Open Access | Case Report
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Robotic-assisted partial nephrectomy nightmare: poor clamping and tumor rupture
* Corresponding author: Artur de Oliveira Paludo
Mailing address: Hospital Moinhos de Vento, Rua Tiradentes
333, 12° andar, Porto Alegre, Rio Grande do Sul, Brazil.
Email: arturpaludo@gmail.com
This article belongs to the Special Issue: Nightmare and complex cases in Urology
Received: 08 March 2023 / Revised: 02 April 2023 / Accepted: 06 April 2023 / Published: 30 June 2023
DOI: 10.31491/UTJ.2023.06.008
Abstract
An 82-year-old Caucasian male patient was referred to our center because of the incidental finding of a nodule in the middle third of the right kidney during a screening abdominal ultrasound. No local or systemic symptom could be associated with the renal mass on initial evaluation. The patient underwent an abdominal contrastenhanced CT scan, which revealed a mass in the outer middle third of the right kidney measuring 4.5 × 3.5 cm, predominantly exophytic, with contact to the collecting system and an enhancement pattern suspicious for primary renal malignancy. Tumor complexity was classified as PADUA score 9 and Renal Score 9x. Clinical stage was cT1bN0M0. Total procedure time was 130 min. Estimated blood loss was 800 mL and warm ischemia time was 24 min. Intraoperative complications were tumor rupture and increased bleeding. The postoperative course was uneventful. Meticulous preoperative planning including contrast enhanced CT with 1 mm thick slices and 3D reconstruction imaging is critical. Comprehensive dissection of the renal hilum must be performed to access the exact number of vessels vascularizing the kidney and to provide adequate ischemia of the renal parenchyma for clean visualization during tumor resection to avoid nightmares such as positive margins and tumor rupture.
Keywords
Partial nephrectomy, robotic surgery, complications
Introduction
Partial nephrectomy is the optimal surgical strategy for
T1a renal masses [1]. In recent years, thanks to the evolution of the technique and the progressive implementation
of robotic surgery, it has been possible to safely expand
the indications for partial nephrectomy to patients with
larger tumors, namely T1b and/or T2 [2].
However, careful preoperative planning is necessary to
achieve good postoperative and oncologic outcomes. Triphasic, contrast-enhanced computed tomography (CT)
with 1 mm thick slices is fundamental for understanding
the vascular anatomy and for performing reliable 3D reconstruction, which, when available, is a key tool to support surgical planning. This type of reconstruction allows
us to accurately study the anatomical nuances of different
cases that will be encountered intraoperatively in each
different surgery. Also, a magnetic resonance image can
add valid information about the involvement of structures
adjacent to the tumor and venous thrombus [3].
Despite all this careful preoperative planning, technical
and surgical difficulties can arise during robotic-assisted
partial nephrectomy (RAPN), some of which can be a
nightmare if not properly managed. One of the most unwanted intraoperative complications is bleeding, which
can occur due to inadequate arterial or venous preparation
and clamping. Tumor rupture is another complication that should always be avoided due to the risk of contamination
and consequently tumor recurrence [4].
Case report
In this case report, an 82-year-old Caucasian male patient was referred to our center because of the incidental finding of a nodule in the middle third of the right kidney on an abdominal ultrasound performed for screening purposes. No local or systemic symptom could be associated with the renal mass on initial evaluation. The patient’s medical history included a surgical procedure for aortic valve replacement and myocardial revascularization with subsequent need for percutaneous right coronary angioplasty. Comorbidities included chronic heart failure with low functional capacity (NYHA class II), hypertension, and dyslipidemia (both controlled with medical therapy). Physical examination was unremarkable for palpable flank masses or other findings. His body mass index was 24.5 kg/m2 . The patient had stage IIIb chronic kidney disease prior to surgery (eGFR = 43 mL/min/1.73m2 estimated by the 2021 CKD-EPI creatinine equation [5]). Urinalysis showed no hematuria, proteinuria or other abnormalities. All other biochemical tests were normal.
Tumor Nephrometric Characterization
The patient underwent an abdominal contrast-enhanced CT scan to better define the lesion, which revealed a mass in the outer middle third of the right kidney measuring 4.5 × 3.5 cm, predominantly exophytic, with contact to the collecting system and an enhancement pattern suspicious for primary renal malignancy. Tumor complexity was classified as PADUA score 9 (2 points for “tumor size”; 1 point for “exophytic” rate; 2 points for “collecting system” involvement; 1 point for “sinus” involvement; 1 point for “renal rim”; 2 points for polar location) and Renal Score 9x. Clinical stage was cT1bN0M0.
Preoperative evaluation
The images from the contrast-enhanced CT scan were retrieved in DICOM format and processed in software under development at our institution to obtain a virtual 3D reconstruction of the renal mass and its correlation with major vessels and other important structures (Figure 1).
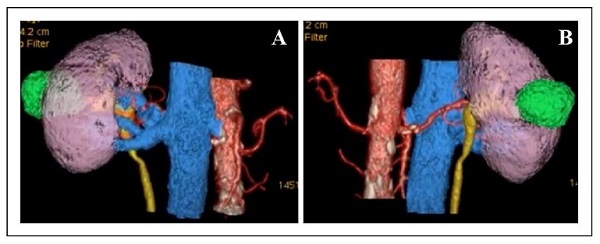
Figure 1. The anterior (A) and posterior (B) aspect of the 3D reconstruction of the tumor and its relationship to the kidney and other key structures.
Surgical technique and setup
(a) Use of the da Vinci Si robotic platform with a fourarm configuration plus two assistant ports [both 12 mm
ports, (in this specific case, we used two 12 mm ports for the assistant to allow him to hem-O-Lok clips from both
ports, maximizing patient safety and coordination with the
primary surgeon) [6] (Figure 2)];
(b) Transperitoneal approach;
(c) Mobilization of right colon and duodenum;
(d) Psoas identification;
(e) Dissection and isolation of the hilum;
(f) Clamping of the main renal artery and vein with bulldogs;
(g) Enucleation/enucleoresection strategy;
(h) Double layer renorrhaphy for renal reconstruction
(declamp after medullary layer reconstruction);
(i) Gerota’s closure.
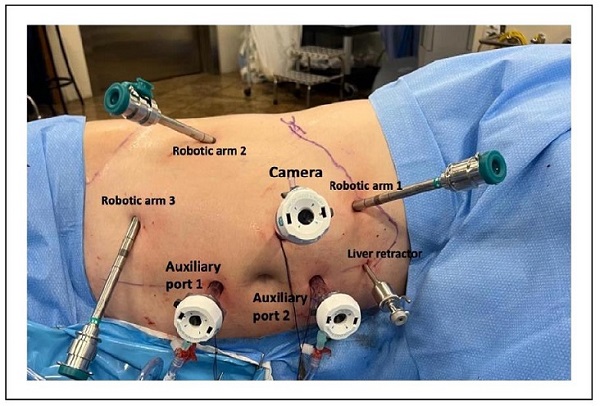
Figure 2. The port placement in our case of robotic-assisted partial nephrectomy (RAPN). The da Vinci Si robotic platform was used with a 30º lens, a four-arm configuration plus two assist ports (both 12 mm ports with 2 gas insufflators).
Intra- and post-operative results
Total procedure time was 130 min. Estimated blood loss was 800 mL and warm ischemia time was 24 min. Intraoperative complications were tumor rupture and increased bleeding (Supplementary Video). The postoperative course was uneventful. The urinary catheter was removed 1 day after surgery. On day 2, the surgical drain was removed and the patient was discharged from the hospital in good condition, although he showed a decline in renal function from eGFR 43 mL/min/1.73m2 to 26 mL/min/1.73m2.
Histopathologic analysis
Sample processing and histopathologic examination were performed by uropathologists according to the standard protocols of our institution. Tumor staging was performed according to the latest TNM criteria, and histopathologic classification was performed according to the latest guidelines of the International Society of Urological Pathology [7, 8]. Histopathologic analysis revealed a renal mass measuring 35 × 30 × 30 mm, G2, renal cell carcinoma, papillary type, with the presence of necrosis in 20% of the neoplasia. Analysis showed no evidence of sarcomatoid/ rhabdoid pattern, angiolymphatic invasion, renal capsule infiltration, or perirenal adipose tissue infiltration. The surgical parenchymal and radial margins were free of neoplastic involvement (pT1aNxMx) (Figure 3).
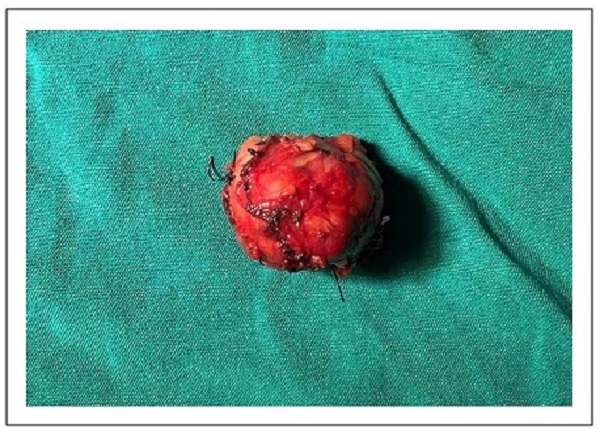
Figure 3. The resected renal lesion was suspicious for renal malignancy. Histopathologic analysis revealed a renal mass measuring 35 × 30 × 30 mm, G2, renal cell carcinoma, papillary type, with the presence of necrosis in 20% of the neoplasia. Margins were free of neoplastic involvement (pT1aNxMx).
Follow-up
At the 6-month follow-up, the patient was asymptomatic and had no postoperative complications. Renal function has returned to baseline levels (eGFR 43 mL/min/1.73m2 ), and the contrast-enhanced CT scan 6 months after surgery showed no evidence of local or systemic recurrence.
Discussion
During the last decade, the prevalence of renal tumors has
been increasing, and more challenging cases are being
performed, potentially increasing the risk of perioperative
complications [9]. In this context, several retrospective
series have demonstrated that RAPN offers a lower rate of
perioperative complications, lower estimated blood loss,
and shorter length of stay than open partial nephrectomy
(OPN), suggesting that RAPN may be an effective alternative to OPN [10].
To further reduce the risks and complications of a surgical technique, it is important to standardize the outcomes collection. In this regard, Sri et al. described the concept
of “trifecta” and “pentafecta” with the goal of identifying the most important outcomes of a surgical procedure.
Pentafecta was defined as achieving the “trifecta” (namely
negative surgical margin, no postoperative complications,
and WIT < 25 min) plus over 90% estimated GFR preservation and no CKD stage progression at 1 year [11].
These concepts have been shown to be easily applicable
to PN patients and provide an internationally comparable
PN outcome as a quality measure.
Despite this premise, complications nightmares can still
occur; therefore, regarding intraoperative bleeding, there
are some maneuvers that can be useful for its prevention
or management. For instance, the “clamp test” consists in
clamping the renal artery for 1 to 3 min during the renal
artery test ischemia prior to the actual ischemia and tumor
resection. The disappearance of blood flow around the renal tumor must be confirmed by color Doppler ultrasound
to proceed with the actual ischemia and tumor resection.
This practice has been shown to prevent massive bleeding during tumor resection and to avoid dissection of
non-feeding arteries around the tumor [12]. Furthermore,
Nepple et al. described a comprehensive checklist for intraoperative bleeding control during RAPN clamping [13].
Regarding arterial hemorrhage, the authors suggested
that controlled reduction of blood pressure may improve
visualization in patients with significant hypertension.
Furthermore, another temporizing option is to unclamp
the renal vein to allow venous outflow, thus improving
visualization of the partial nephrectomy bed. Another tool
that can be used is indocyanine green, which is useful to
ensure renal ischemia after clamping the renal artery [14].
Regarding the clamping of the renal vein during partial
nephrectomy, it depends on the surgeon’s preference, but
most surgeons agree that we need to clamp the vein only
during right partial nephrectomy in tumors adjacent to the
renal sinus. In this particular case, clamping of the renal
vein without proper arterial clamping probably resulted in
increased intraoperative bleeding.
Another feared complication is tumor rupture, especially
when there is a cystic component, which is a relatively
common situation but with limited oncologic implications
[15]. Pradere et al. evaluated a retrospective cohort of patients with cystic tumor rupture and reported that at a median follow-up of 32 months, 5 patients (2.5%) had local
recurrence, while progression to metastasis was observed
in only 2% of patients [15]. No peritoneal carcinomatosis
or port site metastasis was described. Also, no local or
metastatic recurrence was reported in the subgroup with
intraoperative cyst rupture. Estimated 5-year recurrencefree survival was not significantly different between
patients with and without intraoperative cyst rupture at
100% vs. 92.7% (P = 0.2). However, if tumor rupture occurs, it is necessary to avoid extensive contamination of
the cavity by aspirating and carefully removing any residual tumor fragments. Irrigation of the area with at least
1 L of saline solution is also indicated.
Several techniques can be used to resect these tumors,
ranging from polar nephrectomy, wide resection, enucleation, or pure enucleation, which is the standard in most
cases. Minervini et al. stated that robotic tumor enucleation is safe and achieves negative surgical margins in the
vast majority of patients, even in the case of complete PC
invasion [16]. If we have any doubts about the margins
after resection, we can perform a biopsy of the tumor bed.
Literature shows that in clinical T1b renal cell carcinoma,
tumor infiltration on the tumor bed was detected in 6 cases
(3.4%) and satellite lesion was detected in 3 cases (1.7%)
[17].
We believe that this nightmare was mainly caused by
incorrect clamping of the renal pedicle, which led to increased bleeding with poor visualization and tumor rupture. The fact that the tumor had a large area of necrosis
may also have facilitated tumor rupture.
A trained and experienced team is essential to avoid and
resolve these complications. Two aspirators must be available, as well as extra wires for renorrhaphy. Key points
when such complications occur are: to remain calm, but to
be effective and quick in tumor resection and renal suturing to avoid extensive bleeding.
Conclusions
Careful preoperative planning, including contrast-enhanced CT with 1-mm-thick slices and 3D reconstruction imaging, is essential. During surgery, a comprehensive dissection of the renal hilum must be attempted to access the exact number of vessels vascularizing the kidney and their bifurcation point to properly evaluate whether clamping of the artery would be sufficient to provide adequate ischemia of the renal parenchyma for clean visualization during tumor resection to avoid nightmares such as positive margins and tumor rupture.
Declarations
Authors’ contributions
Study concept and design: Paludo A.
Acquisition of data: Paludo A, Knijnik P, Duarte Jr. D.
Analysis and interpretation of data: Paludo A, Berger M,
Neto B.
Drafting of the manuscript: Paludo A, Knijnik P.
Critical revision of the manuscript for important intellectual content: Paludo A, Mazzone E, Puliatti S, Berger M,
Berger A, Neto B.
Statistical analysis: Paludo A.
Supervision: Berger A, Neto B, Berger M.
Financial support and sponsorship
None.
Conflicts of interest
The authors report no conflicts of interest.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the patient.
Availability of data and materials
Not applicable.
References
1.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol, 2011, 59(4): 543-552. [Crossref]
2. Mir MC, Derweesh I, Porpiglia F, Zargar H, Mottrie A, & Autorino R. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol, 2017, 71(4): 606-617. [Crossref]
3. Abou Elkassem AM, Lo SS, Gunn AJ, Shuch BM, DewittFoy ME, Abouassaly R, et al. Role of Imaging in Renal Cell Carcinoma: A Multidisciplinary Perspective. Radiographics, 2021, 41(5): 1387-1407. [Crossref]
4. Tanagho YS, Kaouk JH, Allaf ME, Rogers CG, Stifelman MD, Kaczmarek BF, et al. Perioperative complications of robot-assisted partial nephrectomy: analysis of 886 patients at 5 United States centers. Urology, 2013, 81(3): 573-579. [Crossref]
5. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med, 2021, 385(19): 1737-1749. [Crossref]
6. Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, & Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology, 2004, 64(5): 914-918. [Crossref]
7. Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol, 2013, 37(10): 1469-1489. [Crossref]
8. Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol, 2013, 37(10): 1490-1504. [Crossref]
9. Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, et al. Epidemiology of Renal Cell Carcinoma. World J Oncol, 2020, 11(3): 79-87. [Crossref]
10. Bravi CA, Rosiello G, Mazzone E, Minervini A, Mari A, Di Maida F, et al. The IRON Study: Investigation of Robotassisted Versus Open Nephron-sparing Surgery. Eur Urol Open Sci, 2023, 49: 71-77. [Crossref]
11. Sri D, Thakkar R, Patel HRH, Lazarus J, Berger F, McArthur R, et al. Robotic-assisted partial nephrectomy (RAPN) and standardization of outcome reporting: a prospective, observational study on reaching the “Trifecta and Pentafecta”. J Robot Surg, 2021, 15(4): 571-577. [Crossref]
12. Nohara T, Kadomoto S, Iwamoto H, Yaegashi H, Iijima M, Kawaguchi S, et al. Test clamp procedure in robot-assisted partial nephrectomy: is it a safe procedure? J Robot Surg, 2022, 16(3): 633-639. [Crossref]
13. Nepple KG, Sandhu GS, Rogers CG, Allaf ME, Kaouk JH, Figenshau RS, et al. Description of a multicenter safety checklist for intraoperative hemorrhage control while clamped during robotic partial nephrectomy. Patient Saf Surg, 2012, 6: 8-15. [Crossref]
14. Pandey A, Dell’Oglio P, Mazzone E, Mottrie A, & Geert De N. Usefulness of the Indocyanine Green (ICG) Immunofluorescence in laparoscopic and robotic partial nephrectomy. Arch Esp Urol, 2019, 72(8): 723-728. Cite this article as: Paludo AO, Knijnik PG, Duarte Jr DM, Mazzone E, Puliatti S, Berger AK, et al. Roboticassisted partial nephrectomy nightmare: poor clamping and tumor rupture. Uro-Technology Journal, 2023, 7(2): 08-12. doi: 1031491/UTJ.2023.06.008
15. Pradere B, Peyronnet B, Delporte G, Manach Q, Khene ZE, Moulin M, et al. Intraoperative Cyst Rupture during Partial Nephrectomy for Cystic Renal Masses-Does it Increase the Risk of Recurrence? J Urol, 2018, 200(6): 1200-1206. [Crossref]
16. Minervini A, Campi R, Di Maida F, Mari A, Montagnani I, Tellini R, et al. Tumor-parenchyma interface and longterm oncologic outcomes after robotic tumor enucleation for sporadic renal cell carcinoma. Urol Oncol, 2018, 36(12): 527.e521-527.e511. [Crossref]
17. Lu Q, Ji C, Zhao X, Fu Y, Guo S, Liu G, et al. Histopathologic analysis of tumor bed and peritumoral pseudocapsule after in vitro tumor enucleation on radical nephrectomy specimen for clinical T1b renal cell carcinoma. Urol Oncol, 2017, 35(10): 603.e615-603.e620. [Crossref]