Open Access | Brief Correspondence
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Neoadjuvant chemotherapy should only be considered in patients with cT3-T4a muscle-invasive bladder cancer
* Corresponding author: Sytse C. van Beek
Mailing address: department of Urology, Erasmus University Medical Centre, Rotterdam, Netherlands.
Email: scvanbeek1985@gmail.com
This article belongs to the Special Issue: Technologic advances on robot-assisted radical cystectomy
Received: 30 July 2024 / Revised: 12 August 2024 / Accepted: 29 August 2024 / Published: 24 September 2024
DOI: 10.31491/UTJ.2024.09.025
The European Association of Urology (EAU) guideline on muscle-invasive bladder cancer (MIBC) recommends offering neoadjuvant
chemotherapy (NAC) to cisplatinfit patients with stage cT2-T4aN0M0 MIBC [1]. This
recommendation is based on a systematic review, including 1,596 randomized patients who demonstrated an 8% improvement in overall
survival [2]. Notably, within this review no subgroup analysis for clinical tumor stage
was conducted. However, recent findings indicated that NAC in cT3-T4a MIBC patients significantly improved overall survival while
it did not in cT2 patients [3, 4]. The aim of our
study was to assess overall survival of cT2 and of cT3-4a MIBC patients treated with NAC before radical cystectomy versus those
without NAC.
We conducted a nationwide retrospective study in 19 hospitals including 965 cT2-4aN0M0 MIBC patients undergoing radical surgery
between January 1st, 2012 and December 31st, 2015 [5,
6]. Case-control matching was done to compare overall survival of patients who underwent
NAC versus those who did not undergo NAC in cT2 patients and in cT3-4a. Matching was done using the prognostic variables age,
gender, clinical tumor stage, Charlson comorbidity index and kidney function. Clinical tumor stage was determined by the primary
physician based on findings of clinical, pathological and radiological investigations and agreed upon by a multidisciplinary tumor
board. No additional staging was done in the light of our study, thereby representing regular clinical practice. Previous results
of this cohort have been published elsewhere [7,
8].
After case-control matching, 206 patients were treated with NAC before cystectomy and 206 without. The baseline characteristics
that were used as matching variables did not differ between both cohorts, nor did body mass index (BMI), presence of carcinoma in
situ (CIS) and the American Society of Anesthesiologists (ASA) physical status classification system score
(Table 1). Only the preoperative hemoglobin level differed significantly between both
cohorts. In cT2 patients, 79% (76/96) received cisplatin/gemcitabine and 64% (58/90) finished the complete course. The pathological
complete response (pCR) rate (ypT0N0) after NAC was 29% (95%CI 21–39%) and the occurrence of pT0N0 without NAC was 17% (95%CI
10–25%). In cT3-T4a patients, 79% (70/110) received cisplatin/gemcitabine and 67% (68/102) finished the complete course. The pCR
after NAC was 34% (95%CI 25–43%) and the occurrence of pT0N0 without NAC was 13% (95%CI 8–20%).
Table 1
Baseline characteristics of 412 case-control cT2 and cT3-4a matched patients with MIBC who underwent radical surgery, either
with neoadjuvant chemotherapy (NAC) or without NAC.
| cT2 | cT3-T4a | |||
| NAC (n = 96) | No NAC (n = 96) | NAC (n = 110) | No NAC (n = 110) | |
| Age (years) | 65 (59–72) | 65 (60–73) | 66 (59–71) | 68 (62–74) |
| Male | 72 (75%) | 72 (75%) | 77 (70%) | 77 (70%) |
| CCI | ||||
| Scores 1–4 | 81 (84%) | 81 (84%) | 84 (76%) | 84 (76%) |
| Scores 5–6 | 12 (13%) | 12 (13%) | 23 (21%) | 23 (21%) |
| Scores ≥ 7 | 3 (3%) | 3 (3%) | 3 (3%) | 3 (3%) |
| ASA | ||||
| ≤ 2 | 79 (84%) | 76 (80%) | 84 (78%) | 79 (78%) |
| > 2 | 15 (16%) | 19 (20%) | 23 (22%) | 22 (22%) |
| Missing | 2 | 1 | 3 | 9 |
| BMI (kg/m2) | 25.1 (23.3–28.1) | 26.3 (23.3–29.3) | 25.1 (23.7–28.3) | 24.9 (22.5–28.5) |
| Hb (pre-operative, mmol/L) | 7.3 (6.7–8.0) | 8.6 (7.6–9.1) | 7.1 (6.5–7.9) | 8.2 (7.1–8.8) |
| Creatinine (pre-operative, µmol/L) | 93 (77–110) | 91 (73–106) | 97 (80–112) | 97 (80–117) |
| Clinical T-stage | ||||
| cT2 | 96 (100%) | 96 (100%) | - | - |
| cT3 | - | - | 94 (85%) | 91 (83%) |
| cT4a | - | - | 16 (15%) | 19 (17%) |
| Chemotherapy type | ||||
| Cis/gem | 76 (79%) | - | 70 (64%) | - |
| Carb/gem | 9 (9%) | - | 25 (23%) | - |
| MVAC | 11 (12%) | - | 15 (14%) | - |
| Chemotherapy cycles | ||||
| incomplete (< 4) | 32 (36%) | - | 34 (33%) | - |
| complete (4 or more) | 58 (64%) | - | 68 (67%) | - |
| missing | 6 | - | 8 | - |
| pathological T-stage | ||||
| (y)pT0N0 | 28 (29%) | 16 (17%) | 37 (34%) | 14 (13%) |
| pT1/cisN0 | 16 (17%) | 11 (12%) | 8 (7%) | 9 (8%) |
| pT2-4a/N+ | 52 (54%) | 69 (71%) | 65 (59%) | 87 (79%) |
Note: CCI: Charlson Comorbidity Index; ASA: American Society of Anesthesiologists physical score; BMI: Body Mass Index; Hb: hemoglobin; Cis/gem: cisplatin/gemcitabine; carb/gem: carboplatin/gemcitabine; MVAC: methotrexate, vinblastine, doxorubicin, cisplatin. Continuous data are described as median with surrounding interquartile range.
In patients with cT2 disease, the 5-year survival rate after NAC was 57% (95%CI 48–68%) and without NAC was 56% (95%CI 48–68%, P = 0.97, Figure 1A). The 5-year recurrence-free survival after NAC was 48% (95%CI 37–60%) and without NAC was 51% (95%CI 41–62%). In patients with cT3-T4a MIBC, the 5-year survival rate after NAC was 61% (95%CI 53–71%) and without NAC was 39% (95%CI 32–50%, P < 0.01, Figure 1B). The 5-year recurrence-free survival after NAC was 54% (95%CI 44–64%) and without NAC was 30% (95%CI 21–39%). These outcomes should be interpreted with caution given the observational nature of our study. Although we aimed to adjust for imbalances in prognostic factors, there remains a risk of residual confounding. Moreover, data on focality and variant histology were missing. Despite these limitations, our survival rates suggest that in contrary to the current EAU guideline, NAC should only be considered in cT3-T4a patients with MIBC and not in cT2 patients.
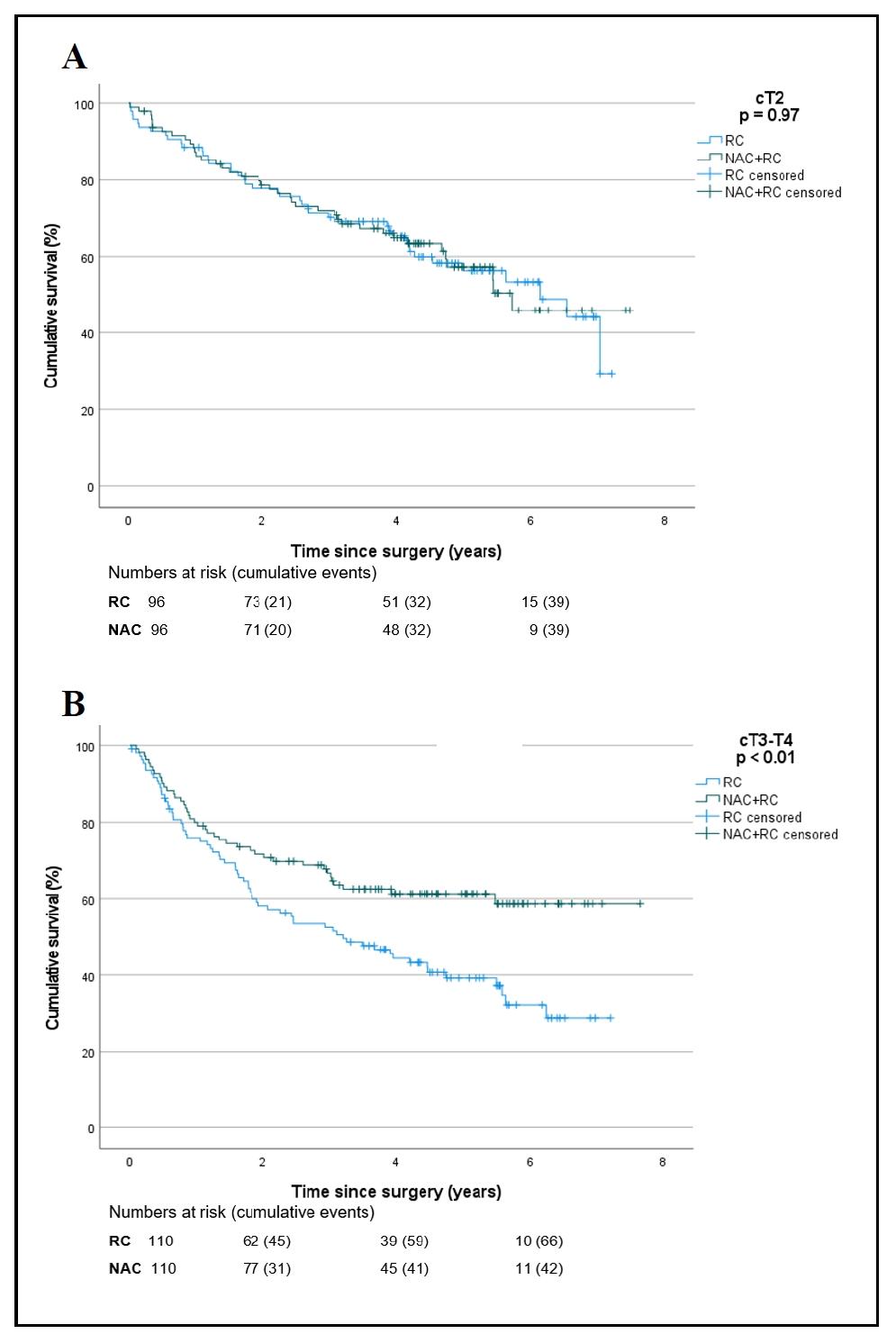
Figure 1. Kaplan-Meier curves of 192 patients with cT2 bladder cancer (A) and 220 patients with cT3-T4 bladder cancer (B) who underwent radical cystectomy (RC) with or without neoadjuvant chemotherapy (NAC).
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Not applicable.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: dummary of the 2020 guidelines. Eur Urol, 2021, 79(1): 82-104. [Crossref]
2. Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step metaanalysis. Oncologist, 2016, 21(6): 708-715. [Crossref]
3. Liu S, Yao Y, Guan F, Sun L, & Zhang G. Neoadjuvant chemotherapy for different stages of muscle-invasive bladder cancer: a systematic review and meta-analysis. Dis Markers, 2022, 2022: 8493519. [Crossref]
4. Soria F, Black PC, Fairey AS, Cookson MS, Yu EY, Kassouf W, et al. Neoadjuvant chemotherapy plus radical cystectomy versus radical cystectomy alone in clinical T2 bladder cancer without hydronephrosis. BJU Int, 2021, 128(1): 79-87. [Crossref]
5. van Ginkel N, Hermans TJN, Meijer D, Boormans JL, Voortman J, Mertens L, et al. Survival outcomes of patients with muscle-invasive bladder cancer according to pathological response at radical cystectomy with or without neo-adjuvant chemotherapy: a case-control matching study. Int Urol Nephrol, 2022, 54(12): 3145- 3152. [Crossref]
6. Hinsenveld FJ, Boormans JL, van der Poel HG, van der Schoot DKE, Vis AN, Aben KKH, et al. Intermediate-term survival of robot-assisted versus open radical cystectomy for muscle-invasive and high-risk non-muscle invasive bladder cancer in The Netherlands. Urol Oncol, 2022, 40(2): 60.e61-60.e69. [Crossref]
7. van Ginkel N, Meijer D, Boormans JL, Mertens LS, van Beek SC, Vis AN, et al. Predictors of major complications and the association with oncological outcomes after radical cystectomy for bladder cancer: A nationwide registry study. Medical Research Archives, 2023, 11(6): 3978-3989. [Crossref]
8. van Ginkel N, Vis AN, Boormans JL, van der Poel HG, van der Schoot DK, Aben KK, et al. Middellangetermijnoverleving na open versus robotgeassisteerde radicale cystectomie in nederland: resultaten van de ‘SNAPSHOT’cystectomie. Tijdschrift voor Urologie, 2023, 13(8): 164-175. [Crossref]