Open Access | Research
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
The effects of 5-ARIs on prostate volume in patients with or without heart failure and benign prostate hyperplasia: prospective, comparative study
* Corresponding author: Denis Krakhotkin
Mailing address: Outpatient Department, Central District Hospital, Kamenolomni, Rostov Region, Russia.
Email: den_surgeon@mail.ru
Received: 17 June 2024 / Revised: 04 July 2024 / Accepted: 09 July 2024 / Published: 24 September 2024
DOI: 10.31491/UTJ.2024.09.023
Abstract
Objective: Compare the effects of finasteride and dutasteride on prostate volume in men with or without chronic heart failure (CHF) and benign prostate hyperplasia (BPH).
Materials and Methods: Five hundred and seventy-nine patients were recruited with symptomatic BPH receiving inhibitors of 5-alpha reductase from December 2022 to January 2024. Five hundred and forty-six patients were followed up during 12 months. The study included analysis of four groups of patients: Group I (n = 136) received dutasteride and had chronic cardiac failure; Group II (n = 137) received finasteride and had chronic cardiac failure; Group III (n = 136) received dutasteride and had no chronic cardiac failure; Group IV (n = 137) received finasteride and had no chronic cardiac failure. Prostate volume, PSA level, total IPSS and its voiding and storage subscores, Qmax, post-voided residual urine volume (PVR) were evaluated at baseline and after 6 and 12 months. Echocardiography, electrocardiography and brain natriuretic peptide (BNP) testing were performed for diagnosis of chronic cardiac failure in all patients. Statistical significance was set at p < 0.05.
Results: The IPSS (total, storage, and voiding symptom) score was significantly decreased after 6 and 12 months of treatment in group III and IV (p < 0.01). The reducing of prostate volume was effective in groups III and IV in patients without chronic cardiac failure. There were not statistically significant differences in reducing of prostate volume at 6 and 12 months of treatment in all groups (p > 0.05). However, the patients with chronic heart failure had a worse effect of inhibitors 5-alpha reductase on prostate volume. The patients with BN p > 100 pg/mL and left ventricular ejection fraction (LVEF) ≤ 40% had the most minimal reducing of prostate volume at 6- and 12-month treatment. In groups III and IV, the postvoid residual urine volume (PVR) and Qmax were significantly decreased (p < 0.05) at 6 and 12 months of treatment compared with the baseline values. The patients with CHF did not receive any benefits in regarding to PVR, Qmax and IPSS score during 12 months of treatment.
Conclusion: Dutasteride and finasteride had no effect on prostate volume in patients with CHF, BNP > 100 pg/mL and with severely abnormal (< 30%) and moderately abnormal (≥ 30% ≤ 40%) LVEF. The patients with BPH and CHF did not receive any benefits in regarding to PVR, Qmax and IPSS score improvement after treatment with dutasteride or finasteride.
Keywords
Benign prostate hyperplasia; chronic heart failure; left ventricular ejection fraction; lower urinary tract symptoms; 5-alpha reductase inhibitors
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases in aging men and one of the most common causes of lower urinary tract symptoms (LUTS) [1]. The incidence of LUTS/BPH increases with age and reaches more 50% in men by the 60 and 80% in men aged > 70 years. BPH is defined as the abnormal and uncontrolled proliferation of epithelial and stromal cells in prostatic tissue [2-4].The pathogenesis of BPH is complicated and includes the interaction of androgen-dependent (levels of dihydrotestosterone) and androgen-independent (ischemia, oxidative stress, metabolic syndrome, infection, autoimmune reactions, and inflammation) factors [5-7]. Currently, α-blockers (ABs) and/or 5α-reductase inhibitors (5-ARIs) are still the main therapeutic agents for the management of LUTS secondary to BPH [8]. In men suffering from BPH, 5-ARIs are therapeutical option with prostate volume greater than 30–40 mL and duration of treatment longer than 1 year. In the same category patients with BPH, combination of 5-ARIs with ABs is a more effective treatment option for improvement and reduction of disease progression if the profile of adverse effects is favorable [9]. The older men with LUTS and BPH have a high prevalence of cardiovascular disease (CVD) than the general population [10]. The using of ABs is associated with increased risk of adverse cardiovascular events compared with 5-ARIs [11]. In one study, Lusty et al. [12] using multivariable competing risk analysis demonstrated that the men treated with 5-ARIs alone appeared to have less cardiac failure than those prescribed ABs, either as ABs alone or as combination therapy. In population-based cohort study, Ayele et al. [13] showed that the administration of 5-ARIs in patients with BPH was not associated with an increased risk of hospitalization for stroke, heart failure and myocardial infarction compared with non-use. By literature data, several studies demonstrated that treatment with 5-ARIs in patients with BPH significantly decreased the obstructive LUTS and prostate volume and increased the maximum urinary flow [14, 15]. In our study, we chose the chronic heart failure because one is clinical syndrome underlining many cardiovascular diseases which are closely related to impaired organ perfusion, and accordingly in some extent with prostate hypoxia as well. In this study, we performed comparative analysis of the effects of finasteride and dutasteride on prostate volume in men with or without chronic heart failure (CHF) and BPH during 12 months of treatment.
Materials and methods
Study design and patient population
This open-label, prospective, comparative study included 579 patients with BPH/LUTS who were investigated from December 2022 to January 2024. All participants provided written informed consent (IRB approval number 29052273). The criteria inclusion and exclusion for the study are shown in Table 1. Patients who met the inclusion criteria were assigned into the CHF group (defined as BNP > 100 pg/mL) or non-CHF group (defined as BNP < 100 pg/mL) and then randomly allocated by computergenerated random numbers into the dutasteride 0.5 mg group or finasteride 5 mg group for 12-month of treatment. By the data echocardiography, each group with CHF were divided into three subgroups with left ventricular ejection fraction LVEF < 30%, LVEF ≥ 30% ≤ 40% and LVEF ≥ 40% ≤ 51% for separate analysis of change of prostate volume in patients taking dutasteride 0.5 mg and finasteride 5 mg. For all participants, we conducted history taking, including total IPSS and its voiding and storage subscores; a measurement of serum prostate-specific antigen (PSA); transrectal ultrasonography to assess the prostate volume and abdominal ultrasound investigation for evaluation of post-voiding residual (PVR) urine; uroflowmetry; echocardiography, electrocardiography and brain natriuretic peptide (BNP) testing. The study was performed in according to the principles of the 1964 Declaration of Helsinki and was approved by Institutional Review Board of Central District Hospital Kamenolomni.
Table 1
Final items for the treatment satisfaction questionnaire for
medication (TSQM).
| Inclusion criteria | Exclusion criteria |
|---|---|
| -The age of men ≥ 50 years -Diagnosis of BPH by history and physical examination -Prostate volume measured by TRUS ≥ 30 cm3 -IPSS score ≥ 8 (moderate-to-severe symptoms) -Qmax ≤ 15 mL/s -Serum PSA level ≥ 1.5 ng/mL -Symptoms of chronic heart failure -Absence of heart failure complications (arrhythmias, pulmonary and hepatic congestion, Pulmonary hypertension, thromboembolism) -BNP levels over 100 pg/mL |
-Postvoid residual volume > 250 mL -History of prostate cancer, urethral stricture, pelvic irradiation, recurrent urinary tract infections, carcinoma in situ of the urinary bladder, urinary incontinence. -Previous prostate surgery (TURP, open adenomectomy) -Use of an α1-blocker within 1 month or any previous use of a 5-ARI -Serum PSA level ≥ 10 ng/mL |
Note: BPH, benign prostate hyperplasia. BNP, brain natriuretic peptide. TURP, transurethral resection of prostate. PSA, prostate specific antigen. IPSS, International Prostate Symptom Score.
Study end-points and assessments
The primary end-point of this study was to determine the effects of dutasteride 0.5 mg and 5 mg finasteride on prostate volume in patients with or without CHF. This parameter was assessed by analyzing changes from baseline in prostate volume at 6 and 12 months. Secondary end-points were to analyze the improvement in total IPSS and its voiding and storage subscores, quality of life (QoL) score, PVR, PSA level and maximum urinary flow rate (Qmax) in men with or without CHF at 6 and 12 months.
Statistical analysis
Data are expressed as mean ± standard deviation (SD), and statistical significance was accepted at p < 0.05. The Wilcoxon rank sum test and two-sample t-test were conducted to analyze continuous variables, and the chi-square test was used to analyze categorical variables. Statistical analysis was performed with software SPSS 20.0 (SPSS, Chicago IL, USA) and Prism software (version 5.00; GraphPad Instat, San Diego, CA, USA).
Results
Patient demographics and background
Four hundred and seventy-nine men with BPH/LUTS were enrolled for the study from December 2022 to January 2024.During the recruitment period, 30 patients were excluded from the study due to prostate cancer (n = 11), radical prostatectomy (n = 3), pelvic radiotherapy (n = 6) and recurrent urinary tract infections (n = 10). A total of 546 men were followed up for 12 months. Of these, 264 CHF patients and 282 non-CHF patients were randomized to receive dutasteride 0.5 mg or finasteride 5 mg (group I, CHF, dutasteride 0.5 mg; group 2, CHF finasteride 5 mg; group 3, non-CHF, dutasteride 0.5 mg; group 4, non-CHF, finasteride 5 mg). In the group I, 126 patients completed the study and 4 men were withdrawn due to adverse events (AEs). In the group II, 129 men finished the study and 5 patients were withdrawn due to AEs. In the group III, all patients completed the study. In the group IV, 138 finished the study and one patient discontinued because of loss during 12-month follow-up period (Figure 1). Baseline demographic and clinical parameters of the patients are shown in Table 2. There was no statistically significant difference between the four treatment groups in regarding to baseline demographic and clinical parameters. However, there was statistically significant differences between CHF and non-CHF patients in relation to the BNP level and value of LVEF (p < 0.05).
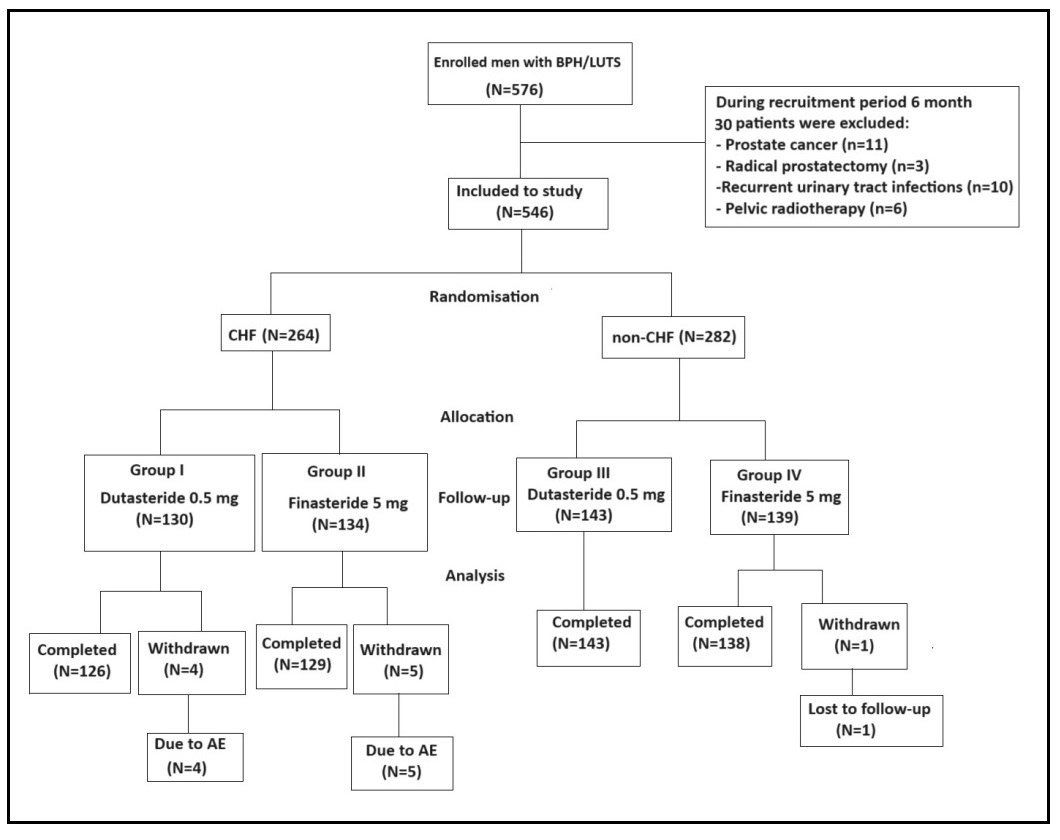
Figure 1. The flow-chart of the study.
Table 2
Patient demographics and baseline parameters in men with BPH/LUTS.
| Chronic heart failure | Non-chronic heart failure | P-value | |||
| Group I Dutasteride 0.5 mg |
Group II Finasteride 5 mg |
Group I Dutasteride 0.5 mg |
Group II Finasteride 5 mg |
||
| No. of patient, n | 130 | 134 | 143 | 139 | |
| Age, years | 64 ± 3.4 | 62 ± 3.9 | 63 ± 3.6 | 64 ± 3.7 | 0.126 |
| Height, cm | 169 ± 4 | 170 ± 3.6 | 168 ± 4.1 | 170 ± 3.9 | 0.457 |
| BMI, kg/m2 | 24 ± 3.1 | 25 ± 3.2 | 24 ± 3.4 | 25 ± 3.3 | 0.321 |
| BNP, pg/mL | 690 ± 54 | 612 ± 65 | 85 ± 24 | 81 ± 22 | 0.012 |
| LVEF, % | 37 ± 4.5 | 38 ± 4.9 | 67 ± 3.4 | 65 ± 4.2 | 0.024 |
| Prostate volume, cc | 85 ± 7.9 | 83 ± 7.4 | 84 ± 8.2 | 82 ± 7.7 | 0.732 |
| PSA, ng/mL | 1.5 ± 1.2 | 1.4 ± 1.3 | 1.5 ± 1.4 | 1.6 ± 1.4 | 0.523 |
| PVR, mL | 103.5 ± 8.5 | 104.9 ± 8.7 | 102.2 ± 7.9 | 103.4 ± 8.1 | 0.391 |
| IPSS (baseline) | |||||
| Total | 20.3 ± 5.7 | 20.5 ± 6.2 | 21.2 ± 5.9 | 20.9 ± 6.4 | 0.675 |
| Voiding subscore | 15.6 ± 4.1 | 16.2 ± 4.5 | 15.9 ± 4.6 | 16.5 ± 4.8 | 0.529 |
| Storage subscore | 4.7 ± 1.6 | 4.3 ± 1.7 | 5.3 ± 1.3 | 4.4 ± 1.6 | 0.734 |
| QoL score | 4.5 ± 0.9 | 4.6 ± 0.7 | 4.4 ± 0.8 | 4.5 ± 0.6 | 0.432 |
| Qmax (mL/s) | 9.8 ± 2.7 | 10.1 ± 2.1 | 9.9 ± 2.3 | 10.3 ± 2.2 | 0.589 |
Note: SD, standard deviation. BMI, body mass index. BNP, brain natriuretic peptide. LVEF, left ventricular ejection fraction. PSA, prostate-specific antigen. PVR, post-void residual volume. IPSS, International Prostate Symptom Score. QoL, quality of life.
Primary endpoint
The long-term follow-up results demonstrate that the prostate volume significantly decreased in patients without CHF in group III and IV during 12 months of treatment. However, the patients with CHF taking both dutasteride 0.5 mg and finasteride 5 mg had no benefits in reduction of prostate volume at 6 and 12 months of treatment (Table 3). The subgroup analysis of change of prostate volume in patients with CHF showed that the administration both dutasteride 0.5 mg and finasteride 5 mg in men with severely abnormal (< 30%) and moderately abnormal (≥ 30% ≤ 40%) left ventricular ejection fraction did not have any effects to prostate volume. The men with mildly abnormal (≥ 40% ≤ 51%) LVEF had minimal reduction of prostate volume—10.7 % for A3-subgroup and—10.5% for B3 subgroup at 12-month of follow-up (Table 4). The men with CHF the reduction of prostate volume at 12-month of follow-up was—4.7% for dutasteride 0.5 mg group and—4.8% for finasteride 5 mg group. At the same time, in the patients without CHF, reduction of prostate volume at 12-month was—23.8% and—25.6% for dutasteride and finasteride group, respectively (Table 3).
Table 3
Clinical characteristics of the patients.
| (A) Chronic heart failure | Prostate volume (cc) | |||
| Baseline | After 6 months of therapy | After 12 months of therapy | P-value | |
| Group I Dutasteride 0.5 mg |
85 ± 7.9 | 83 ± 4.5 | 81 ± 4.2 | 0.932 |
| Group II Finasteride 5 mg |
83 ± 7.4 | 81 ± 4.3 | 79 ± 4.1 | 0.824 |
| (B) Non-Chronic heart failure | Prostate volume (cc) | |||
| Baseline | After 6 months of therapy | After 12 months of therapy | P-value | |
| Group III Dutasteride 0.5 mg |
84 ± 8.2 | 73 ± 6.5 | 64 ± 4.9 | 0.021 |
| Group IV Finasteride 5 mg |
82 ± 7.7 | 71 ± 6.3 | 61 ± 4.4 | 0.015 |
Table 4
The subgroup analysis of change of the prostate volume in patients with CHF taking dutasteride (A) and finasteride (B) in depending of value
of LVEF (%) during 12 months of follow-up.
| (A) Dutasteride 0.5 mg | Prostate volume (cc) | |||
| No. of patients (n = 130) | Baseline | After 6 months of therapy | After 12 months of therapy | P-value |
| A1 subgroup LVEF < 30% (n = 43) | 83 ± 4.5 | 82 ± 4.4 | 81 ± 4.6 | 0.876 |
| A2 subgroup (LVEF) ≥ 30% ≤ 40% (n = 44) | 84 ± 4.2 | 83 ± 4.4 | 82 ± 4.1 | 0.632 |
| A3 subgroup (LVEF) ≥ 40% ≤ 51% (n = 43) | 84 ± 4.3 | 79 ± 4.1 | 75 ± 3.9 | 0.032 |
| (B) Finasteride 5 mg | Prostate volume (cc) | |||
| No. of patients (n = 134) | Baseline | After 6 months of therapy | After 12 months of therapy | P-value |
| B1 subgroup LVEF < 30% (n = 45) | 85 ± 4.4 | 83 ± 4.3 | 82 ± 4.5 | 0.743 |
| B2 subgroup (LVEF) ≥ 30% ≤ 40% (n = 44) | 86 ± 4.3 | 83 ± 4.2 | 82 ± 4.6 | 0.812 |
| B3 subgroup (LVEF) ≥ 40% ≤ 51% (n = 45) | 85 ± 4.2 | 78 ± 3.9 | 76 ± 3.3 | 0.024 |
Note: LVEF, Left ventricular ejection fraction.
Secondary endpoints
IPSS and its subscores
A statistically significant improvement (p < 0.05) in total IPSS score was observed from baseline in group III and IV without chronic heart failure who received dutasteride 0.5 mg and finasteride 5 mg, respectively mg (Table 5). The mean change from baseline in group III was 7.0 ± 2.0 and 11.0 ± 2.0 for 6 and 12 months of follow-up, respectively. The mean change in group IV was 6.0 ± 3.0 and 10.3 ± 4.0 for 6 and 12 months of follow-up, respectively. The patients with CHF did not have significant improvements in total IPSS and had more pronounced obstructive symptoms during 12 months of treatment compared with patients with normal heart function (Table 5).
Table 5
Comparison of the treatment outcomes at the follow-up assessment and mean changes in values from baseline to 6 months (A) and 12 months
(B) in four groups of patients.
| (A) | Chronic heart failure | Non-chronic heart failure | P-value | ||
| Group I Dutasteride 0.5 mg |
Group II Finasteride 5 mg |
Group III Dutasteride 0.5 mg |
Group IV Finasteride 5 mg |
||
| PSA, ng/mL | 0.5 ± 0.4 | 0.7 ± 0.3 | 0.8 ± 0.5 | 0.7 ± 0.4 | 0.523 |
| PVR, mL | 100.4 ± 7.5 | 101.9 ± 7.7 | 60.2 ± 5.8 | 62.4 ± 5.1 | 0.014 |
| IPSS (baseline) | |||||
| Total IPSS | 18.3 ± 5.7 | 18.5 ± 5.2 | 14.2 ± 3.9 | 14.9 ± 3.4 | 0.023 |
| Voiding subscore | 14.6 ± 3.1 | 14.2 ± 3.5 | 10.9 ± 2.6 | 10.5 ± 2.8 | 0.018 |
| Storage subscore | 3.7 ± 2.6 | 4.3 ± 1.7 | 3.3 ± 1.3 | 4.4 ± 0.6 | 0.453 |
| QoL score | 3.5 ± 0.6 | 3.6 ± 0.5 | 2.4 ± 0.8 | 2.5 ± 0.4 | 0.032 |
| Qmax (mL/s) | 10.8 ± 2.2 | 10.7 ± 2.6 | 12.9 ± 2.4 | 12.3 ± 2.4 | 0.010 |
| (B) | Chronic heart failure | Non-chronic heart failure | P-value | ||
| Group I Dutasteride 0.5 mg |
Group II Finasteride 5 mg |
Group III Dutasteride 0.5 mg |
Group IV Finasteride 5 mg |
||
| PSA, ng/mL | 0.4 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.5 ± 1.4 | 0.782 |
| PVR, mL | 100.1 ± 8.5 | 102.9 ± 8.7 | 40.2 ± 4.9 | 43.4 ± 4.1 | 0.026 |
| IPSS (baseline) | |||||
| Total IPSS | 17.3 ± 4.7 | 17.6 ± 5.2 | 10.2 ± 2.9 | 10.3 ± 2.4 | 0.014 |
| Voiding subscore | 13.6 ± 2.1 | 13.2 ± 2.5 | 7.9 ± 1.6 | 7.5 ± 1.8 | 0.029 |
| Storage subscore | 3.7 ± 2.6 | 4.4 ± 2.7 | 2.3 ± 1.3 | 2.8 ± 0.6 | 0.014 |
| QoL score | 3.4 ± 0.5 | 3.5 ± 0.6 | 1.9 ± 0.5 | 1.7 ± 0.4 | 0.038 |
| Qmax (mL/s) | 11.1 ± 2.4 | 10.9 ± 2.8 | 14.9 ± 2.6 | 14.3 ± 2.7 | 0.004 |
PVR
The patients of group III and IV had statistically significant improvement of PVR after 6 and 12 months of treatment (p < 0.05). The mean change in PVR from baseline in group III was 42 ± 2.1 mL and 62 ± 3.0 mL for 6 and 12 months of follow-up, respectively. The mean change in PVR from baseline in group IV was 42 ± 2.1 mL and 62 ± 3.0 mL for 6 and 12 months of follow-up, respectively. However, the patients with CHF did not have any improvements in PVR during 12 months of treatment (Table 5).
Qmax
The men in group III and IV had a statistically significant increase (p < 0.05) in maximum flow rate Qmax (mL/s) compared with patients in groups I and II, who had CHF (Table 3). The mean change from baseline in group III was 3.0 ± 0.1 and 5.0 ± 0.3 for 6 and 12 months of followup, respectively. The mean change in group IV was 2.0 ± 0.2 and 4.0 ± 0.5 for 6 and 12 months of follow-up, respectively. The men with CHF had no significant improvement in maximum flow rate Qmax (Table 5).
PSA level
The PSA level had decreased by 50% in all group and there were not statistically significant differences between the four groups after 6 and 12 months of treatment (Table 5).
Discussion
This study was conducted to compare the effects of 5-ARIs (dutasteride 0.5 mg and finasteride 5 mg) on
prostate volume in men with BPH with or without concomitant chronic heart failure. The results demonstrate
that both treatment options improved LUTS after 12 months in terms of total IPSS and its subscores in patients
with normal heart function. In addition, PVR and Qmax were significantly improved in men without
concomitant chronic heart failure after 12 months of treatment. At the same time, the men with concomitant
CHF had more pronounced obstructive LUTS even at 12-month of treatment. Furthermore, the men with CHF had
higher values of PVR and lower Qmax at 6 and 12 months of treatment. In the current recommendations
of the European Association of Urology (EAU), use of 5-ARIs is indicated in patients with enlarged prostate >
40 cc and moderate to severe LUTS [16,
17]. The main effect of 5-ARIs is associated with induction of
apoptosis of epithelial cells in the prostate gland leading to prostate size reduction of about 18-28% and a
decrease in circulating PSA levels of about 50% after 6 to 12 months of therapy
[18, 19]. Gittelman
et al. reported that dutasteride decreased a prostate volume by 26.2% from baseline to 48 months in
men with prostate volume of 40 cc or greater [20]. Jeong et
al. demonstrated that a prostate volume was reduced by 24.5% in finasteride-treated group and by 26.1%
dutasteride-treated group after 12 months of treatment [21]. In one
of the long-term clinical studies was found that finasteride therapy to reduce prostate volume by
approximately 27% compared to baseline after 36 months of treatment
[22].
In the present study, the patients with BPH and without concomitant chronic heart failure had a similar
reduction of prostate size—23.8% for dutasteride 0.5 mg and—25.6% for finasteride 5 mg after 12 months of
treatment. However, the men with concomitant CHF had a different pattern of reduction of prostate volume in
depending of value of the LVEF. The patients with severely abnormal (< 30%) and moderately abnormal
(≥ 30% ≤ 40%) LVEF had no effect on prostate volume during 12 months of treatment. In contrast, the men with
mildly abnormal (≥ 40% ≤ 51%) LVEF had a minimal reduction of prostate volume by 10.7% for dutasteride group
and by 10.5% for finasteride group at 12-month of follow-up. In large population-based study Ayele et
al. demonstrated that the use of 5-ARIs was not associated with an increased risk of hospitalization for
cardiovascular diseases such as heart failure, stroke, myocardial infarction in men with BPH
[13]. In another study, Hsieh et al. reported that the
longterm use of 5-alpha-reductase inhibitors did not increase the risk of cardiovascular events in men with
BPH [23]. In cross-sectional study, Russo et al.
demonstrated an increase of more than five-fold of having a Framingham CVD risk score of ≥ 10% in men with
BPH and moderatesevere LUTS [24]. The diverse effects of 5-ARIs on
prostate volume in current study can be explained by hemodynamic differences in patients with or without
concomitant CHF. In study, Chen et al. demonstrated resistive indexes of the periurethral arteries
have a positive correlation with the cardiovascular risk factors. In addition, they reported that the
periurethral artery resistive index positively correlated with both prostate and transitional zones volumes
[25]. In context of BPH, the increase in the resistive index
indicates to vascular resistance suggesting about relationship between underperfusion of prostate and
cardiovascular disease. Anatomically, the diameter of periurethral arteries is smaller than the other branches
of the prostatic vessels and its resistive index is susceptible parameter to hemodynamic changes. Several
studies have demonstrated that cardiovascular risk factors may cause enlargement of the prostate volume by
causing chronic prostatic ischemia [26,
27]. In our study, we demonstrate that administration of 5-ARIs in
men with CHF did not improve BPH-related LUTS and does not affect prostate volume in patients with severely
abnormal (< 30%) and moderately abnormal (≥ 30% ≤ 40%) LVEF. Elsherbini et al. reported that
preoperative 5-ARI is not associated with any clinically significant different perioperative or functional
outcomes for GreenLight photovaporization of prostate (PVP) using the XPS-180W system. Thus, they demonstrate
that there is no role for the initiation or discontinuation of 5-alpha reductase inhibitors prior to
GreenLight PVP [28]. Thus, such category of patients' needs
minimal-invasive surgical treatment for achievement of improvement of LUTS and good quality of life
considering all operative-related risk factors.
Conclusions
Dutasteride and finasteride had no effect on prostate volume in patients with CHF, BNP > 100 pg/mL and with severely abnormal (< 30%) and moderately abnormal (≥ 30% ≤ 40%) LVEF. The patients with BPH and CHF did not receive any benefits in regarding to PVR, Qmax and IPSS score improvement after treatment with dutasteride or finasteride. Accordingly, the patient with severe LUTS and concomitant chronic heart failure need minimal-invasive surgical treatment considering all operative-related risk factors due-to ineffectiveness of 5-ARIs for reducing of prostate volume.
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Krakhotkin DV, Chernylovskiy VA, Francesco Greco, Aly M Abdel-Karim and Ali Serdar Gözen are members of the editorial board of UroTechnology Journal. The authors declare that they have no conflicts and were not involved in the journal's review or decision regarding this manuscript.
Ethical statement
This study was approved by the Institutional Review Board of Central District Hospital Kamenolomni. All patients were informed of the procedures and provided written informed consent.
Consent for publication
Not applicable.
References
1. Van Asseldonk B, Barkin J, & Elterman DS. Medical therapy for benign prostatic hyperplasia: a review. Can J Urol, 2015, 22 Suppl 1: 7-17.
2. O'Quin C, White KL, Campbell JR, Myers SH, Patil S, Chandler D, et al. Pharmacological approaches in managing symptomatic relief of benign prostatic hyperplasia: a comprehensive review. Cureus, 2023, 15(12): e51314. [Crossref]
3. Mahon JT, & Welliver C. National trends in the management of lower urinary tract symptoms associated with benign prostatic hyperplasia. Curr Urol Rep, 2020, 21(12): 63-73. [Crossref]
4. Su YT, Chen HL, Teoh JY, Chan VW, Wu WJ, & Lee HY. Comparison of add-on medications for persistent storage symptoms after α-blocker treatment in BPH patients - a network meta-analysis. BMC Urol, 2023, 23(1): 154-164. [Crossref]
5. Managing benign prostatic hyperplasia and prostate cancer – the challenges today. Journal of Men's Health, 2010, 7(2): 113-124. [Crossref]
6. He Q, Wang Z, Liu G, Daneshgari F, MacLennan GT, & Gupta S. Metabolic syndrome, inflammation and lower urinary tract symptoms: possible translational links. Prostate Cancer Prostatic Dis, 2016, 19(1): 7-13. [Crossref]
7. Devlin CM, Simms MS, & Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int, 2021, 127(4): 389-399. [Crossref]
8. Zitoun OA, Farhat AM, Mohamed MA, Hamad MR, Aramini B, & Haider KH. Management of benign prostate hyperplasia (BPH) by combinatorial approach using alpha-1-adrenergic antagonists and 5-alpha-reductase inhibitors. Eur J Pharmacol, 2020, 883: 173301. [Crossref]
9. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, & Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int, 2005, 96(4): 572-577. [Crossref]
10. Karatas OF, Bayrak O, Cimentepe E, & Unal D. An insidious risk factor for cardiovascular disease: benign prostatic hyperplasia. Int J Cardiol, 2010, 144(3): 452-462. [Crossref]
11. Zhang J, Latour CD, Olawore O, Pate V, Friedlander DF, Stürmer T, et al. Cardiovascular outcomes of α-blockers vs 5-α reductase inhibitors for benign prostatic hyperplasia. JAMA Netw Open, 2023, 6(11): e2343299. [Crossref]
12. Lusty A, Siemens DR, Tohidi M, Whitehead M, Tranmer J, & Nickel JC. Cardiac failure associated with medical therapy of benign prostatic hyperplasia: a population based study. J Urol, 2021, 205(5): 1430-1437. [Crossref]
13. Ayele HT, Reynier P, Azoulay L, Platt RW, Benayoun S, & Filion KB. The cardiovascular safety of five-alphareductase inhibitors among men with benign prostatic hyperplasia: a population-based cohort study. Am J Med, 2023, 136(10): 1000-1010.e1007. [Crossref]
14. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. The finasteride study group. N Engl J Med, 1992, 327(17): 1185-1191. [Crossref]
15. Matsukawa Y, Takai S, Funahashi Y, Majima T, Kato M, Yamamoto T, et al. Effects of withdrawing α1-blocker from combination therapy with α1-blocker and 5α-reductase inhibitor in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a prospective and comparative trial using urodynamics. J Urol, 2017, 198(4): 905-912. [Crossref]
16. Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol, 2013, 64(1): 118-140. [Crossref]
17. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, & de la Rosette JJ. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol, 2004, 46(5): 547-554. [Crossref]
18. Naslund MJ, & Miner M. A review of the clinical efficacy and safety of 5-alpha-reductase inhibitors for the enlarged prostate. Clin Ther, 2007, 29(1): 17-25. [Crossref]
19. Rittmaster RS, Norman RW, Thomas LN, & Rowden G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J Clin Endocrinol Metab, 1996, 81(2): 814-819. [Crossref]
20. Gittelman M, Ramsdell J, Young J, & McNicholas T. Dutasteride improves objective and subjective disease measures in men with benign prostatic hyperplasia and modest or severe prostate enlargement. J Urol, 2006, 176(3): 1045-1050; discussion 1050. [Crossref]
21. Jeong YB, Kwon KS, Kim SD, & Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology, 2009, 73(4): 802-806. [Crossref]
22. Stoner E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology, 1994, 43(3): 284-292; discussion 292-284. [Crossref]
23. Hsieh TF, Yang YW, Lee SS, Lin TH, Liu HH, Tsai TH, et al. Use of 5-alpha-reductase inhibitors did not increase the risk of cardiovascular diseases in patients with benign prostate hyperplasia: a five-year follow-up study. PLoS One, 2015, 10(3): e0119694. [Crossref]
24. Russo GI, Castelli T, Privitera S, Fragalà E, Favilla V, Reale G, et al. Increase of Framingham cardiovascular disease risk score is associated with severity of lower urinary tract symptoms. BJU Int, 2015, 116(5): 791-796. [Crossref]
25. Chen IH, Tsai YS, & Tong YC. Correlations among cardiovascular risk factors, prostate blood flow, and prostate volume in patients with clinical benign prostatic hyperplasia. Urology, 2012, 79(2): 409-414. [Crossref]
26. Berger AP, Bartsch G, Deibl M, Alber H, Pachinger O, Fritsche G, et al. Atherosclerosis as a risk factor for benign prostatic hyperplasia. BJU Int, 2006, 98(5): 1038-1042. [Crossref]
27. Haga N, Akaihata H, Hata J, Aikawa K, Yanagida T, Matsuoka K, et al. The association between local atherosclerosis of the prostatic artery and benign prostatic enlargement in humans: putative mechanism of chronic ischemia for prostatic enlargement. Prostate, 2018, 78(13): 1001-1012. [Crossref]
28. Elsherbini T, Bouhadana D, Sadri I, Nguyen DD, Law KW, Arezki A, et al. The impact of 5-ARI on perioperative and functional outcomes of greenlight PVP: an analysis of the global greenlight group database. Can J Urol, 2023, 30(2): 11473-11479.