Open Access | Case Report
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Telesurgery with cognitive 3D model guidance during robotassisted partial nephrectomy: first experience across Europe
# These authors contributed equally to the first authorship.
¶ These authors contributed equally to the senior authorship.
* Corresponding author: Michele Sica
Mailing address: Department of Oncology, Division of Urology,
University of Turin, San Luigi Gonzaga Hospital, Regione Gonzole, 10, 10043 Orbassano TO, Italy.
Email: michele.sica1991@gmail.com
This article belongs to the Special Issue: Nightmare and complex cases in Urology
Received: 15 January 2023 / Revised: 17 March 2023 / Accepted: 27 March 2023 / Published: 30 March 2023
DOI: 10.31491/UTJ.2023.03.007
Abstract
Telesurgery has found application in the modern era using 3D technology to improve surgical performance and organ preservation outcomes. Virtual congresses were created during the Covid-19 pandemic, giving to the participants the possibility to interact through digital platforms. This study aimed to describe the feasibility of using remotely guided telesurgery while applying 3D technology to aid the surgeon with intraoperative navigation as well as preoperative planning. The generated three-dimensional reconstruction (from the CT scan and ‘segmentation' performed by a dedicated software) was refined by a biomedical engineer under the supervision of the urologist to obtain a detailed 3D model of the organ and surrounding structures. Intraoperatively, the 3D virtual model was displayed and consulted during the intervention by the first surgeon in a cognitive manner aimed to maximize the benefits of real-time navigation. During the Techno Urology Meeting (TUM) of 2021 we offered this technology to two surgeons using the real-time connection provided by Zoom through the Tile-Pro software while an expert surgeon from Italy aided with intraoperative navigation. The patients were aged 49 and 58 years old, respectively, while tumor maximum diameters were 60 and 30 mm for the first case, and 46 mm for the second case. No major complications occurred in either case or blood transfusion was necessary intra or postoperatively. Telementoring provided the possibility to operate with the assistance of an expert surgeon who was not present in the operating room but could virtually supervise and help during critical steps.
Keywords
Telesurgey, 3D models, robotics, kidney cancer
Introduction
The concept of “precision surgery” is intrinsic to the management of genitourinary tumors today [1]. A detailed
understanding of the surgical anatomy is the key point for
tailored treatment planning, especially for renal surgery
and preservation of renal function, which covers a key
role today [2-4].
In this context, 3D reconstruction of standard two-dimensional cross-sectional imaging has been increasingly popular as it allows a better representation and understanding
of the surgical anatomy resulting in a tailor-made surgery
for each patient [5].
This technology is perceived as a useful tool in patient
counseling, surgical planning, and training, as it avoids
the “building-in-mind” process of two-dimensional crosssectional imaging, allowing a better understanding of the
anatomy, vasculature, and location of organs.
Importantly, the correct interpretation of information obtained from standard preoperative 2D images (contrastenhanced CT or MRI) requires extensive anatomical
knowledge and clinical experience. In addition, the mental
transformation from 2D to 3D is not a simple process.
Therefore, following these principles and trying to overcome these problems, 3D technology finds its role, gradually becoming an important tool in daily clinical practice
[6].
In the past, automatic rendering of 2D images resulted
in low-quality 3D reconstructions, which were of little
use for accurate preoperative planning or intraoperative
navigation. Today, thanks to technological innovations,
the development of dedicated software, and collaboration
between bioengineers, radiologists, and urologists, it is
possible to obtain high-definition 3D models.
Virtual 3D models have several fields of application: preoperative patient counseling (such as 3D printed models),
surgical training and simulations, preoperative surgical
planning, and intraoperative surgical navigation [7, 8].
Such technology, however, is currently available only in a
few specialized centers, and their fruition is not common.
Several studies have shown how such technology could
play an essential role in preoperative planning in all settings to pursue the path of precision surgery [9-12].
In an era of technological boom and concomitant confinement due to the COVID-19 pandemic, platforms and software which allow patients' remote management has been
developed: telemedicine is one example that has invaded
the urological setting as well [13, 14]. Telesurgery, which
in the past had been explored for laparoscopic techniques
[15] has found application in the modern era using 3D
technology to improve surgical performance and to ensure
organ preservation even in difficult neoplasms [16].
The COVID-19 pandemic bent all scientific circles, making not only patient management (diagnosis, treatment,
follow-up) difficult, but also the scientific reports being
developed through congresses. Therefore, virtual congresses where participants could interact through digital
platforms for professional development were also created
in this area [17]. The use of this technology confirmed the
advantages related to 3D-guided surgery, as highlighted in
our previous works [9, 18-20]. In particular, the surgeon
was able to study the case preoperatively after sharing the
model.
This study aimed to describe the feasibility of using remotely guided telesurgery by also applying 3D technology
to aid the surgeon in intraoperative navigation as well as
preoperative planning.
Case report
3D models creation
To realize this project, we started with the creation of the
3D model following a rigorous approach [9].
The first step is the sharing of the images on dedicated
and authorized cloud platforms (www.mymedics3d.com).
On such platforms it is possible to upload CT-scan DICOM images, making them accessible from anywhere in
the world. Using DICOM image visualization software,
it is necessary to analyze the object, select the most useful images (e.g., arterial, or late phase images of a CT
scan), and modify and adjust specific parameters (e.g.,
image contrast and brightness) according to the needs of
the project. This phase is referred to as the “preprocessing phase.” Next, a rendering of the volume is created and
then “segmentation'' is performed, which is a process being performed semi-automatically by dedicated software.
At the end of the process, the generated three-dimensional
reconstruction is usually refined by a biomedical engineer
under the supervision of the urologist. The goal is to obtain a 3D model, a detailed reproduction of the organ and
surrounding structures. This is made possible by so-called
thresholding, which is based on selecting a specific range
of a defined parameter (e.g., grayscale). The final steps
in the process are the creation of a transcription code for
visualizing the reconstruction in an interactive 3D-PDF
format to improve understanding of the relationships between the tumor and surrounding structures, and the conversion of each part into stereolithographic (STL) format.
Then, thanks to the same cloud platform, virtual reconstructions can be downloaded and displayed on an electronic device for use in preoperative planning (Figure 1
and 2).
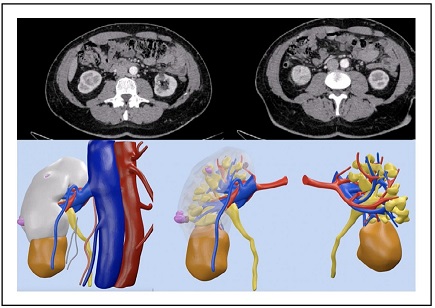
Figure 1. CT-scan and 3D reconstruction of case 1.
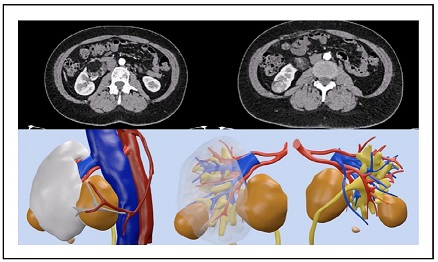
Figure 2. CT-scan and 3D reconstruction of case 2.
Intraoperative navigation
Intraoperatively, the 3D virtual models can be displayed and consulted during the intervention by the first surgeon,
in a cognitive (on-demand) manner, aimed to maximize
the benefits of real-time navigation.
During the 2021 edition of the Techno Urology Meeting
(TUM), taking advantage of the changeover to the virtual
format of the Congress, we offered this technology to
surgeons invited to operate even during procedures performed remotely.
Thanks to a real-time connection provided by Zoom
(https://zoom.us), an expert 3D-model navigator was able
to interact with the surgeon, modifying the virtual images
displayed directly inside the robotic console, thanks to the
Tile-pro software.
For the first time, two cognitive 3D robot-assisted partial
nephrectomy operations were performed, guided live by
one urologist expert in intraoperative navigation (D.A.)
who assisted the surgeons (A.M. and A.B.) in the most
difficult phases of the operation (Figure 3).
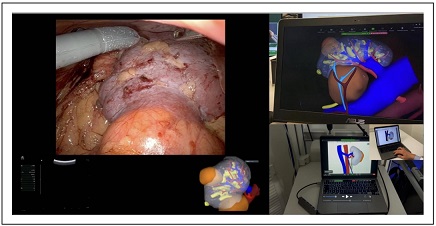
Figure 3. Intraoperative navigation with 3D models cognitive assistance.
Clinical Cases
The first case was a 49-year-old female patient. Her past
medical history included arterial hypertension, dyslipidemia, and a prior appendectomy. Incidental ultrasound
found two right-sided renal masses at the lower pole measuring 60 and 30 mm at the CT scan study (PADUA Score
10 and 7, R.E.N.A.L score 8 and 6). The patient was admitted to the Department of Urology O.L.V Hospital in
Aalst, Belgium.
The second case was a 58-year-old male patient. His
previous medical history included arterial hypertension,
a history of peptic ulcer, removal of a dorsal cutaneous
squamous carcinoma, and a rhino septoplasty. An incidental ultrasound finding of a right-sided renal mass was
confirmed by a CT scan and a 46 mm solid right renal
mass located at the inferior pole was evaluated (PADUA
score 8, R.E.N.A.L. score 7). The patient was admitted to
the Department of Uro-Oncology at Fundació Puigvert,
Barcelona.
For both patients, consent was obtained for surgery, including the use of a telesurgery platform to remotely
involve another consultant surgeon during the procedure.
The connection between the sites was easy to establish
and was maintained during all the procedures without the
occurrence of technical issues.
The size of the tumors was respectively 60 mm and 33
mm in the first case and 46 mm in the second case. The
operative time was respectively 98 min and 82 min.
Blood loss was 120 cc in the first patient and 130 cc in the
second one. No blood transfusion was necessary intra or
postoperatively in both case. The value of hemoglobin at
admission was 12.8 g/dl and the value of hemoglobin at
discharge was 12.4 g/dl in the first patient and 13.3 g/dl
and 13.2 g/dl in the second one, respectively. The duration
of hospital stay was 4 and 5 days, respectively; the bladder catheter was removed on the 2nd postoperative day for
the same patients. The characteristics of patients undergoing surgery are summarized and divided by case (case 1
patient of Fundació Puigvert and case 2 patient of OLV
Hospital) in Table 1.
Table 1
Characteristics of clinical cases.
| Case 1 | Case 2 | |
|---|---|---|
| Preoperative Features | ||
| Sex | Male | Female |
| Age (years) | 58 | 49 |
| BMI | 28 | 31.5 |
| Age-adjusted Charlson's Index | 3 | 1 |
| PADUA score | 8 | 10 and 7 (double mass) |
| R.E.N.A.L. score | 7 | 8 and 6 (double mass) |
| cTNM | T1b | T1b |
| Intraoperative Features | ||
| Connection time (s) | 20 | 28 |
| Latency time (s) | 1 | 2 |
| Type of Anesthesia | General anesthesia | General anesthesia |
| Type of clamping | Super selective clamping | Super selective clamping |
| Time of clamping (min) | 18 | 14 |
| Operative time (min) | 98 | 82 |
| Blood loss (ml) | 120 | 130 |
| Postoperative Features | ||
| Drainage removal (days) | None | None | Removal catheter (days) | 2 | 2 | Hospitalization (days) | 4 | 5 | Histological type | RCC | RCC | pTNM | pT1bNxMx | pT1aNxMx | Clavien-Dindo POD | 0 | 0 |
Conclusions
Robot-assisted Partial Nephrectomy (RAPN) outcomes
are strictly related to the surgeon's experience and a good
method of increasing the learning curve is having the opportunity to connect with more experienced surgeons.
In our series, we introduced the possibility to have 3D
Tele assistance during a broadcasted RAPN provided by a
skilled robotic surgeon confirming, as highlighted in our
previous works, that the use of 3D Virtual Models gives
the surgeon a more accurate pre- and intraoperative understanding of the renal mass nephelometry details and surgical complexity.
Surely this technology must face some issues: first, a
stable and high-performance internet connection (e.g.,
5G) is needed to allow the dataflow between the two parties involved. Secondly, the connection must be protected
and, maybe, encrypted, to protect patients' data from
informatic attacks. Lastly, the urologist maneuvering the
model, although he/she may be self-taught, would benefit from dedicated training, which could be fulfilled using the
same concept of telementoring proposed for live surgery.
In conclusion, our experience suggests that telementoring with 3D models cognitive assistance can be easily
performed, supporting the surgeon during the crucial
steps of complex procedures such as RAPN. In the actual
technology-driven era, the implementation of telesurgery
will allow for reducing the physical barriers and distance
avoiding the need for physical travel.
Declarations
Authors' contributions
Sica M: Study concepts, Study design, Formal analysis and interpretation, Manuscript preparation, Manuscript editing. Meziere J: Study concepts, Study design, Formal analysis and interpretation, Manuscript preparation, Manuscript editing. Verri P: Data acquisition, Data Formal analysis. Daniele Amparore: Study design, Manuscript review. Piramide F: Data acquisition, Data Formal analysis, and interpretation. Volpi G: Data acquisition, Data Formal analysis. De Cillis S: Data Formal analysis and interpretation. Breda A: Study design, Manuscript review. Mottrie A: Study design, Manuscript review. Porpiglia F: Study concepts, Study design, Manuscript editing, Manuscript review. Checcucci E: Study concepts, Study design, Manuscript editing, Manuscript review.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Informed consent for publication was obtained from all patients.
References
1. Autorino R, Porpiglia F, Dasgupta P, Rassweiler J, Catto JW, Hampton LJ, et al. Precision surgery and genitourinary cancers. Eur J Surg Oncol, 2017, 43(5): 893-908. [Crossref]
2. Grivas N, Kalampokis N, Larcher A, Tyritzis S, Rha KH, Ficarra V, et al. Robot-assisted versus open partial nephrectomy: comparison of outcomes. A systematic review. Minerva Urol Nefrol, 2019, 71(2): 113-120. [Crossref]
3. Bertolo RG, Zargar H, Autorino R, Fiori C, Kaouk JH, Russo P, et al. Estimated glomerular filtration rate, renal scan and volumetric assessment of the kidney before and after partial nephrectomy: a review of the current literature. Minerva Urol Nefrol, 2017, 69(6): 539-547. [Crossref]
4. Bertolo R, Fiori C, Piramide F, Amparore D, Barrera M, Sardo D, et al. Assessment of the relationship between renal volume and renal function after minimally-invasive partial nephrectomy: the role of computed tomography and nuclear renal scan. Minerva Urol Nefrol, 2018, 70(5): 509-517. [Crossref]
5. Bertolo R, Autorino R, Simone G, Derweesh I, Garisto JD, Minervini A, et al. Outcomes of Robot-assisted Partial Nephrectomy for Clinical T2 Renal Tumors: A Multicenter Analysis (ROSULA Collaborative Group). Eur Urol, 2018, 74(2): 226-232. [Crossref]
6. Checcucci E, De Cillis S, & Porpiglia F. 3D-printed models and virtual reality as new tools for image-guided robotassisted nephron-sparing surgery: a systematic review of the newest evidences. Curr Opin Urol, 2020, 30(1): 55- 64. [Crossref]
7. Porpiglia F, Amparore D, Checcucci E, Autorino R, Manfredi M, Iannizzi G, et al. Current Use of Threedimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur Urol Focus, 2018, 4(5): 652-656. [Crossref]
8. Parikh N, & Sharma P. Three-Dimensional Printing in Urology: History, Current Applications, and Future Directions. Urology, 2018, 121: 3-10. [Crossref]
9. Checcucci E, Amparore D, Pecoraro A, Peretti D, Aimar R, S DEC, et al. 3D mixed reality holograms for preoperative surgical planning of nephron-sparing surgery: evaluation of surgeons' perception. Minerva Urol Nephrol, 2021, 73(3): 367-375. [Crossref]
10. Amparore D, Pecoraro A, Checcucci E, S DEC, Piramide F, Volpi G, et al. 3D imaging technologies in minimally invasive kidney and prostate cancer surgery: which is the urologists' perception? Minerva Urol Nephrol, 2022, 74(2): 178-185. [Crossref]
11. Porpiglia F, Bertolo R, Checcucci E, Amparore D, Autorino R, Dasgupta P, et al. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: urologists' and patients' perception. World J Urol, 2018, 36(2): 201-207. [Crossref]
12. Checcucci E, Amparore D, Fiori C, Manfredi M, Ivano M, Di Dio M, et al. 3D imaging applications for robotic urologic surgery: an ESUT YAUWP review. World J Urol, 2020, 38(4): 869-881. [Crossref]
13. Checcucci E, De Luca S, Alessio P, Verri P, Granato S, De Cillis S, et al. Implementing telemedicine for the management of benign urologic conditions: a single centre experience in Italy. World J Urol, 2021, 39(8): 3109-3115. [Crossref]
14. Bruschi M, Micali S, Porpiglia F, Celia A, De Stefani S, Grande M, et al. Laparoscopic telementored adrenalectomy: the Italian experience. Surg Endosc, 2005, 19(6): 836-840. [Crossref]
15. Shin DH, Dalag L, Azhar RA, Santomauro M, Satkunasivam R, Metcalfe C, et al. A novel interface for the telementoring of robotic surgery. BJU Int, 2015, 116(2): 302-308. [Crossref]
16. Porpiglia F, Checcucci E, Autorino R, Amparore D, Cooperberg MR, Ficarra V, et al. Traditional and Virtual Congress Meetings During the COVID-19 Pandemic and the Post-COVID-19 Era: Is it Time to Change the Paradigm? Eur Urol, 2020, 78(3): 301-303. [Crossref]
17. Amparore D, Pecoraro A, Checcucci E, Piramide F, Verri P, De Cillis S, et al. Three-dimensional Virtual Models' Assistance During Minimally Invasive Partial Nephrectomy Minimizes the Impairment of Kidney Function. Eur Urol Oncol, 2022, 5(1): 104-108. [Crossref]
18. Piramide F, Kowalewski KF, Cacciamani G, Rivero Belenchon I, Taratkin M, Carbonara U, et al. Three-dimensional Model-assisted Minimally Invasive Partial Nephrectomy: A Systematic Review with Meta-analysis of Comparative Studies. Eur Urol Oncol, 2022, 5(6): 640-650. [Crossref]
19. Amparore D, Pecoraro A, Piramide F, Verri P, Checcucci E, De Cillis S, et al. Three-dimensional imaging reconstruction of the kidney's anatomy for a tailored minimally invasive partial nephrectomy: A pilot study. Asian J Urol, 2022, 9(3): 263-271. [Crossref]
20. Checcucci E, Amparore D, Volpi G, & Porpiglia F. A snapshot into the future of image-guided surgery for renal cancer. Asian J Urol, 2022, 9(3): 201-203. [Crossref]