy, urinary incontinence, urethral-bladder anastomosis, anterior and posterior reconstructions, lateral approach
Introduction
Radical prostatectomy is a surgical procedure performed in case of prostate cancer and consists of the complete removal of prostate and seminal vesicles and subsequent anastomosis of the bladder neck with the urethra. It is one of the active treatments for localized prostate cancer and has an excellent oncology efficacy according to the type and stage of the disease. Some short and long-term complications are possible. Most frequent are erectile dysfunction (about 20–30% after 12 months) and urinary incontinence (about 3–16% after 12 months) [1, 2]. Urinary incontinence can have a significant impact on quality of life at any age, affecting psychological and social aspects of the patient’s life [3]. Techniques have been developed to reduce the risk of this complication over time and the advent of minimally invasive techniques, especially robotic surgery, has improved outcomes. There are several factors that can influence urinary continence recovery. Herein we describe those have shown more influence and how robotic surgery may have helped to improve them.
Influence factors
Vesico-urethral anastomosis technique and use of tension-free sutures
The anastomosis aims to reconstruct the continuity between bladder and urethra after removal of the prostate, preserving the integrity of the urethral rhabdosphincter. In open surgery, anastomosis techniques were used with 6 interrupted sutures positioned circumferentially. The development of laparoscopic and robotic minimally invasive techniques made it possible to use new continuous and barbed suture techniques (Figure 1). These new techniques have shown reduced anastomosis time and operating time, as well as lower catheterization times and lower rates of extravasation [4, 5]. In addition, a lower rate of urinary incontinence associated with the use of tension-free techniques has also been reported [6].
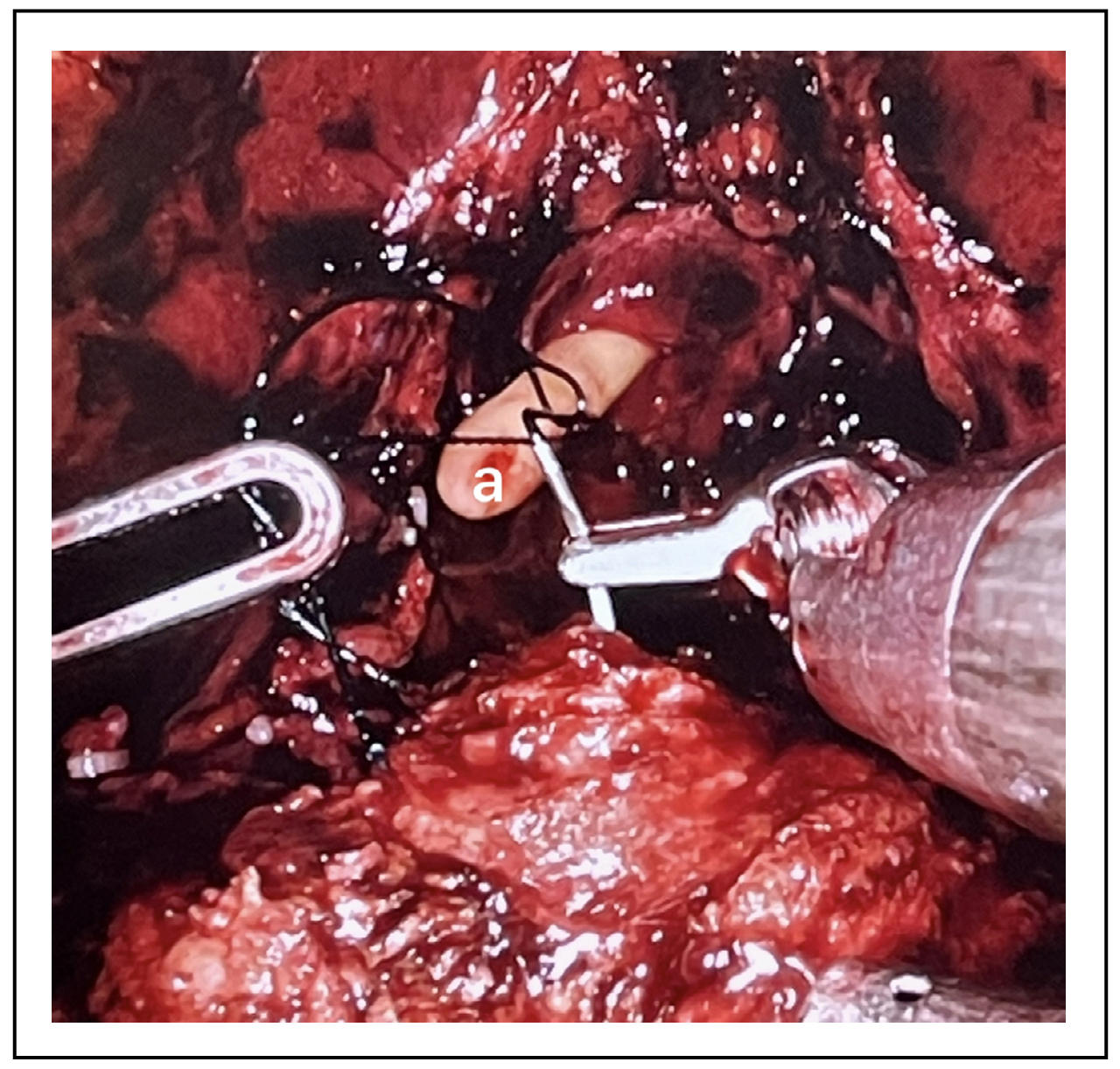
Figure 1. a-Continuous barbed suture.
Bladder neck preservation
Preservation of the bladder neck may improve continence rate after radical
prostatectomy. In particular, the use of robotic surgery has allowed to
perform the preservation of the neck in a more accurate way and this
affects urinary continence outcomes [7].
The neck is preserved effectively when it appears to have a diameter
almost equal to that of the urethra and does not need reconstruction.
Unfortunately, there are cases in which it is not possible to save the
bladder neck due to the presence of a voluminous median prostatic lobe
or previous transurethral resection of the prostate.
Unfortunately, sometimes preserving the bladder neck can increase
the possibility of positive margins, particularly for tumors of
the base of the prostate.
Lateral approach: About bladder neck preservation, among the
various techniques of neck incision, some have shown different
results on urinary continence. In particular, the lateral approach
to dissection of the bladder neck is described, which in some manuscripts
seems to show a greater return to urinary continence after 3 months
or more from the surgery [8] other than reduce the risk of postoperative
anastomosis leakage. The lateral approach to the incision of the bladder
neck requires the recognition of some anatomical structures, including
the tissue that covers the anterior prostate and bladder neck which is
called "detrusor apron" [9]. Laterally to such tissue and the bladder
neck is identifiable a fat triangular space bounded by the neck of the
bladder, base of the prostate and prostatic peduncle. Performing the
dissection at this level will access the front face of the seminal
vesicles that will be the depth landmark of the dissection. Starting
from the left, the "detrusor apron" can be cut transversely, accessing
the triangle of the left, until seeing the bladder neck and urethra fibers,
taking care to not damage the prostate and once reached the anterior plane
of the seminal vesicles the bladder neck’s anterior wall can be incised
(Figure 2 and Figure 3).
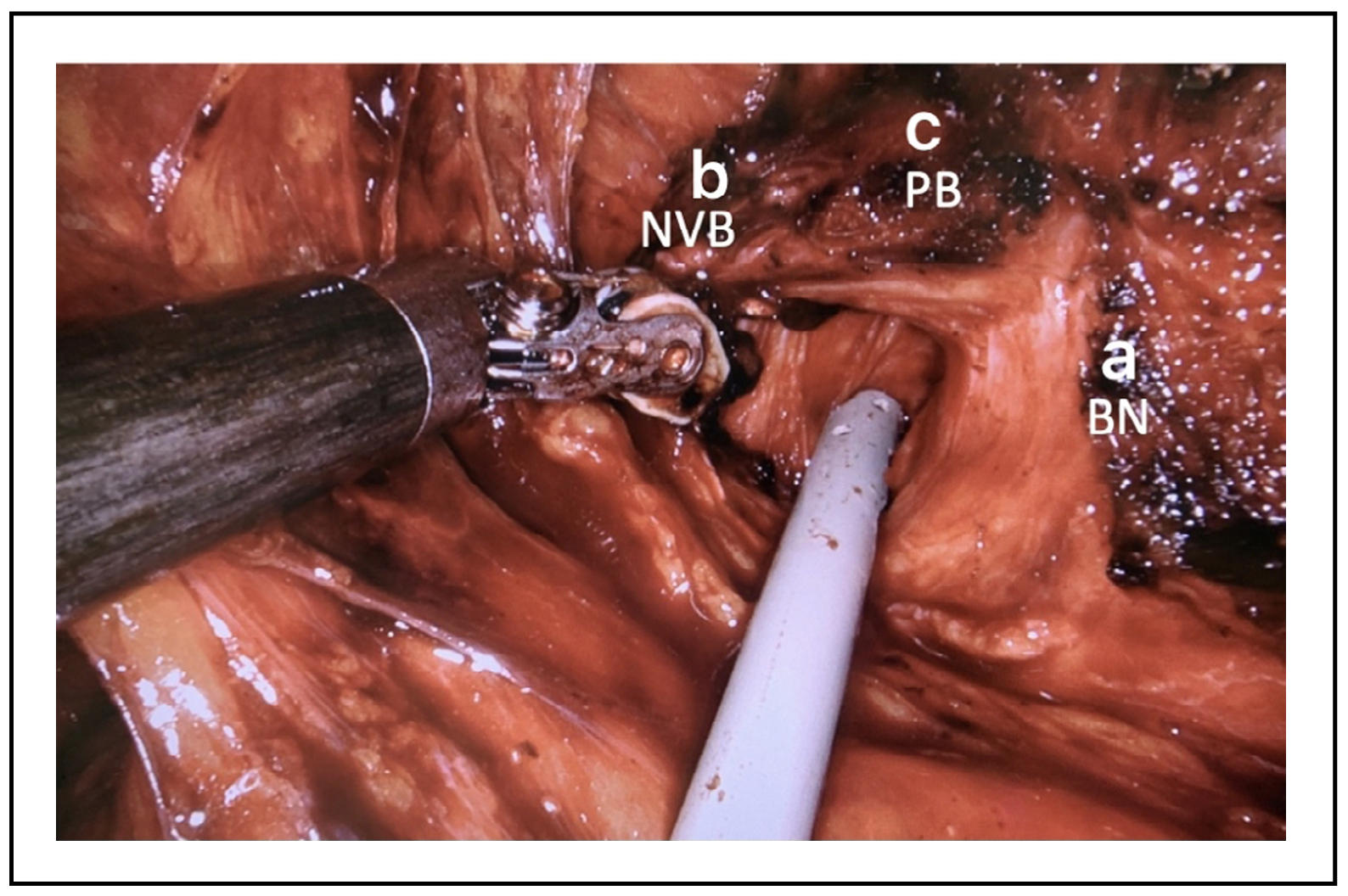
Figure 2. “Left triangle”. a-bladder neck (BN), b-prostate pedicle/neurovascular bundle (NVB), c-base of the prostate (PB).
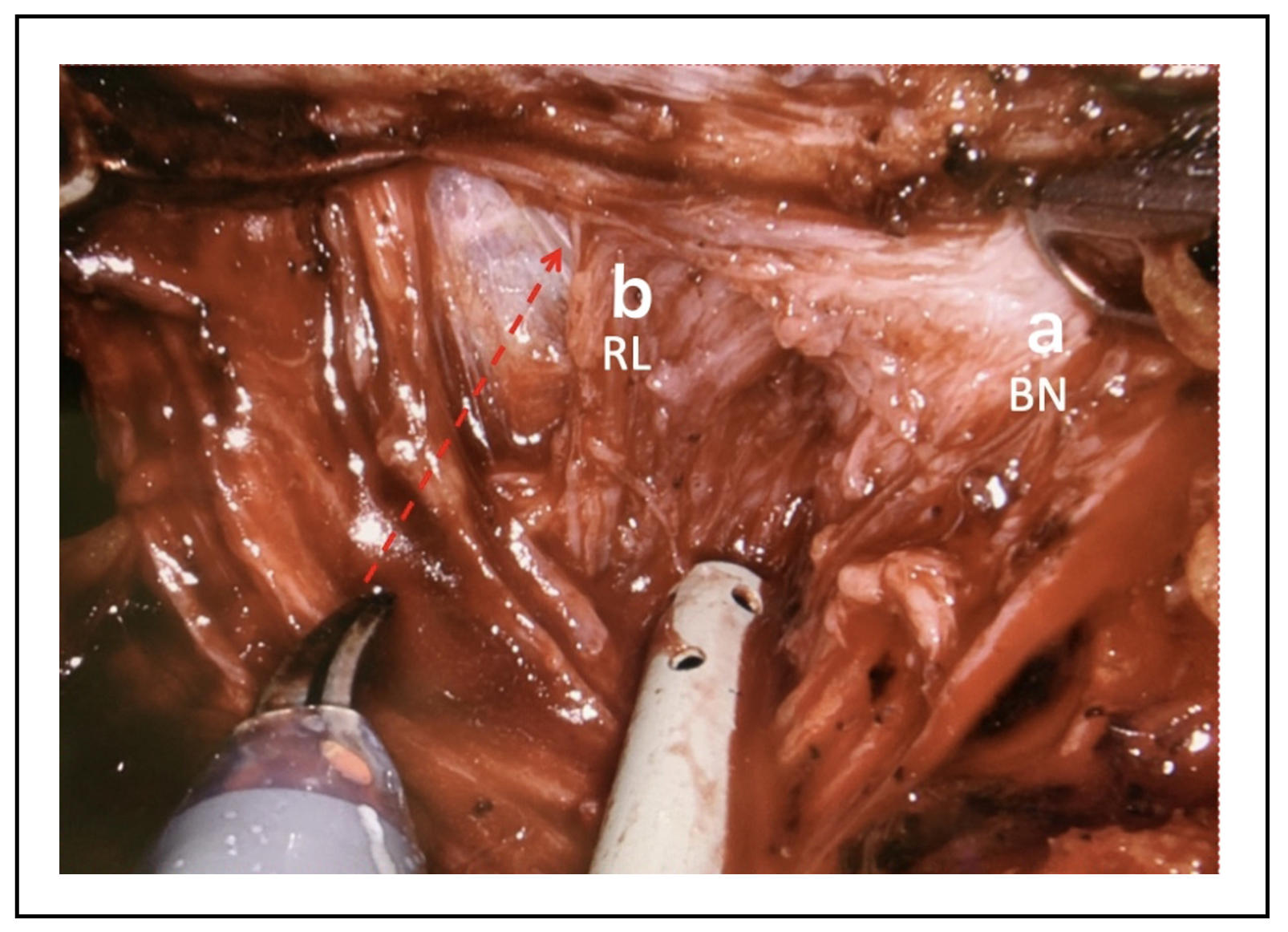
Figure 3. Dissection of the a-bladder neck (BN) maintaining, b-retrotrigonal layer (RL) of the right side over the trigone.
Preservation of the urethral length
Membranous urethra is located immediately apical to the prostate and the preservation of its length during radical prostatectomy has been seen to be directly related with a higher probability of urinary continence post-surgery (Figure 4). In addition, pre-operative length of the membranous urethra evaluation, which can be studied with MRI, is directly proportional to the probability of avoiding incontinence after surgery [10]. The use of robotic surgery, with magnification and improvement of image quality and reduction of gross motion errors would allow a greater capacity to preserve this length of urethra. It may happen that preserving the urethral length can cause a greater probability of positive margins in the case of prostatic apex cancers.
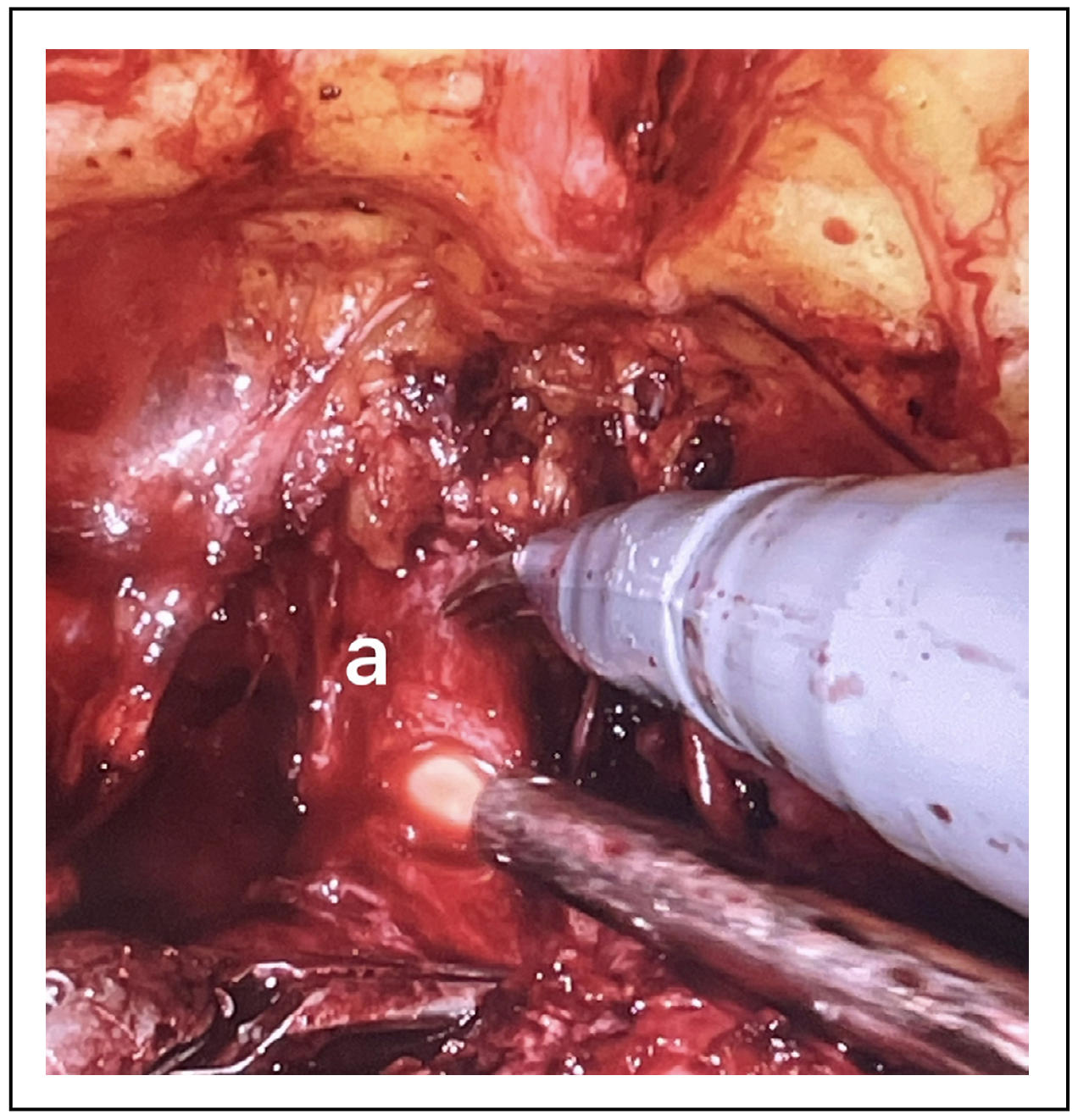
Figure 4. a-Urethral length preserved after anterior urethral incision.
Transurethral bladder catheterization
A shorter catheterization may reduce the rates of early urinary incontinence. Thus, indirectly, robotic surgery allowing shorter vesical catheterization time due to the safety of vesico-urethral anastomosis with positive impact on urinary continence recovery [11]. In addition, early catheter removal has been associated with lower rates of post-operative detrusor hyperactivity [12]. Moreover, the early removal of the bladder catheter did not show a lower seal of the vesico-urethral suture and did not increase the incidence of acute urinary retention [13].
Lateral pelvic fascia preservation technique
The lateral pelvic fascia (also called lateral prostatic fascia) preservation had shown to be effective in reducing urinary incontinence rates. It has found its greatest application in robotic surgery, which has made it possible to perform it simply, safely and precisely [14].
Anterior and posterior reconstructions techniques
The use of posterior reconstruction and anterior reconstruction techniques had shown to be effective in reducing incontinence rate. The posterior reconstruction can be performed as a Rocco’s stitch, that is a suture that brings together the denonvilliers' band, the muscle detrusor back and the rhabdosfincter rear, with the aim of reducing the tension of the anastomosis and creating a support for the anastomosis itself [15]. In some cases, always in order to reduce the tension of the anastomosis can be performed the modified technique sec. Patel, consisting in suspending the bladder neck from the pelvic fascia to avoid the dorsal-caudal movement of the anastomosis [14]. Anterior reconstruction is a procedure that consists in suspending the urethra to the anterior bladder neck, by passing the suture at the level of the periosteum of the pubis or on the complex formed by the dorsal venous complex bound and the puboprostatic ligaments [16] (Figure 5). In particular, the posterior reconstruction technique has been shown to allow a greater return to early continence. In addition, many studies, despite the difficulty of bias and causal multifactoriality, have shown positive results on urinary continence when both anterior and posterior reconstructions are used [17].
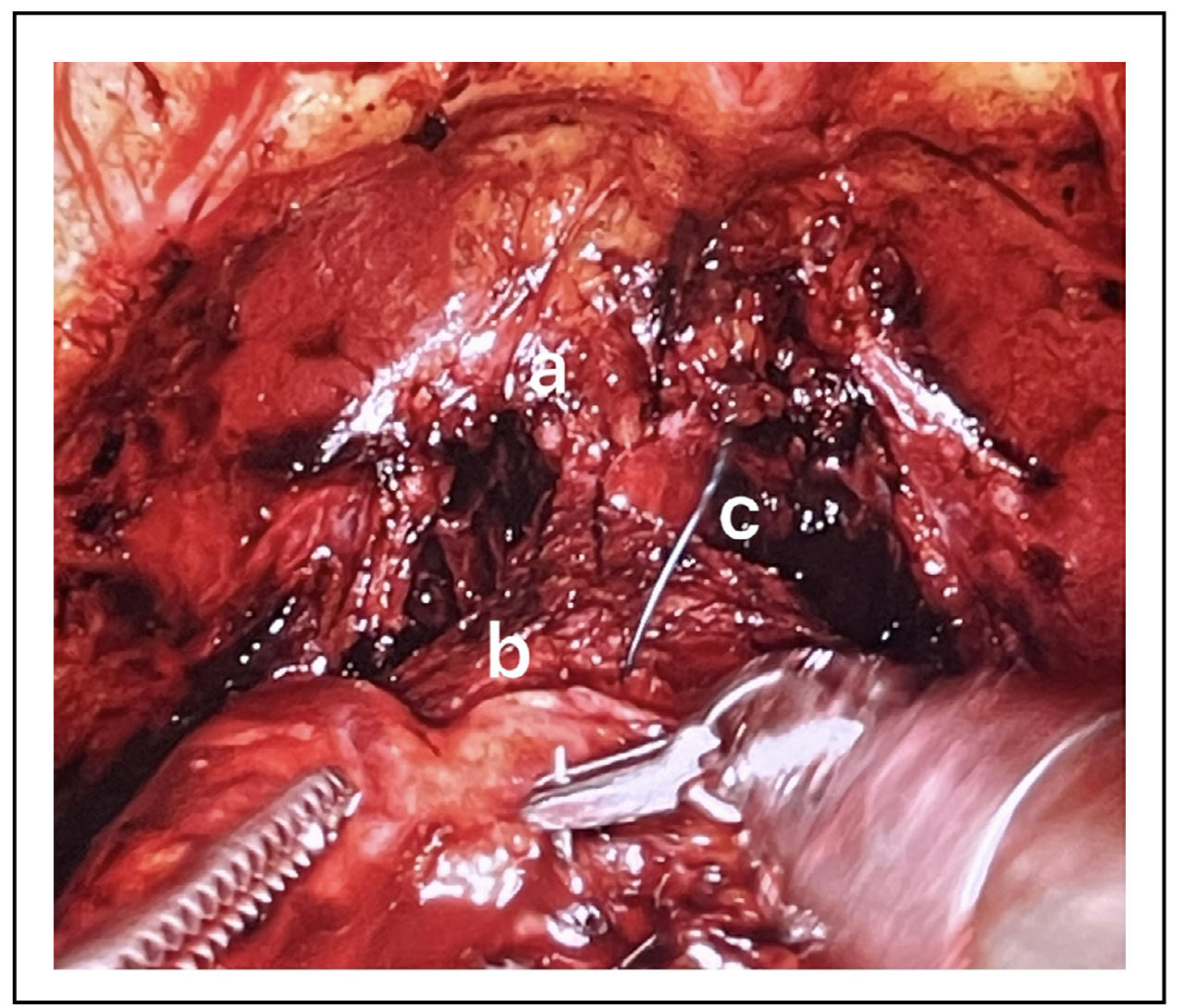
Figure 5. a-ligated dorsal venous complex, b-bladder neck anterior wall, c-anterior reconstruction..
Retzius-sparing techniques
It consists of a posterior approach through the space of the douglas that saves the anterior structures, involved in urinary continence. Robotic retzius-sparing radical prostatectomy (RS-RARP) techniques show better recovery rates of early urinary continence than standard robot-assisted radical prostatectomy.
Some limitations of the techniques
In despite of their efficacy on the urinary continence certain
procedures have limitations, such as:
- Sometimes preserving the bladder neck can increase the
possibility of positive margins, particularly for tumors of
the base of the prostate. Moreover, there are cases in which
it is not possible to save the bladder neck due to the presence
of a voluminous median prostatic lobe or previous transurethral
resection of the prostate;
- It may happen that preserving the urethral length can
cause a greater probability of positive margins in the
case of prostatic apex cancers.
- Although RS-RARP has shown in some studies a more early
recovery of continence, however showed higher positive
margin rates [18, 19].
Conclusions
Many new techniques aimed at improving urinary continence
outcomes have been found to be easy to introduce and/or better
applied through the use of robotic surgery.
Every technique where the use of improved images in quality
and size and a greater finesse of movements is crucial effectiveness
for the correct execution of the technique itself has found in robotic
surgery the easiest way to be able to develop and be applied.
This short narrative review allows to evaluate the factors that affect
urinary continence after robotic radical prostatectomy and how the
latter may have therefore allowed to improve the results also functional
thanks to the characteristics mentioned above. You must always keep in
mind that some of these techniques, as written before, may have limitations
due to the type of tumor and/or the patient’s anatomical condition, so it
is essential to perform a procedure that is as much as possible tailored to
the patient’s clinical case.
Declarations
Funding
None.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
None.
Competing interests
All authors declare that they have no competing interests.
References
1. Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol, 2012, 62(3): 418-430. [Crossref]
2. Ficarra V, Novara G, Rosen RC, Artibani W, Carroll PR, Costello A, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol, 2012, 62(3): 405-417. [Crossref]
3. Kohada Y, Hieda K, Miyamoto S, Tasaka R, Asami A, Akiyama K, et al. Retrospective evaluation of the improvement in the urinary status-related quality of life after robot-assisted radical prostatectomy. Int J Urol, 2023, 30(11): 1020-1027. [Crossref]
4. Wiatr T, Golabek T, Dudek P, Belch L, Przydacz M, Bukowczan J, et al. Single running suture versus single-knot running suture for vesicourethral anastomosis in laparoscopic radical prostatectomy: a prospective randomised comparative study. Urol Int, 2015, 95(4): 445-451. [Crossref]
5. Matsuyama H, Matsumoto H, Nagao K, Harada N, Hara T, & Sakano S. Running suture versus interrupted suture for vesicourethral anastomosis in retropubic radical prostatectomy: a randomized study. International Journal of Urology, 2015, 22(3): 271-277. [Crossref]
6. Tan G, Srivastava A, Grover S, Peters D, Dorsey P, Jr., Scott A, et al. Optimizing vesicourethral anastomosis healing after robot-assisted laparoscopic radical prostatectomy: lessons learned from three techniques in 1900 patients. J Endourol, 2010, 24(12): 1975-1983. [Crossref]
7. Ma X, Tang K, Yang C, Wu G, Xu N, Wang M, et al. Bladder neck preservation improves time to continence after radical prostatectomy: a systematic review and meta-analysis. Oncotarget, 2016, 7(41): 67463-67475. [Crossref]
8. Hashimoto T, Yoshioka K, Gondo T, Hasama K, Hirasawa Y, Nakashima J, et al. The Impact of lateral bladder neck preservation on urinary continence recovery after robot-assisted radical prostatectomy. J Endourol, 2018, 32(1): 40-45. [Crossref]
9. Myers RP. Detrusor apron, associated vascular plexus, and avascular plane: relevance to radical retropubic prostatectomy--anatomic and surgical commentary. Urology, 2002, 59(4): 472-479. [Crossref]
10. Rico L, Vitagliano G, López FM, Rios Pita H, Bonanno N, Nazar ME, et al. Urinary continence prediction after laparoscopic radical prostatectomy according to preoperative urethral length measurement in multiparametric prostate resonance. Arch Esp Urol, 2020, 73(9): 777-783.
11. Rossanese M, Crestani A, Palumbo V, Giannarini G, Inferrera A, Novara G, et al. Time of catheterization as an independent predictor of early urinary continence recovery after radical prostatectomy. Minerva Urol Nefrol, 2018, 70(4): 401-407. [Crossref]
12. Hao H, Chen X, Liu Y, Si L, Chen Y, Zhang M, et al. The impact of catheter removal time on urinary continence and overactive bladder symptoms after robot-assisted radical prostatectomy: a retrospective analysis of consecutive 432 cases from a single institution. Transl Androl Urol, 2022, 11(10): 1389-1398. [Crossref]
13. Kodzokov MA, Shpot EV, Akopyan GN, Proskura AV, Gasanov EN, & Gazimiev MA. Early urethral catheter removal after robot-assisted radical prostatectomy. Urologiia, 2022, (4): 5-9.
14. Shiota M, Tsukahara S, Ueda S, Mutaguchi J, Goto S, Kobayashi S, et al. Improved urinary continence recovery after robot-assisted radical prostatectomy with lateral pelvic fascia preservation. J Robot Surg, 2023, 17(6): 2721-2728. [Crossref]
15. Rocco F, Carmignani L, Acquati P, Gadda F, Dell'Orto P, Rocco B, et al. Restoration of posterior aspect of rhabdosphincter shortens continence time after radical retropubic prostatectomy. J Urol, 2006, 175(6): 2201-2206. [Crossref]
16. Vis AN, van der Poel HG, Ruiter AEC, Hu JC, Tewari AK, Rocco B, et al. Posterior, anterior, and periurethral surgical reconstruction of urinary continence mechanisms in robot-assisted radical prostatectomy: a description and video compilation of commonly performed surgical techniques. Eur Urol, 2019, 76(6): 814-822. [Crossref]
17. Rinaldi M, Porreca A, Di Lena S, Di Gianfrancesco L, Zazzara M, Scarcia M, et al. A matched-analysis on short-term and long-term (up to 5 years of follow-up) urinary incontinence outcomes after robot-assisted radical prostatectomy with and without anterior and posterior reconstruction: data on 1358 patients. Int Urol Nephrol, 2024, 56(1): 121-127. [Crossref]
18. Barakat B, Othman H, Gauger U, Wolff I, Hadaschik B, & Rehme C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: which technique is more beneficial for prostate cancer patients (MASTER Study)? A systematic review and meta-analysis. Eur Urol Focus, 2022, 8(4): 1060-1071. [Crossref]
19. Asimakopoulos AD, Miano R, Galfano A, Bocciardi AM, Vespasiani G, Spera E, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: critical appraisal of the anatomic landmarks for a complete intrafascial approach. Clin Anat, 2015, 28(7): 896-902. [Crossref]