Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Robot-assisted surgery vs. laparoscopic surgery in urology: a critical review
* Corresponding author: Vivek Vasudeo
Mailing address: Department of Urology, Max Superspeciality Hospital, Saket, New Delhi, 110017, India.
Email: vasudeo.vivek@gmail.com
This article belongs to the Special Issue: Robot-assisted surgery vs. laparoscopy surgery; which is better?
Received: 26 August 2024 / Revised: 12 September 2024 / Accepted: 09 October 2024 / Published: 27 December 2024
DOI: 10.31491/UTJ.2024.12.025
Abstract
The increasing use of robotic platforms in urology requires a thorough examination of their financial implications and advantages over conventional laparoscopic methods, especially in the context of a developing country. A review, albeit non-systematic, of the relevant literature spanning the last three decades was undertaken to shed light on the advantages and disadvantages of robot-assisted and traditional laparoscopic urological surgery. This review covered a variety of procedures including but not limited to radical prostatectomy, partial and radical nephrectomy, radical cystectomy, retroperitoneal and inguinal lymph node dissection and kidney transplantation. Recent advances in laparoscopic or robotic urology are also presented. The review showed almost parallel outcomes between robotic and conventional laparoscopic urological surgery for these procedures. However, robotic procedures were found to be significantly more expensive than their laparoscopic counterparts. Given the lack of definitive advantages of robotic procedures over traditional laparoscopy, coupled with the limited availability and significant costs associated with robotic technologies, laparoscopic surgery remains an important part of the medical landscape in developing countries.
Keywords
Laparoscopy, robot assisted, minimally invasive surgery, uro-oncology, single port
Introduction
Since its inception, surgery has undergone significant advances. Among these, minimally invasive surgery (MIS) is a notable development [1], with laparoscopy establishing itself as the preferred method for a wide range of urological procedures in the fields of uro-oncology, ablative urology and reconstructive urology [2, 3]. The applicability of laparoscopy continues to expand and is often preferred over traditional open surgical approaches due to its advantages such as reduced pain, shorter recovery times, faster return to normal activities, improved aesthetic outcomes and lower complication rates [4]. However, conventional laparoscopy presents challenges including limited range of motion, reliance on two-dimensional imaging, transmission of physiological tremor and fulcrum effect, longer learning curve and surgeon fatigue. The advent of robotic surgical techniques has addressed many of these issues. However, robotic surgery is not without its own limitations, yet it has been widely adopted both domestically and internationally. Due to the high initial and recurring costs, robotic surgery has not been widely adopted in many developing countries. It is important to ensure that these technological advances are based on scientific evidence so that informed decisions can be made about their risks and benefits. Therefore, this review aims to summarize the latest research on the effectiveness and status of laparoscopic and robotic surgery for various urological procedures.
Materials and methods
This study conducted a systematic literature search of the National Library of Medicine database (PubMed) using search terms related to various surgical procedures, including laparoscopic surgery, robotic surgery, urology, and specific procedures such as radical prostatectomy, radical and partial nephrectomy, radical cystectomy, neobladder, pyeloplasty, retroperitoneal and inguinal lymph node dissection, donor nephrectomy, and kidney transplantation. Appropriate MeSH terms and appropriate nesting and Boolean operators were used to modify the search. From January 1990 to March 2024, the search yielded 2061 articles. All comparative studies of lap and robotic surgery were selected. Reviews and meta-analyses were also included. Editorials, commentaries and letters to the editor were excluded. Case reports/series were only included if they were deemed necessary and contained relevant information. Additional studies were identified by cross-referencing, resulting in 152 articles that were considered relevant and subsequently analyzed (Figure 1). Some older publications were also referenced to provide historical context. Publications were excluded from the review if they were not accessible via PubMed or were not written in English. Additional searches were conducted for advances in laparoscopy and robotic surgery. Information from company websites and catalogues was used to describe newer or upcoming surgical tools or devices.
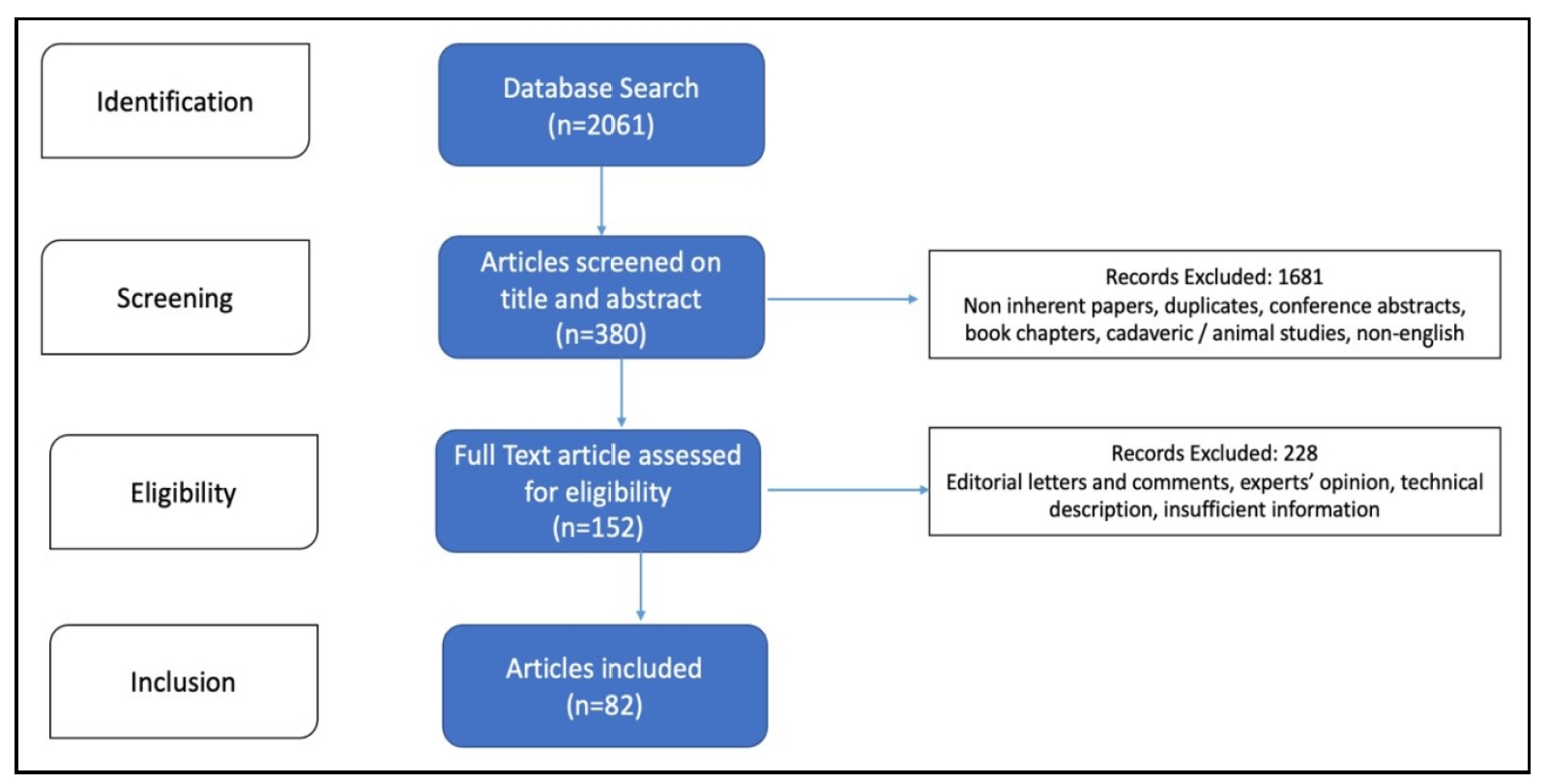
Figure 1. Preferred reporting items for systematic review and meta-analysis (PRISMA) flow diagram.
Result
Prostate cancer
The open perineal approach to radical prostatectomy (RP), first introduced by Young in 1904, remained the preferred
surgical route for almost one hundred years until Walsh revolutionized practice by introducing open
retropubic RP in 1982 [5]. The advent of laparoscopic RP (LRP) in 1997 marked
a significant advance, followed by the pioneering introduction of robot-assisted RP (RARP) using the da
Vinci Surgical System® by Binder in 2002 [6]. Due to its complexity
and steep learning curve, LRP was limited to those with extensive laparoscopic experience. The
integration of robotics is thought to make this complex procedure easier to perform. The number of randomized
controlled trials (RCTs) directly comparing these techniques is limited. Observational studies suggest that RARP
and LRP result in less estimated blood loss (EBL) and fewer transfusions than open RP, with comparable or better
complication rates [6]. In addition, analyses of observational studies
suggest improved urinary and sexual function outcomes after RARP compared with open RP [7].
RARP has also been reported to be less stressful for surgeons than traditional open RP [8].
Three randomized prospective trials of LRP versus RARP have been published. Two of these have published their extended
follow-ups, making a total of five. Of the three, one is a multicenter and patient-blinded study
conducted in four centers in Germany [9]. The other two are singlecenter
studies from Italy [10,
11]. There is a recent metaanalysis that includes the three trials
[12]. In terms of functional outcomes, RARP performed better than LRP in
terms of overall recovery of continence (OR = 1.60, 95%CI 1.16–2.20, P = 0.004), overall recovery of erectile
function (OR = 4.07, 95%CI 2.51–6.60, P < 0.001). This was also true for recovery of continence
and potency at 3 months (OR = 1.51 and 4.25), 6 months (OR = 2.66 and 3.52) and 12 months (OR = 3.52 and 3.59).
Both groups were similar in terms of blood loss, catheter indwelling time, overall complication rate, overall
positive surgical margin and biochemical recurrence rate. Advances in surgical techniques such as the development
of the hood technique, Retzius sparing, etc. have also contributed to the improved results with RARP
[13, 14].
According to current global standards, no single surgical technique is recommended as superior [3],
suggesting that surgeon expertise and hospital volume are more critical factors in
optimizing functional and oncological outcomes [6].
Kidney cancer
For localized kidney cancer, laparoscopic radical nephrectomy is considered the standard of care
[2]. Studies have shown that the robotic approach to radical nephrectomy offers no
clear advantage over conventional laparoscopy and is not cost-effective [15].
The most recent global standard [2] advocates partial nephrectomy (PN) as
the preferred treatment for T1 renal tumors, citing its improved oncologic outcomes for T2 tumors in certain
cases [16]. The initial introduction of laparoscopic partial nephrectomy
(LPN) by Winfield and colleagues in 1993 was a major advance, offering significant advantages over
traditional open PN (OPN) by reducing wound complications, decreasing postoperative pain, and facilitating earlier
hospital discharge [2]. The longterm oncologic efficacy of LPN has
been confirmed [17], establishing it as first-line therapy for T1a renal
tumors. However, performing LPN poses a significant challenge to surgeons, both mentally and physically, due
to the demanding nature of intricate laparoscopic procedures that require intracorporeal suturing within a limited
time frame to prevent ischemic damage to the kidney while maintaining effective hemostasis [18].
Gettman and colleagues first introduced robot-assisted partial nephrectomy (RAPN) in 2004 [19].
This surgical technique increases the precision of tumor removal and simplifies
suturing within the body [18]. However, the evidence supporting RAPN
comes from observational studies, and there are no head-to-head RCTs comparing RAPN with LPN. The success of
these surgical options for partial nephrectomy is evaluated based on triple outcomes, including clear surgical
margins, minimal reduction in renal function, and perioperative safety [20].
In a comprehensive meta-analysis by Cacciamani et al. [21], which
included 98 studies and 20,282 patients, RAPN patients were more likely to have hilar tumors and higher
RENAL scores. RAPN outperformed LPN in several areas: warm ischemia time (WIT) was reduced (OR = 4.21, 95% CI 2.24–6.17,
P < 0.00001), there was less need for transfusion (OR = 1.37, 95% CI 2.23–7.20, P <
0. 00001), fewer operative complications (OR = 2.05, 95%CI 1.51–2.80, P < 0.00001), and lower rates of
conversion to OPN (OR = 2.61, 95% CI 1.11–6.15, P = 0.03) and radical nephrectomy (OR = 4.00, 95% CI
2.23–7.20, P < 0.00001). Furthermore, RAPN was associated with a lower positive surgical margin (PSM)
rate (OR = 2.01, 95%CI 1.52–2.66, P < 0.00001) and a lower percentage decline in estimated glomerular
filtration rate (eGFR) (OR = -1.97, 95% CI -3.57– 0.36, P = 0.02), indicating a benefit over LPN. Operative
time, estimated blood loss (EBL), 30-day readmission rates, cancer recurrence and cancerrelated mortality
did not differ significantly between the two cohorts.
A separate meta-analysis by Leow and colleagues [22] found a reduction in
complications, WIT and PSM rates with RAPN compared to LPN. In addition, the learning curve for RAPN has
been reported to be shorter than for LPN [23], and RAPN has facilitated
the introduction of innovative renorrhaphy techniques, such as the sliding clip technique [24],
which have contributed to a reduction in WIT. However, RAPN is significantly more expensive than LPN
[25], which limits its widespread
use, especially in resource-limited settings. In addition, the long-term oncologic safety of RAPN has not been
fully evaluated, and existing studies have had limited follow-up [2].
In a recent systematic review, robotic partial nephrectomy was associated with lower WIT, longer operative time, and
no difference in Clavein III and IV complications. RAPN also appears to improve oncologic control and
surgeon ergonomics without increasing postoperative complications [26].
However, there are no completed or ongoing RCTs to provide high-level evidence in favor of either approach.
Off-clamp minimally invasive PN is not new, initially performed under controlled hypotension and later mainly by
"super-selective microdissection" and control prior to tumor excision. It eliminates the possibility of
ischemic renal injury. Long-term results for off-clamp lap PN are reported from a single high-volume center
[27]. There are many studies comparing on- versus off-clamp PN, but the
data are conflicting. A randomized trial comparing onversus off-clamp laparoscopic PN found no difference in perioperative
and early functional outcomes [28]. A multicenter study
found no difference in preservation of renal function, but off-clamp RAPN was associated with increased conversion to
radical nephrectomy and blood transfusion [29]. A recent review
and meta-analysis concluded that off-clamp RAPN may offer better renal function preservation and lower margin positivity
rates [30]. We could not find any study comparing
laparoscopic and robotic off-clamp PN.
Urinary bladder and upper tract urothelial carcinoma
The advent of laparoscopic radical cystectomy (LRC) was first documented by Sanchez and colleagues in 1993
[31], followed a decade later by the introduction of robot-assisted radical
cystectomy (RARC) by Menon and colleagues in 2003 [32]. Initially supported by
limited series and single center reports, the body of evidence for LRC and RARC has expanded to include
a growing corpus of RCTs and meta-analyses, facilitating direct comparisons with open radical cystectomy (ORC) and
among themselves [33,
34]. The evaluation of LRC and RARC versus ORC involves a tripartite analysis
of outcomes: perioperative surgical performance, completeness of the surgical procedure, and long-term
cancer control. Perioperative metrics include operative time, EBL, need for transfusion, rate of perioperative complications,
and length of hospital stay (LOS). Surgical thoroughness is measured by the PSM rate, which ranges
from 1% to 6.3% in ORC [35, 36],
and lymph node (LN) sampling, with an optimal number of 10 to 14 nodes required for accurate disease
staging [37].
A direct comparison between RARC and LRC was performed in a meta-analysis by Feng et al.
[34], which included 2 RCTs and 8 observational studies. This study found that RARC
had better outcomes in terms of LOS, complication rate, and LN yield compared to LRC, although LRC was associated with
reduced EBL. The rate of PSM was comparable between the two groups. Longterm oncologic outcomes over a
5-year period were recently reported in the CORAL trial [38], which showed
no significant differences between ORC, LRC, and RARC in these metrics. The study was limited by its small
sample size and single-center design.
Kowalewski et al. performed a systematic review and network meta-analysis of RCTs comparing the three
approaches with the primary outcomes of overall survival (OS) and relapse-free survival (RFS), which would only
be performed if there was sufficient evidence to compare all three approaches [39].
There was insufficient evidence to perform a network meta-analysis of all three approaches for the
primary outcomes. Although the data showed no significant differences in OS between RARC and ORC, there was insufficient
evidence to make a comparison with LRC. This indirectly shows the trend towards increased use of RARC
for minimally invasive radical cystectomy. Regarding total intracorporeal urinary diversion, we could not find a
head-to-head comparison between laparoscopic and robotic intracorporeal diversion. Although laparoscopic
intracorporeal ileal neobladder was introduced more than 20 years ago [40],
it has not become routine due to the complexity of the procedure as evidenced by the limited number of
small series [41]. At the same time, robotic cystectomy came later, but robotic
intracorporeal diversion continued to increase, probably because of easier and less time-consuming
suturing with the robot [42, 43].
Radical nephroureterectomy (RNU) with bladder cuff excision (BCE) and lymphadenectomy is the standard of care for
localized high-risk upper tract urothelial carcinoma (UTUC). The evidence for this rare condition comes only
from retrospective/prospective observational studies. Studies comparing laparoscopic RNU + BCE with robotic RNU
suggest that the robotic approach has better lymphadenectomy rates and probably better cancer-specific survival
rates [44-46]. The better
survival may be attributed to better staging and receipt of adjuvant therapy due to better lymphadenectomy rates.
Another advantage of the robotic approach is the ability to perform partial bladder cuff excision robotically.
It is not that it cannot be done laparoscopically, but the literature shows few studies that have done total
laparoscopic RNU and BCE [47], while the majority go with a mixed approach
of laparoscopic RNU and open BCE [48,
49].
Testicular cancer
The primary scenarios in which retroperitoneal lymph node dissection (RPLND) is currently recommended for Ca Testis
include stage 2A and early stages of stage 2B disease, as well as its use as a salvage treatment after
chemotherapy in cases where tumor markers have returned to normal [50].
In this context, open RPLND is the benchmark procedure. However, it is associated with significant adverse
effects and a prolonged recovery period, leading to the exploration of less invasive techniques for RPLND. The
concept of laparoscopic RPLND (L-RPLND) was introduced in 1992 [51].
Despite initial skepticism regarding its efficacy, the comprehensive review by Rassweiler and colleagues, which
included data from more than 800 individuals, established the efficacy of L-RPLND [52].
This review showed that performing lymph node dissection using modified templates resulted in an average of 16
lymph nodes retrieved, with a range of 5 to 36 nodes. When
compared with the traditional open technique, no significant differences were observed between laparoscopic
retroperitoneal lymph node dissection (L-RPLND) in terms of recurrence rates, the proportion of patients receiving
chemotherapy (29% for L-RPLND vs. 31% for open surgery), and the frequency of subsequent salvage
surgery (1.2% for L-RPLND vs. 1.5% for open surgery). Nevertheless, L-RPLND presents a significant learning
curve and challenges such as management of bleeding near the major vessels, in addition to its limited applicability
in cases requiring bilateral template RPLND and surgery after chemotherapy [53].
Therefore, the widespread adoption of L-RPLND awaits further validation, and it is currently recommended only for
specialists in whom it demonstrates equivalent efficacy to open
RPLND with less morbidity [50].
The introduction of robot-assisted RPLND (RA-RPLND) represents the forefront of innovation in the field, driven
by the benefits of enhanced three-dimensional visualization and improved robotic dexterity [53].
However, the evidence supporting RA-RPLND comes primarily from case studies and limited retrospective analyses
[54]. A review by Tselos
et al. of retrospective studies involving 116 patients found that outcomes for RA-RPLND were similar to
those for L-RPLND, including a median lymph node count of 22.3, an overall positive detection rate of 26%, and
a complication rate of 8% [55].
Direct comparisons between L-RPLND and RA-RPLND are rare, but a study by Harris et al. showed comparable
results in terms of perioperative morbidity and short-term cancer control [56].
However, notable disadvantages of robotic surgery include significantly higher costs and potential delays in bleeding
control for significant vascular injury as the lead surgeon
operates remotely from the patient [56].
Robotic and laparoscopic RPLND for testicular cancer in the post-chemotherapy setting is also safe and feasible.
These laparoscopic cases should be appropriately planned and performed in low to moderate volume residual
masses. Similarly, RA RPLND in the post-chemotherapy setting should be limited to surgeons with good experience
in robotic surgery and a well-accessible location and limited volume of residual tumor
[57-59].
Penile cancer
Although rare, penile cancer typically requires removal of the penile tumor and dissection of the inguinal lymph nodes as the primary treatment strategy. The traditional method of open inguinal lymph node dissection carries a morbidity risk of approximately 50%, including complications such as lymphedema (21%), lymphocele (21%), wound infection (26%), and flap necrosis (41%) [60]. The introduction of video endoscopic inguinal lymphadenectomy (VEIL) using conventional laparoscopic instruments in 2005 has shown a decrease in surgical morbidity while maintaining the integrity of cancer treatment outcomes [61]. Despite these advances, VEIL remains a complicated and physically demanding procedure. The introduction of robot-assisted VEIL (RA VEIL) by Josephson and colleagues in 2009 marked a significant milestone [62]. Initial observations have validated the feasibility of RA VEIL, suggesting a reduction in complications and length of hospital stay [63]. However, comparative analyses of VEIL and RA VEIL are scarce and mostly retrospective. A retrospective evaluation by Russel and his team showed that the results of VEIL and RA VEIL are similar [64]. Currently, there is no clear advantage of robotic surgery over laparoscopic surgery for lymph node dissection in penile cancer, highlighting the critical need for further research in this area.
Benign conditions: ureteropelvic junction obstruction
The surgical technique known as Anderson-Hynes dismembered pyeloplasty was originally introduced in 1949 as a method
to treat obstructions at the ureteropelvic junction (UPJ). It was first described in a case of a retrocaval
ureter [65]. Initially performed through an open surgical approach, this
method has significant disadvantages, including the significant discomfort and potential complications of a
flank incision, as well as postoperative pain [66 ]. These challenges have
paved the way for the introduction of laparoscopic pyeloplasty (LP), which represents a significant
improvement by reducing morbidity, decreasing the need for postoperative pain management, and facilitating faster
hospital discharge, although it requires longer operative times [67]. However,
the laparoscopic approach requires a high level of proficiency in laparoscopic suturing techniques and involves a
significant learning curve [68]. With the growing interest in robotic surgery
due to its precision, minimized tremor, and increased surgical flexibility, an increasing number of centers are now offering
robot-assisted pyeloplasty (RAP) for both adults and children.
While most studies comparing LP and RAP are observational [69], there are a
limited number of randomized trials directly comparing the two methods [70].
Previous reviews and analyses comparing LP with RAP have yielded mixed results [71].
However, a recent network meta-analysis by Uhlig and colleagues, focusing primarily on adult patients diagnosed with UPJO,
analyzed the outcomes of open pyeloplasty, LP, RAP, and endopyelotomy [70]. This
study found that RAP had a significantly higher rate of surgical success than LP, although the likelihood of perioperative
problems, instances of urine leakage, need for additional surgeries, frequency of blood transfusions, and length of hospital
stay (LOS) were similar between the two methods. When the analysis was limited to studies that included 1-year follow-up
(13 of 24 studies), LP and RAP showed no difference in success rates. The majority of these studies
were retrospective, and only one randomized trial was included. The analysis also showed that RAP was significantly more
expensive than LP. In addition, another meta-analysis of 14 observational studies comparing LP and RAP
in pediatric patients found that RAP not only had a higher success rate but also a shorter LOS [72].
Ureteric reconstruction
Traditional open ureteral reimplantation remains the standard of care for benign conditions such as vesicoureteral
reflux (VUR), megaureter, or obstruction. Laparoscopic ureteral reimplantation (LUR) has been shown to reduce
EBL, hospital LOS, and patient discomfort compared to the traditional open approach [73].
Robot-assisted ureteral reimplantation (RAUR), introduced in 2003, has been the subject of
several observational studies. These studies typically find no significant differences in mean operative time, EBL, and LOS when
comparing RAUR to LUR, with both methods achieving success rates close to 100% [73].
However, RAUR has a shorter follow-up period in these studies compared to LUR. In pediatric cases of VUR, RAUR has been studied,
but there is no consensus, with some reports suggesting that it has higher complication rates and less favorable outcomes
than open surgery [74].
Simple prostatectomy (SP) is another urological procedure where MIS has become increasingly common. A large multi-institutional
study of laparoscopic and robotic SP evaluated the factors associated with favorable trifecta
outcomes (International Prostate Symptom Score (IPSS) < 8, maximum flow rate (Qmax) > 15 mL/s, and no perioperative
complications). They found that the trifecta outcome was not significantly influenced by the two
approaches [75]. A recent systematic review and meta-analysis aimed to perform
a pooled analysis to compare the safety and efficacy profiles of laparoscopic and robotic SP. They
found that robotic SP had a higher Qmax (WMD = 2.15 mL/s, 95%CI 3.75–0.55, P = 0.0009) and comparable IPSS
and sexual health inventory (SHIM) compared to laparoscopic SP [76].
Donor nephrectomy and kidney transplantation
Laparoscopic donor nephrectomy (LDN) has become the preferred method for harvesting kidneys for transplantation, offering
advantages such as reduced blood loss, less postoperative pain, and faster hospital discharge
[77]. However, LDN requires considerable skill due to its complex nature
[78]. Robot-assisted donor nephrectomy (RADN), introduced by Horgan
and colleagues [79], is currently performed in a limited number of centers
worldwide. However, the definitive advantage of RADN over LDN remains to be proven. Comparative RCTs are
scarce [80] and often involve small numbers of participants. A meta-analysis by
Wang et al. [81], which included 2 RCTs and 5
retrospective studies with 514 patients, showed that LDN was associated with significantly shorter operative time, lower
estimated blood loss, and shorter warm ischemia time. Factors such as removal of the kidney by an
assisting surgeon and removal of the robotic arms prior to extraction may increase warm ischemia time in RADN procedures.
In addition, RADN has been reported to be associated with higher costs [82]. We
do not see any advantage of RADN over LDN. RADN is just a more expensive way to do the same procedure.
The first laparoscopic kidney transplant (LKT) from a living related donor was performed in April 2009
[83]. Modi et al. further demonstrated the safety and efficacy of LKT
in 2011, and subsequent studies have confirmed these findings, indicating that LKT outcomes, including eGFR at both 1
month and 1 year, are comparable to those of open kidney transplantation (OKT) [84].
However, the LKT literature is limited to a few case reports.
In 2014, Menon et al. presented a detailed account of robot-assisted kidney transplantation (RAKT) using regional
hypothermia and found that creatinine clearance rates from the first postoperative day were
comparable to OKT in cases performed by surgeons experienced in robotic procedures [61].
Bansal et al. described an independent analysis of the learning curve and some key
modifications to the RAKT technique. The learning curve for technical outcome (total anastomosis time) was 24 cases and for
functional outcome (serum creatinine at days 7 and 30) was 15 to 25 cases. Major technical
modifications included arterial and ureteral spatulation on the table, use of polypropylene 50 suture in the graft vessels
to facilitate intraoperative handling, keeping the anterior arterial wall smaller to visualize the
posterior arterial wall anastomosis, and leaving a small amount of fatty tissue on the supero-lateral surface of the kidney
for handling after jacket removal [85]. The same group
also reported the results of robot-assisted pediatric renal transplantation. They found it to be safe and had similar
outcomes with less pain and better cosmesis compared to the open technique [86].
Although the adoption of RAKT has increased nationally, the complexity and sensitivity of the surgical process, particularly
the vascular anastomosis, has limited the expansion of minimally invasive renal transplantation
techniques. Since RAKT's inception, no significant new research on LKT has emerged post-2014. Nevertheless, OKT remains the
benchmark procedure, even as the clinical indications for choosing RAKT continue to grow.
Advances in robotics
Advances in MIS include the use of single port laparoscopy and robotic technology. The first reports of single port
laparoscopic urologic surgery were found in 2008 [87]. The
technique is better known as laparoendoscopic single site (LESS) surgery. Despite reports of improved perioperative
and cosmetic benefits, LESS surgery with a purely laparoscopic approach never became popular and was not
adopted by many centers. This was primarily due to the long learning curve and inherent instrumentation limitations.
Eventually, a dedicated single-port robotic system was developed with the da Vinci SP robotic system, which
received FDA clearance for use in urologic surgery in 2018. The SP da Vinci system has been used for radical prostatectomy,
nephrectomy and cystectomy, and reconstructive surgery [88]. Recently, a
dedicated single-port robotic system, the SHURUI Robotic Surgical System (SHURUI Robotics, Beijing, China), has reported
its safety and feasibility in radical prostatectomy and retroperitoneal partial nephrectomy
[89, 90]. It has a dualcontinuum
structure instead of cable-driven wrists, which they
claim has a higher payload capacity and greater reliability. It also has the shortest insertion length. Finally, the
cost is as low as laparoscopic surgery.
The latest multiport system from intuitive surgical is the da Vinci 5 robotic system. This claims to provide an enhanced
surgical sensation by enabling force feedback technology, further improved vision system and improved
ergonomics and will be an exciting development to watch in the future [91].
Following the 20-year dominance of the da Vinci robotic platform, numerous multiport robotic surgical
systems have been developed and introduced into clinical practice over the past decade. These platforms include the
Senhance robotic system, the CMR-Versius robotic system, and the Hugo RAS, Avatera, Hinotori, Mantra, and
Dexter [92].
The Hinotori Surgical Robot System (Medicaroid, Japan) has multiple robotic arms extending from a single bedside
patient cart and an enclosed surgeon's console like a da Vinci robot. The robotic arms have eight axes of
operation, which is expected to reduce inter-arm or arm-toassistant interference and provide a smoother operation.
Another feature is its dockless design, in which the pivot (instrument support point) is set by software.
This results in a clear workspace around the trocar. The surgeon's console is also said to be more ergonomic
[93]. The Avatera system (Avateramedical GmbH, Germany), also a multiarm
surgical robot, is particularly space saving. The surgical robot has four arms with slender instruments (5 mm diameter).
The control unit has a slim eyepiece and does not obstruct the surgeon's mouth or ears, improving
communication between the surgeon and the team. They have a strict single-use concept for instruments, so surgeons
always work with new, reliable instruments, eliminating the risk of cross-contamination and the need for
cleaning and sterilization procedure [94].
Other multiport robotic systems such as Hugo, Versius, Senhance, SSI Mantra and Dexter have independent arm carriages,
unlike the systems mentioned above. The HugoTM Robotic Assisted Surgery (RAS) system
(Medtronic, Minneapolis, USA) is designed to provide a more ergonomic and personalized working environment. Notable
benefits include superior dexterity and range of motion, HD 3D visualization, open console, haptic feedback
and remote telemetry. Advanced safety features include force sensing, collision avoidance and joint lockout
[95]. The Versius surgical robotic system was introduced by Cambridge
Medical Robotics (CMR, UK). It features an open console that allows the surgeon to operate in a sitting or standing
position, reducing stress and fatigue. Up to five lightweight robotic arms can be used as separate units.
VWrist technology provides 360 degrees of wrist motion, 7 DOF and haptic feedback [96].
The Senhance Surgical System (Ascensus Surgical, NC, USA), uses familiar laparoscopic surgical
techniques to improve safety and efficiency without a steep learning curve. It also has an open console architecture
with hand controls similar to laparoscopic instrument handles. It is equipped with haptic sensing,
eye-tracking camera control, a 3 and 5 mm reusable instrument portfolio, and a digital fulcrum to minimize torque
at the incision site [97]. The Swiss Dexter robotic system
(Distalmotion Switzerland) features a compact, modular and mobile design that allows it to be easily moved and shared
between operating rooms. It is compatible with all OR equipment, including imaging systems and energy
devices. Thus, the system integrates into the intraoperative workflow and protects existing investments
[98]. Another low-cost surgical robot comes from India. The SSi Mantra 3 (SS
Innovations, India) has a surgeon command center, patient-side robotic arm carts, and a vision cart. The surgeon
command center has an open console design and is equipped with a large 4K 3D monitor, 2D touch monitor system
controls, an ergonomic hand control device, and a head tracking safety feature. The vision cart has the same monitor as
the command center, providing the same 3D vision to the bedside assistant. The articulating endoscope is
used for different levels of vision without changing the scope [92]. The development
of these robotic platforms will definitely bring down the cost and gain wide acceptance.
Advances in laparoscopy
The biggest challenge in conventional laparoscopy is hand-eye coordination in a three-dimensional (3D) field observed
on a 2D display. The introduction of 3D laparoscopic vision improves surgical precision and hand-eye
coordination, making laparoscopy more acceptable, safe and cost-effective. It has low investment and maintenance costs
and reduces the learning curve. The 3D feature of laparoscopy brings it closer to robotic systems [93].
The next limitation of laparoscopy is the need for the assistant to hold the camera and its inherent problems such as
tremors and improper direction and angle. The endoscope holders/manipulators solve this problem to a large
extent, where the control of the camera is only with the operating surgeon. SOLOASSIST II (Aktormed GmbH, Germany) provides
stable and precise positioning of the endoscope for a steady image with unique range of motion with
unobstructed access to the patient site with manual or voice control [94].
Emaro (Riverfield, Japan) is a pneumatic endoscope manipulator that moves with the surgeon's head. An
inertial measurement unit (IMU) is mounted on the surgeon's head to detect movement [95].
Freehand has developed two collaborative robots (cobots)—Vista and Panorama—for different
types of surgery. Vista was developed for urological surgery. The camera is held by a 3-joint modular arm that provides
a stable platform. It also consists of Instinctive Motion Control (IMC) through a headset to select the
direction of movement based on the surgeon's head movement [96].
Laparoscopic surgery is made easier and more precise with the use of articulating instruments. These offer greater
dexterity and degrees of freedom compared to conventional instruments. ArtiSenial (LIVSMED, Republic of
Korea) is one such instrument that allows intuitive movement of the endoscope to match the surgeon's fingers and wrist.
There is also a "hold" mode in which the end effector can be frozen at any desired angle. The difficult
task of laparoscopic suturing can be made as easy as robotics with this tool [97].
HandX from Human Xtensions (Meril Life, India) is a handheld, fully articulating, software-driven
laparoscopic tool that translates basic hand movements into complex movements within the surgical field. It has an
interchangeable articulating head that fits through 5 mm ports. The tool is quick to set up and offers
ergonomic advantages [98].
The LevitaTM Magnetic Surgical System (LMSS) (MARSTM from Levita Magnetics) is another recent addition.
It is a first-of-its-kind device that uses magnets to assist in trocarless retraction, reducing the number of
ports in laparoscopic and robotic procedures. Initially used in laparoscopic cholecystectomy and bariatric surgery,
its first application in urology was robotic radical prostatectomy. Fulla and colleagues published their
initial clinical experience in patients undergoing either laparoscopic or robotic (da Vinci Xi or single port)
renal surgery. The first prospective, multicenter, single-arm, open-label study of the safety and feasibility of
the robotic magnetic surgical system was published in November 2022. The study included 30 patients undergoing
laparoscopic cholecystectomy and laparoscopic bariatric surgery and found the system to be safe and feasible with
reduced incisions and increased surgeon control [92].
The future
The future of minimally invasive surgery is very exciting and will see many new developments. The penetration of various robotic systems is increasing and is likely to continue to increase due to lower costs and widespread availability. The integration of virtual reality (VR), augmented reality (AR) and mixed reality into surgery is already underway. Artificial intelligence and machine learning will come in a big way. The latest generation da Vinci 5 has 10,000 times the processing power of the previous generation. It is designed to enable the future of AI and machine learning in surgery. The next game changer could be tele-mentoring in surgery. There are many ethical issues associated with tele-surgery. With advances in telecommunications and the internet, tele-mentoring can be a viable option where the trainee's console is connected to the proctor's console, even if both consoles are in different locations. This allows the proctor to train many trainees without having to travel to his hospital. Finally, in the future, biochemical, molecular, or isotopic imaging will be available to preferentially illuminate the lesion and help the surgeon perform a precise dissection.
Discussion
When evaluating traditional laparoscopy versus robotassisted surgical approaches, it's important to consider
their impact from three critical perspectives: the nature of the surgery, the patient's outlook, and the surgeon's
experience. Robot-assisted techniques offer several significant technological improvements over conventional
laparoscopy. Equipped with a sophisticated dual 3-chip camera, these systems provide a three-dimensional image
coupled with a magnification range of 10-12X, significantly improving depth perception. In addition, the integration
of "Endo Wrist" technology into robotic instruments introduces an innovative pivot point near the tip of the
instrument, which significantly enhances movement flexibility. The tremor filtering and motion scaling capabilities,
which can reach a ratio of up to 3:1, are critical for performing intricate maneuvers within the surgical
field [99]. These advancements are particularly beneficial for surgeries
performed in confined spaces such as the pelvis, leading to the predominant use of robotic surgery in
procedures such as radical prostatectomy (RP) and various gynecologic surgeries. The addition of 4K and 3D vision
and the use of Firefly technology in laparoscopic surgery has improved laparoscopic surgery and brought it
closer to robotic surgery.
However, despite the accumulation of more than three decades of research data, there remains a noticeable lack
of clear benefits of robotic assistance on key surgical outcomes such as operative time (OT), EBL, PSM rates, and
LOS. In addition, the lack of tactile feedback is a notable limitation of the robotic platform.
The use of robotic techniques in nearly all aspects of urologic surgery has been shown to be feasible; however,
feasibility should not be confused with superiority. The desire for robotic procedures is largely driven by
patient preference, a trend strongly influenced by direct-to-consumer advertising and the absence of competing
technologies, which may lead to inflated expectations of robotic efficacy. An increasing number of patients are
requesting robotic surgery; many hospitals are adopting and promoting robotic surgery even though its benefits
have not been conclusively demonstrated. An Italian group evaluated the rate of lost patents due to the absence
of a robotic platform at their center. They found that the presence of a robotic platform would have increased the
surgical volume of radical prostatectomy by 49%, as these patients chose to undergo robotic surgery
elsewhere. Despite no technique showing superiority in terms of oncologic or functional outcomes, eligible patients
choose to be operated on elsewhere due to the lack of a robotic system [100].
The da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA) has an initial price tag of about $2 million,
with annual maintenance costs of about $100,000 and disposable costs of about $1,500 per case
[101-103]. Few hospitals can
afford such costs without increasing the volume and cost of robotic surgery, which is a critical issue,
especially in developing countries. In addition, the cost of robotic surgery is not covered by all health insurance
plans. The investment for a single robotic unit is comparable to the cost of establishing 15-20 laparoscopic
centers, which may be a more economical approach. Given the backlog of urologic patients awaiting surgery, judicious
resource allocation is prudent. New technologies must meet five criteria for widespread adoption:
affordability, acceptability, accessibility, availability, and appropriateness for the general population. In
addition, comprehensive long-term safety and efficiency data for robotic systems in urologic oncology procedures
compared to conventional laparoscopy remain elusive. It's important to remember that robots, as mechanical entities,
are prone to operational failures that are significantly underreported. In emergencies, the time required
to switch from robotic to laparoscopic or open surgery could be critically delayed [102].
Robotic systems have been highlighted as beneficial to surgeons. When surgeons operate from a master console away
from the patient, the surgical process is ergonomically improved and less stressful for the surgeon. However,
there are conflicting reports, with over 50% of surgeons surveyed experiencing symptoms such as neck stiffness and
fatigue in the fingers and eyes, which correlate with the amount of time spent at the console. In addition,
surgeons who perform a greater number of robotic procedures per year report increased low back stiffness
[101]. The ergonomic benefits of robotic surgery for surgeons are still
under investigation and require thorough evaluation using standardized questionnaires and metrics. While robotic
assistance may shorten the learning curve for certain procedures, its benefits must be weighed against the
costs associated with health care and patient expenses, particularly in developing countries. In addition, shortening
the learning curve for laparoscopy could also be achieved through adequate training in residency programs
from the outset. Dependence on technology should not replace the need for expertise. High patient volumes in developing
countries, such as India, can help surgeons improve their skills, increase their proficiency, and
achieve better outcomes in laparoscopic procedures [103]. Ongoing advances
in laparoscopic technology, including the introduction of 4K ultra-high-definition systems with 3D vision,
sophisticated sealing tools, robotic laparoscopic instruments, and ergonomically designed platforms with chest supports,
armrests, and camera mounts, may provide a cost-effective alternative that achieves comparable efficacy
to robotic technologies [1, 104].
Current guidelines within the field of urology do not favor the use of robotic surgery over laparoscopic techniques
and vice versa. This view is shared by specialties outside of urology as well as several governmental
organizations. The National Health Service in England has stated that there is insufficient evidence to justify the
expenditure on robot-assisted radical cystectomy [101].
Similarly, the American College of Obstetricians and Gynecologists has stated in a committee opinion that the
effectiveness of robotic assistance in performing hysterectomies for nonmalignant conditions remains equivocal,
indicating a need for additional research to support its adoption [105].
In addition, a safety and effectiveness analysis conducted by the Society of American Gastrointestinal and
Endoscopic Surgeons found that although robotic assistance in gastrointestinal surgery is considered safe and provides
outcomes comparable to traditional laparoscopic methods, it does not necessarily provide better outcomes
and may result in increased costs for certain gastrointestinal procedures [106].
In developing countries with limited availability of robotics in centers and long waiting lists,
surgeons need to acquire adequate laparoscopic skills to perform cases in a minimally invasive manner for the greater
benefit of the poor population. Therefore, the adoption of laparoscopic surgery by such urologists is
important even in the era of robotic surgery.
Conclusions
This review aims to critically evaluate the comparative studies between laparoscopy and robotic surgical techniques, especially in the context of developing countries where access to robotic surgery remains limited. Current evidence suggests that outcomes for patients undergoing laparoscopic surgery are broadly comparable to those of robot-assisted surgery for a variety of urologic procedures. While robotic surgery offers improved visualization, ergonomic advantages, and the ability to perform more precise maneuvers, it remains unclear whether these advantages translate into significantly better outcomes in clinical practice and further comprehensive studies are needed. Despite the clear advantages of robotic surgery over conventional laparoscopy, the high cost of robotic systems limits their widespread adoption. Innovations in laparoscopic technology, such as wristed instruments and 3D imaging, may address some of laparoscopy's shortcomings at a fraction of the cost of robotic alternatives. Currently, laparoscopic approaches to urological surgery are gaining popularity, especially in developing countries with limited resources. Investment in new surgical technologies should be guided by critical analysis and evidence-based decision making.
Declarations
Authors' Contributions
Made substantial contributions to conception, design of the study and proof reading: Kumar A, Data analysis, interpretation and manuscript writing: Vasudeo V.
Availability of Data and Materials
Not applicable.
Financial Support and Sponsorship
None.
Conflict of Interest
All authors declared that there are no conflicts of interest.
Ethical Approval and Informed consent
Not applicable.
Consent for Publication
Not applicable.
References
1. Rassweiler JJ, & Teber D. Advances in laparoscopic surgery in urology. Nat Rev Urol, 2016, 13(7): 387-399. [Crossref]
2. Renal cell carcinoma. 2024, from https://uroweb.org/guidelines/renal-cell-carcinoma.
3. Prostate cancer. 2024, from https://uroweb.org/guidelines/prostate-cancer.
4. Luk ACO, Pandian RMK, & Heer R. Laparoscopic renal surgery is here to stay. Arab J Urol, 2018, 16(3): 314-320. [Crossref]
5. Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol, 1998, 160(6 Pt 2): 2418-2424. [Crossref]
6. Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, & Frydenberg M. Laparoscopic and robot-assisted vs open radical prostatectomy for the treatment of localized prostate cancer: a cochrane systematic review. BJU Int, 2018, 121(6): 845-853. [Crossref]
7. Moran PS, O'Neill M, Teljeur C, Flattery M, Murphy LA, Smyth G, et al. Robot-assisted radical prostatectomy compared with open and laparoscopic approaches: a systematic review and meta-analysis. Int J Urol, 2013, 20(3): 312-321. [Crossref]
8. Porcaro AB, Molinari A, Terrin A, De Luyk N, Baldassarre R, Brunelli M, et al. Robotic-assisted radical prostatectomy is less stressful than the open approach: results of a contemporary prospective study evaluating pathophysiology of cortisol stress-related kinetics in prostate cancer surgery. J Robot Surg, 2015, 9(3): 249-255. [Crossref]
9. Stolzenburg J-U, Holze S, Neuhaus P, Do HM, Haney CM, Dietel A, et al. Robotic-assisted versus laparoscopic radical prostatectomy: 12-month outcomes of the multicentre randomised controlled LAP-01 trial. European Urology Focus, 2022, 8(6): 1583-1590. [Crossref]
10. Porpiglia F, Fiori C, Bertolo R, Manfredi M, Mele F, Checcucci E, et al. Five-year outcomes for a prospective randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol Focus, 2018, 4(1): 80-86. [Crossref]
11. Asimakopoulos AD, Pereira Fraga CT, Annino F, Pasqualetti P, Calado AA, & Mugnier C. Randomized comparison between laparoscopic and robot-assisted nervesparing radical prostatectomy. J Sex Med, 2011, 8(5): 1503-1512. [Crossref]
12. Ma J, Xu W, Chen R, Zhu Y, Wang Y, Cao W, et al. Roboticassisted versus laparoscopic radical prostatectomy for prostate cancer: the first separate systematic review and meta-analysis of randomised controlled trials and nonrandomised studies. Int J Surg, 2023, 109(5): 1350-1359. [Crossref]
13. Galfano A, Di Trapani D, Sozzi F, Strada E, Petralia G, Bramerio M, et al. Beyond the learning curve of the Retziussparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with ≥ 1 year of follow-up. Eur Urol, 2013, 64(6): 974-980. [Crossref]
14. Wagaskar VG, Mittal A, Sobotka S, Ratnani P, Lantz A, Falagario UG, et al. Hood technique for robotic radical prostatectomy-preserving periurethral anatomical structures in the space of retzius and sparing the pouch of douglas, enabling early return of continence without compromising surgical margin rates. Eur Urol, 2021, 80(2): 213-221. [Crossref]
15. Jeong IG, Khandwala YS, Kim JH, Han DH, Li S, Wang Y, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA, 2017, 318(16): 1561-1568. [Crossref]
16. Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, & Autorino R. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol, 2017, 71(4): 606-617. [Crossref]
17. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol, 2007, 178(1): 41-46. [Crossref]
18. Shiroki R, Fukami N, Fukaya K, Kusaka M, Natsume T, Ichihara T, et al. Robot-assisted partial nephrectomy: superiority over laparoscopic partial nephrectomy. Int J Urol, 2016, 23(2): 122-131. [Crossref]
19. Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, & Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with daVinci robotic system. Urology, 2004, 64(5): 914-918. [Crossref]
20. Hung AJ, Cai J, Simmons MN, & Gill IS. “Trifecta” in partial nephrectomy. J Urol, 2013, 189(1): 36-42. [Crossref]
21. Cacciamani GE, Medina LG, Gill T, Abreu A, Sotelo R, Artibani W, et al. Impact of surgical factors on robotic partial nephrectomy outcomes: comprehensive systematic review and meta-analysis. J Urol, 2018, 200(2): 258-274. [Crossref]
22. Leow JJ, Heah NH, Chang SL, Chong YL, & Png KS. Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4,919 patients. J Urol , 2016, 196(5): 1371-1377. [Crossref]
23. Hanzly M, Frederick A, Creighton T, Atwood K, Mehedint D, Kauffman EC, et al. Learning curves for robot-assisted and laparoscopic partial nephrectomy. J Endourol, 2015, 29(3): 297-303. [Crossref]
24. Benway BM, Wang AJ, Cabello JM, & Bhayani SB. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol, 2009, 55(3): 592-599. [Crossref]
25. Kızılay F, Turna B, Apaydın E, & Semerci B. Comparison of long-term outcomes of laparoscopic and robot-assisted laparoscopic partial nephrectomy. Kaohsiung J Med Sci, 2019, 35(4): 238-243. [Crossref]
26. Ruiz Guerrero E, Claro AVO, Ledo Cepero MJ, Soto Delgado M, & Álvarez-Ossorio Fernández JL. Robotic versus laparoscopic partial nephrectomy in the eew era: systematic review. Cancers (Basel), 2023, 15(6): 1793-1805. [Crossref]
27. Brassetti A, Anceschi U, Bove AM, Prata F, Costantini M, Ferriero M, et al. Purely off-clamp laparoscopic partial nephrectomy stands the test of time: 15 years functional and oncologic outcomes from a single center experience. Curr Oncol, 2023, 30(1): 1196-1205. [Crossref]
28. Bertolo R, Bove P, Sandri M, Celia A, Cindolo L, Cipriani C, et al. Randomized clinical trial comparing on-clamp versus off-clamp laparoscopic partial nephrectomy for small renal masses (CLOCK II laparoscopic study): a intention-to-treat analysis of perioperative outcomes. Eur Urol Open Sci, 2022, 46: 75-81. [Crossref]
29. Sharma G, Shah M, Ahluwalia P, Dasgupta P, Challacombe BJ, Bhandari M, et al. Off-clamp versus on-clamp robotassisted partial nephrectomy: a propensity-matched analysis. Eur Urol Oncol, 2023, 6(5): 525-530. [Crossref]
30. Fong KY, Gan VHL, Lim BJH, Chan YH, Castellani D, Chen K, et al. Off-clamp vs on-clamp robot-assisted partial nephrectomy: a systematic review and meta-analysis. BJU Int, 2024, 133(4): 375-386. [Crossref]
31. Sanchez de Badajoz E, & JL GP. Radical cystectomy and laparoscopic ileal conduit. Archivos espanoles de urologia, 1993, 46(7): 621-624.
32. Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int, 2003, 92(3): 232-236. [Crossref]
33. Fonseka T, Ahmed K, Froghi S, Khan SA, Dasgupta P, & Shamim Khan M. Comparing robotic, laparoscopic and open cystectomy: a systematic review and meta-analysis. Arch Ital Urol Androl, 2015, 87(1): 41-48. [Crossref]
34. Feng D, Liu S, Tang Y, Yang Y, Wei W, & Han P. Comparison of perioperative and oncologic outcomes between robotassisted and laparoscopic radical cystectomy for bladder cancer: a systematic review and updated meta-analysis. Int Urol Nephrol, 2020, 52(7): 1243-1254. [Crossref]
35. Novara G, Svatek RS, Karakiewicz PI, Skinner E, Ficarra V, Fradet Y, et al. Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy: a multicenter study of more than 4,400 patients. J Urol, 2010, 183(6): 2165-2170. [Crossref]
36. Hadjizacharia P, Stein JP, Cai J, & Miranda G. The impact of positive soft tissue surgical margins following radical cystectomy for high-grade, invasive bladder cancer. World J Urol, 2009, 27(1): 33-38. [Crossref]
37. Herr H, Lee C, Chang S, & Lerner S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: a collaborative group report. J Urol, 2004, 171(5): 1823-1828; discussion 1827-1828. [Crossref]
38. Khan MS, Omar K, Ahmed K, Gan C, Van Hemelrijck M, Nair R, et al. Long-term oncological outcomes from an early phase randomised controlled three-arm trial of open, robotic, and laparoscopic radical cystectomy (CORAL). Eur Urol, 2020, 77(1): 110-118. [Crossref]
39. Kowalewski KF, Wieland VLS, Kriegmair MC, Uysal D, Sicker T, Stolzenburg JU, et al. Robotic-assisted versus laparoscopic versus open radical cystectomy-a systematic review and network meta-analysis of randomized controlled trials. Eur Urol Focus, 2023, 9(3): 480-490. [Crossref]
40. Gill IS, Kaouk JH, Meraney AM, Desai MM, Ulchaker JC, Klein EA, et al. Laparoscopic radical cystectomy and continent orthotopic ileal neobladder performed completely intracorporeally: the initial experience. J Urol, 2002, 168(1): 13-18.
41. Zhang Y, Sun C, Tuo Z, Zhou H, Chen X, & Bi L. Laparoscopic cystectomy with totally intracorporeal versus extracorporeal orthotopic neobladder for bladder cancer: a single center experience. J Laparoendosc Adv Surg Tech A, 2022, 32(6): 659-667. [Crossref]
42. Collins JW, Sooriakumaran P, Sanchez-Salas R, Ahonen R, Nyberg T, Wiklund NP, et al. Robot-assisted radical cystectomy with intracorporeal neobladder diversion: The Karolinska experience. Indian J Urol, 2014, 30(3): 307- 313. [Crossref]
43. Martini A, Falagario UG, Russo A, Mertens LS, Di Gianfrancesco L, Bravi CA, et al. Robot-assisted radical cystectomy with orthotopic neobladder reconstruction: techniques and functional outcomes in males. Eur Urol, 2023, 84(5): 484-490. [Crossref]
44. Kenigsberg AP, Smith W, Meng X, Ghandour R, Rapoport L, Bagrodia A, et al. Robotic nephroureterectomy vs laparoscopic nephroureterectomy: increased utilization, rates of lymphadenectomy, decreased morbidity robotically. J Endourol, 2021, 35(3): 312-318. [Crossref]
45. Stonier T, Simson N, Lee SM, Robertson I, Amer T, Somani BK, et al. Laparoscopic vs robotic nephroureterectomy: is it time to re-establish the standard? Evidence from a systematic review. Arab J Urol, 2017, 15(3): 177-186. [Crossref]
46. Vasudeo V, Singh A, Khanna A, Rawal SK, Pratihar SK, Saurabh N, et al. Surgical and oncological outcomes of robot-assisted versus laparoscopic radical nephroureterectomy for upper-tract urothelial carcinoma: a singlecenter comparative analysis. Indian J Urol, 2023, 39(4): 285-291. [Crossref]
47. Shigeta K, Matsumoto K, Takeda T, Hattori S, Kaneko G, Matsushima M, et al. Evaluating the oncological outcomes of pure laparoscopic radical nephroureterectomy performed for upper-tract urothelial carcinoma patients: a multicenter cohort study adjusted by propensity score matching. Ann Surg Oncol, 2021, 28(1): 465-473. [Crossref]
48. Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol, 2009, 56(3): 520-526. [Crossref]
49. Greco F, Wagner S, Hoda RM, Hamza A, & Fornara P. Laparoscopic vs open radical nephroureterectomy for upper urinary tract urothelial cancer: oncological outcomes and 5-year follow-up. BJU Int, 2009, 104(9): 1274-1278. [Crossref]
50. "EAU guidelines on testicular cancer." 2024, from http://uroweb.org/guidelines/testicular-cancer.
51. Rukstalis DB, & Chodak GW. Laparoscopic retroperitoneal lymph node dissection in a patient with stage 1 testicular carcinoma. J Urol, 1992, 148(6): 1907-1909; discussion 1909-1910. [Crossref]
52. Rassweiler JJ, Scheitlin W, Heidenreich A, Laguna MP, & Janetschek G. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol, 2008, 54(5): 1004-1015. [Crossref]
53. Porter JR. A laparoscopic approach is best for retroperitoneal lymph dode dissection: Yes. J Urol, 2017, 197(6): 1384-1386. [Crossref]
54. Stepanian S, Patel M, & Porter J. Robot-assisted laparoscopic retroperitoneal lymph node dissection for testicular cancer: evolution of the technique. Eur Urol, 2016, 70(4): 661-667. [Crossref]
55. Tselos A, Moris D, Tsilimigras DI, Fragkiadis E, Mpaili E, Sakarellos P, et al. Robot-assisted retroperitoneal lymphadenectomy in testicular cancer treatment: a systematic review. J Laparoendosc Adv Surg Tech A, 2018, 28(6): 682-689. [Crossref]
56. Harris KT, Gorin MA, Ball MW, Pierorazio PM, & Allaf ME. A comparative analysis of robotic vs laparoscopic retroperitoneal lymph node dissection for testicular cancer. BJU Int, 2015, 116(6): 920-923. [Crossref]
57. Li R, Duplisea JJ, Petros FG, González GMN, Tu SM, Karam JA, et al. Robotic postchemotherapy retroperitoneal lymph node dissection for testicular cancer. Eur Urol Oncol, 2021, 4(4): 651-658. [Crossref]
58. Ohlmann CH, Saar M, Pierchalla LC, Zangana M, Bonaventura A, Stöckle M, et al. Indications, feasibility and outcome of robotic retroperitoneal lymph node dissection for metastatic testicular germ cell tumours. Sci Rep, 2021, 11(1): 10700. [Crossref]
59. Vasudeo V, Khanna A, Pratihar SK, Jaipuria J, Chakraborty A, Rawal SK, et al. Robot-assisted retroperitoneal lymph node dissection for post-chemotherapy residual mass in testicular cancer: long-term experience from a tertiary care centre. J Minim Access Surg, 2023, 19(2): 288-295. [Crossref]
60. Favorito LA. The future of inguinal lymphadenecotmy in penile cancer: laparoscopic or robotic? Int Braz J Urol, 2019, 45(2): 208-209. [Crossref]
61. Tobias-Machado M, Tavares A, Molina WR, Jr., Forseto PH, Jr., Juliano RV, & Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int Braz J Urol, 2006, 32(3): 316-321. [Crossref]
62. Josephson DY, Jacobsohn KM, Link BA, & Wilson TG. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology, 2009, 73(1): 167-170; discussion 170-161. [ Crossref]
63. Gkegkes ID, Minis EE, & Iavazzo C. Robotic-assisted inguinal lymphadenectomy: a systematic review. J Robot Surg, 2019, 13(1): 1-8. [Crossref]
64. Russell CM, Salami SS, Niemann A, Weizer AZ, Tomlins SA, Morgan TM, et al. Minimally invasive inguinal lymphadenectomy in the management of penile carcinoma. Urology, 2017, 106: 113-118. [Crossref]
65. Anderson JC, & Hynes W. Retrocaval ureter; a case diagnosed pre-operatively and treated successfully by a plastic operation. Br J Urol, 1949, 21(3): 209-214. [ Crossref]
66. Simforoosh N, Basiri A, Tabibi A, Danesh AK, SharifiAghdas F, Ziaee SA, et al. A comparison between laparoscopic and open pyeloplasty in patients with ureteropelvic junction obstruction. Urol J, 2004, 1(3): 165-169.
67. Pahwa M, Pahwa AR, Girotra M, Abrahm RR, Kathuria S, & Sharma A. Defining the pros and cons of open, conventional laparoscopy, and robot-assisted pyeloplasty in a developing nation. Adv Urol, 2014, 2014: 850156. [Crossref]
68. Calvert RC, Morsy MM, Zelhof B, Rhodes M, & Burgess NA. Comparison of laparoscopic and open pyeloplasty in 100 patients with pelvi-ureteric junction obstruction. Surg Endosc, 2008, 22(2): 411-414. [Crossref]
69. Kumar R, & Nayak B. Robotic versus conventional laparoscopic pyeloplasty: a single surgeon concurrent cohort review. Indian J Urol, 2013, 29(1): 19-21. [Crossref]
70. Uhlig A, Uhlig J, Trojan L, Hinterthaner M, von Hammerstein-Equord A, & Strauss A. Surgical approaches for treatment of ureteropelvic junction obstruction - a systematic review and network meta-analysis. BMC Urol, 2019, 19(1): 112-120. [Crossref]
71. Light A, Karthikeyan S, Maruthan S, Elhage O, Danuser H, & Dasgupta P. Peri-operative outcomes and complications after laparoscopic vs robot-assisted dismembered pyeloplasty: a systematic review and meta-analysis. BJU Int, 2018, 122(2): 181-194. [Crossref]
72. Taktak S, Llewellyn O, Aboelsoud M, Hajibandeh S, & Hajibandeh S. Robot-assisted laparoscopic pyeloplasty versus laparoscopic pyeloplasty for pelvi-ureteric junction obstruction in the paediatric population: a systematic review and meta-analysis. Ther Adv Urol, 2019, 11: 1756287219835704. [Crossref]
73. Asghar AM, Lee RA, Yang KK, Metro M, & Eun DD. Robotassisted distal ureteral reconstruction for benign pathology: current state. Investig Clin Urol, 2020, 61(Suppl 1): S23-s32. [Crossref]
74. Schwaibold H, Wiesend F, & Bach C. The age of robotic surgery - is laparoscopy dead? Arab J Urol, 2018, 16(3): 262-269. [Crossref]
75. Autorino R, Zargar H, Mariano MB, Sanchez-Salas R, Sotelo RJ, Chlosta PL, et al. Perioperative outcomes of robotic and laparoscopic simple prostatectomy: a European-American multi-institutional analysis. Eur Urol, 2015, 68(1): 86-94. [Crossref]
76. Li KP, Chen SY, & Yang L. Laparoscopic simple prostatectomy versus robot-assisted simple prostatectomy for large benign prostatic hyperplasia: a systematic review and meta-analysis of comparative trials. J Robot Surg, 2023, 17(2): 351-364. [Crossref]
77. Cho SJ, Moon HW, Kang SM, Choi SW, Kim KS, Choi YS, et al. Evolution of laparoscopic donor nephrectomy techniques and outcomes: a single-center experience with more than 1000 cases. Ann Transplant, 2020, 25: e918189. [Crossref]
78. Bansal D, Bansal VK, Krishna A, Misra MC, Rajeshwari S, Singh S, et al. Quality improvement in laparoscopic donor nephrectomy by self-imposed proctored preceptorship model. Indian Journal of Surgery, 2020, 82: 163- 168. [Crossref]
79. Horgan S, Vanuno D, Sileri P, Cicalese L, & Benedetti E. Robotic-assisted laparoscopic donor nephrectomy for kidney transplantation. Transplantation, 2002, 73(9): 1474-1479. [Crossref]
80. Bhattu AS, Ganpule A, Sabnis RB, Murali V, Mishra S, & Desai M. Robot-assisted laparoscopic donor nephrectomy vs standard laparoscopic donor nephrectomy: a prospective randomized comparative study. J Endourol, 2015, 29(12): 1334-1340. [Crossref]
81. Wang H, Chen R, Li T, & Peng L. Robot-assisted laparoscopic vs laparoscopic donor nephrectomy in renal transplantation: A meta-analysis. Clin Transplant, 2019, 33(1): e13451. [Crossref]
82. Achit H, Guillemin F, Karam G, Ladrière M, Baumann C, Frimat L, et al. Cost-effectiveness of four living-donor nephrectomy techniques from a hospital perspective. Nephrol Dial Transplant, 2020, 35(11): 2004-2012. [Crossref]
83. Rosales A, Salvador JT, Urdaneta G, Patino D, Montlleó M, Esquena S, et al. Laparoscopic kidney transplantation. European urology, 2010, 57(1): 164-167. [Crossref]
84. Modi P, Rizvi J, Pal B, Bharadwaj R, Trivedi P, Trivedi A, et al. Laparoscopic kidney transplantation: an initial experience. Am J Transplant, 2011, 11(6): 1320-1324. [Crossref]
85. Bansal D, Chaturvedi S, Maheshwari R, Bansal A, & Kumar A. Establishing a robot-assisted kidney transplant program: independent evaluation of the learning curve and surgical nuances. J Endourol, 2021, 35(11): 1650-1658. [Crossref]
86. Bansal A, Maheshwari R, Chaturvedi S, Bansal D, & Kumar A. Comparative analysis of outcomes and long-term follow-up of robot-assisted pediatric kidney transplantation, with open counterpart. Pediatr Transplant, 2021, 25(3): e13917. [Crossref]
87. Kaouk JH, Haber GP, Goel RK, Desai MM, Aron M, Rackley RR, et al. Single-port laparoscopic surgery in urology: initial experience. Urology, 2008, 71(1): 3-6. [Crossref]
88. Morgantini LA, Alzein A, Bharadwaj A, Del Pino MS, Egan E, Ganesh A, et al. A prospective study on single-port versus multiport patient-reported surgical outcomes. BJUI Compass, 2024, 5(1): 84-89. [Crossref]
89. Freehand surgical robotic. 2024, from https://www.freehandsurgeon.com/.
90. Min SH, Cho YS, Park K, Lee Y, Park YS, Ahn SH, et al. Multi-DOF (degree of freedom) articulating laparoscopic instrument is an effective device in performing challenging sutures. J Minim Invasive Surg, 2019, 22(4): 157-163. [Crossref]
91. "HandX - handheld robotic system for laparoscopic surgery." from http://www.merillife.com/medical-devices/endosurgery/robotics/handheld-robotics-system/handx.
92. Romero-Velez G, Robles I, Jiménez J, Cabrera C, Luengas R, Portenier D, et al. Robotic magnetic surgery: results from the first prospective clinical trial. Ann Surg Open, 2022, 3(4): e225. [Crossref]
93. Hinata N, Yamaguchi R, Kusuhara Y, Kanayama H, Kohjimoto Y, Hara I, et al. Hinotori surgical robot system, a novel robot-assisted surgical platform: preclinical and clinical evaluation. Int J Urol, 2022, 29(10): 1213-1220. [Crossref]
94. "Avatera system - avateramedical." 2024, from https://avatera.eu/en/avatera-system.
95. Prata F, Ragusa A, Tempesta C, Iannuzzi A, Tedesco F, Cacciatore L, et al. State of the art in robotic surgery with hugo RAS system: feasibility, safety and clinical applications. J Pers Med, 2023, 13(8): 1233-1243. [Crossref]
96. Alkatout I, Salehiniya H, & Allahqoli L. Assessment of the versius robotic surgical system in minimal access surgery: a systematic review. J Clin Med, 2022, 11(13): 3754-3764. [Crossref]
97. "Senhance® surgical system | Asensus surgical". 2024, from https://www.asensus.com/senhance.
98. "Dexter robotic surgery." 2024, from https://www.distalmotion.com/Dexter.
99. Jain S, & Gautam G. Robotics in urologic oncology. Journal of minimal access surgery, 2015, 11(1): 40-44. [Crossref]
100.Esperto F, Cacciatore L, Tedesco F, Testa A, Callè P, Ragusa A, et al. Impact of robotic technologies on prostate cancer patients' choice for radical treatment. J Pers Med, 2023, 13(5): 794-805. [Crossref]
101.MedknowMandhani A (2018). Let's create facts not perceptions!, Medknow. 34: 95-96.
102.Udwadia TE. Robotic surgery is ready for prime time in India: against the motion. J Minim Access Surg, 2015, 11(1): 5-9. [Crossref]
103.Bora GS, Narain TA, Sharma AP, Mavuduru RS, Devana SK, Singh SK, et al. Robot-assisted surgery in India: a SWOT analysis. Indian J Urol, 2020, 36(1): 1-3. [Crossref]
104.Ramalingam M, Kallappan S, & Nachimuthu S. A prospective comparative study of continuous and interrupted suturing in laparoscopic pyeloplasty in 3D era. J Laparoendosc Adv Surg Tech A, 2018, 28(11): 1275-1278. [Crossref]
105.Matteson KA, & Butts SF. Committee opinion No 701: choosing the route of hysterectomy for benign disease. Obstetrics and Gynecology, 2017, 129(6): E155-E159.
106.Tsuda S, Oleynikov D, Gould J, Azagury D, Sandler B, Hutter M, et al. SAGES TAVAC safety and effectiveness analysis: da Vinci® surgical system (intuitive surgical, sunnyvale, CA). Surg Endosc, 2015, 29(10): 2873-2884. [Crossref]