Open Access | Case Report
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Percutaneous nephrolithotomy in a patient with a ureterosigmoidostomy diversion
* Corresponding author: Irache Abáigar Pedraza
Mailing address: Urology departament Hospital Don Benito Villanueva, carretera Don Benito Villanueva s/N km 3,5 06400, Don
Benito, Badajoz, Spain.
Email: irache.abaigar@salud-juntaex.es
This article belongs to the Special Issue: Nightmare and complex cases in Urology
Received: 30 October 2022 / Revised: 24 November 2022 / Accepted: 08 December 2022 / Published: 29 December 2022
DOI: 10.31491/UTJ.2022.12.005
Abstract
In patients with bladder cancer, ureterosigmoidostomy has been used as a form of urinary diversion, and urinary lithiasis has been reported as a complication. A patient with a large bilateral kidney stone and ureterosigmoidostomy diversion is described. In 2012, a 61-year-old man had a cystectomy due to bladder cancer. He was lost to follow-up after presenting to the emergency department in 2016 with right flank pain and fever. Computed tomography (CT) scan reveals bilateral staghorn calculus. A bilateral percutaneous nephrostomy was performed. The patient was planned for bilateral percutaneous nephrolithotomy (PCNL). He declined, therefore we proposed External Shock Wave Lithotripsy (ESWL). The right kidney stone was removed, but the left kidney stone did not alter after 7 ESWL sessions, thus PCNL was scheduled. The middle calyx was punctured under fluoroscopic guidance through the nephrostomy in Valdivia’s modified position. Two 0.035” hydrophilic guide wires were passed down the renal pelvis and ureter until it ureterosigmoidostomy union was reached. Dilation was carried out with Nephromax. An Amplatz 30 Ch was placed. The holed stone was then fragmented with Laser Holmium. PCNL tubeless was performed. He was discharged two days after surgery. PCNL tubeless was performed. The hospital stay was two days. CT control two months later: Lower pole 5 cm hematoma, the residual stone of 4 mm in the upper calyx. After resolving the renal hematoma, the residual stone will be dealt with ESWL.
Keywords
Ureterosigmoidostomy, renal stone, percutaneous nephrolithotomy
Introduction
Ureterosigmoidostomy has been used as a form of urinary
diversion in patients with bladder cancer. Urinary lithiasis
has been reported as a ureterosigmoidostomy complication in 3-40% of the cases in recent series. The main
causes are bacterial colonization and metabolic derangements due to urinary diversion [1].
Ureterosigmoidostomy was probably first used in about
1,852 by Simon for exstrophy of the urinary bladder [2].
This technique has been criticized for the postoperative
complications, perhaps the most important is that most patients develop pyelonephritis at some time, struvite renal
lithiasis, because they are strongly associated with urinary
tract infections (UTIs) with urea-splitting organisms, hyperchloremic metabolic acidosis and they always have
some anal leakage of a malodorous mixture of feces and
urine [3].
The principal issue with the use of the bowel in the urinary diversion is that the bowel continues to produce
mucus and continues to perform its main physiological
function of secretion and re-absorption [4].
Patients that have ureterosigmoidostomy must be watched
closely. They need a low sodium chloride diet to reduce
their chloride intake to avoid acidosis. They must be given
sodium potassium citrate once or twice per day and an
alkalinizing therapy with oral sodium bicarbonate 1–2 g
three times a day [3].
Case report
We report the case of a 61 years old man who submitted
to cystectomy and ureterosigmoidostomy in 2012 due to
bladder cancer. After the surgery, he was lost to follow up
and in 2016 he presented to the emergency department with right flank pain and fever. The main laboratory findings were anemia, leukocytosis, hyperchloremic metabolic
acidosis, and increased serum creatinine.
A computed tomography scan showed bilateral staghorn
stones.
Two bilateral ultrasound-guided percutaneous nephrostomies were performed to relieve obstruction and fever.
Once the patient recovered from his acute pathology,
bilateral PCNL access was offered to him, but he denied
it. So bilateral ESWL was performed. The right staghorn
lithiasis was completely disintegrated after 5 sessions (for
each session: 3,000 shocks were delivered at a frequency
of 100/min approximately) and the homolateral nephrostomy was removed but, after 7 ESWL sessions over the
left kidney lithiasis, any changes were evidence on X-Ray
(each session: 3,000 shocks were delivered at a frequency
of 100/min approximately, but no expulsive fragments
http://www.antpublisher.com/index.php/UTJ/index
Figure 2. CT coronal plane. Lower calyx lithiasis.
were evidenced). A new computed tomography scan was
performed showing a staghorn stone that filled the renal
pelvis (32 × 20 mm, 975 Hounsfield units (HU), superior
(35 × 18 mm, 1000 UH) and inferior renal calyces (24 ×
23 mm (903 HU) (Figure 1-3).
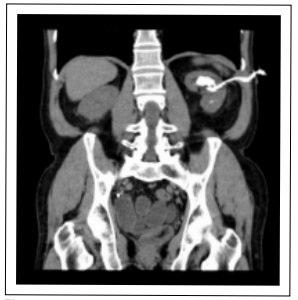
Figure 1. CT coronal plane. Upper calyx lithiasis.
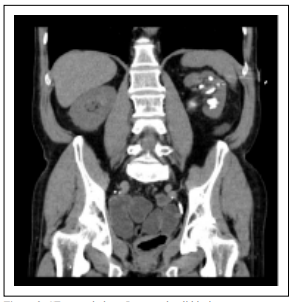
Figure 2. CT coronal plane. Lower calyx lithiasis.
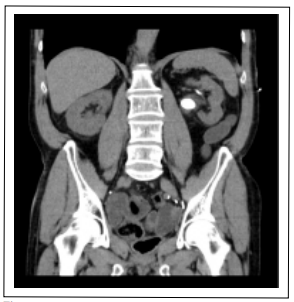
Figure 3. CT coronal plane. Renal pelvis lithiasis.
Once at that point, we advised the patient to reconsider
PCNL and he accepted.
Two weeks previous urine culture revealed multi-drug resistant Klebsiella pneumonia and the antibiotic was started
then according to the results (Meropenem 1 g iv/12h ×
7 d, according to renal function). Five days after having
finished the antibiotic, the urine culture control did not
evidence of any germs.
On the day of the surgery, in the Galdakao-modified
Valdivia position, we performed an antegrade pyelography
through the left nephrostomy tube showing the hydronephrotic changes, the lithiasis, and filling of the rectal ampulla (Figure 4). Because of the location and magnitude
of lithiasis, the lower calyx was chosen to puncture using
the “bull’s eye” technique, but the hydrophilic guidewire
did not progress probably because it was an excluded
calyx, not despite using the ultrasound. So we decided to
insert two ZIP wire™ Hydrophilic Guide Wire®
through
the nephrostomy tube one guided up to the upper pole (for
tract dilation) and the other guided down to the ureter (for
safety). After that, the nephrostomy was replaced and we
introduce a Nefromax®
for a “single-step” dilation technique till 30 F, an Amplatz sheath was then placed.
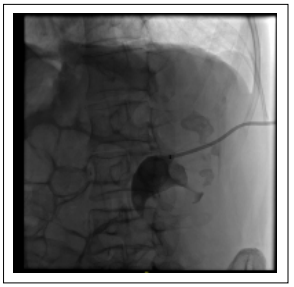
Figure 4. Nephrostomy tube was placed in the renal kidney and upper calyx, lower calyx, and renal pelvis lithiasis.
The calculus in the renal pelvis and upper calyx was
identified by a rigid nephroscope, and fragmentation was
performed by Holmium laser (Auriga 30 W. 2500 MJ, 12
Hz). Significant fragments were retrieved by a grasper.
The lower and the residual upper calyx stones were removed using the flexible cystoscope, Holmium laser
(Auriga 30 W. 2000-2500 MJ, 10 Hz), and a grasper. After
complete stone removal, an inspection of the calyces and
ureter was performed by anterograde pyelography. Once
we evidenced no residual stone, we removed the Amplatz
sheath performing an NPLC tubeless. The postoperative
course was successful. No active bleeding (preoperative
hemoglobin of 13 g/dl and postoperative of 11.4 g/dl) and
no fever. He was discharged two days after surgery.
Stone analysis was performed showing a mixture of magnesium and ammonium phosphate (struvite) 34%, calcium
carbonate apatite 47%, and calcium oxalate (19%), this
last component is rarely developed in corals [5].
Two weeks later post-surgery CT scan control showed a
5 cm hematoma in the lower pole and a 5 mm residual
lithiasis in the upper calyx (Figure 5). The hemoglobin
was 12 g/dl. Conservative management of the hematoma
with ultrasound and a blood test in the second and seventh
month after surgery was decided. Once it was reabsorbed,
the residual lithiasis was resolved with extracorporeal
shock wave lithotripsy.
Stone analysis revealed a mixed type stone, composed of
struvite and apatite.
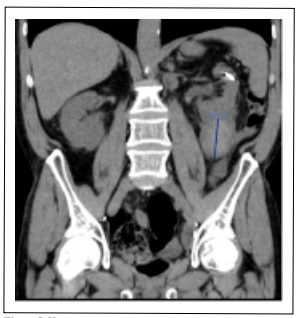
Figure 5. Hematoma of the lower pole.
Discussion
It is well-established that patients undergoing urinary
diversion are at amplified risk of calculi formation. Reported prevalence varies between 3% and 43% [1-4, 6].
When urine is in contact with the bowel wall, ammonia,
hydrogen, and chloride are also reabsorbed. Chronic Acidosis develops from excess reabsorption of ammonium
chloride across the colonic mucosa.
Besides, patients undergoing urinary intestinal diversion
are at increased risk for upper tract stones formation as
well as calculi within the diversion segment for many reasons such as chronic bacteriuria (colonization rates range
from 14 to 96%), urinary reflux, and the possibility of the
presence of foreign bodies such as staples or sutures that
can act as a nidus for stone formation; apart from the hyperchloremic metabolic acidosis patient status [6].
The colon has an abundant luminal anion exchanger that
absorbs chloride and secretes bicarbonate. Thus, when
chloride-rich urine enters the colon, the chloride is absorbed in exchange for bicarbonate, resulting in bicarbonate loss, and chloride retention [7]. The prolonged
contact of urine with the intestinal surface encourages the
exchange of chloride with bicarbonate. The resulting systemic acidosis causes impaired calcium reabsorption from
the proximal tubules and decreased renal production of citrate. There exists also an increase in citrate absorption by
the bowel segments. All of this results in hypercalciuria,
hypocitraturia, alkaline urine, and abundant ammonium
and phosphate ions, each of which promotes stone formation. Besides, the loss of bicarbonate results in acidosis
and hypercalciuria, resulting in calcium stones [1-4, 6].
There are several treatment options for managing urinary
stones. Percutaneous nephrolithotomy is the preferred
option for treating complex kidney stones, large volume
stones, or after the failure of other less invasive therapeutic alternatives [8, 9].
Besides is the best option for treating renal stones in
patients with urinary diversion. Although PCNL is an efficient and safe technique, it may be a demanding procedure in case of urinary diversion.
Despite these newer management techniques, the reconstructed urinary tract poses a variety of challenges, and
gaining percutaneous access is one of them, it is a difficult step. A detailed study of the anatomy previous to
the surgery, cross-sectional imaging with CT and other
techniques, if it is possible, and a thorough study of the
pyelography during the surgery is essential in surgical
planning.
The appropriate management of calculi in patients with
urinary diversions must be individualized. With a priority
on minimally invasive procedures. Little is available in
published reports regarding the outcomes of PCNL in this
specific patient population. Most of the literature is case
reports, there is no large series of patients that allows us to
follow during the procedure.
As we say, there is not exist step-by-step guideline in
these cases. Identifying the neo-ureteral orifices is not
mandatory, in our case we decided not to perform a retrograde pyelography to avoid the risk of bacteriemia [10].
Puncture of the collecting system is necessary to obtain
primary access and to perform a pyelography that allows
surgery. There is no standard position, prone or supine
(Galdakao modified) when performing PCNL. We are
used to the second one, and so we proceeded with this patient.
In normal PCNL we are used to operating with a safety
guide that threads the patient (usually, from de kidney to
the urethra) but in that case, due to the risk of bacteriemia,
we decided not to thread the patient and instead of that,
two guides, one for safety and the other for work, were
used. At that time, we did not have the Miniperc set, so
we used a single dilatation step technique (Nefromax®
) till 30 F.
We are used to and feel safe performing tubeless PNL, so,
as such, we proceeded in the same way, once performing
an anterograde pyelography after having finished the surgery. The patient was discharged two days after surgery.
The post-surgery CT showed a lower pole 5 cm hematoma
and a residual stone of 5 mm in the upper calyx. After resolving the renal hematoma (ultrasound follow), the residual stone will be dealt with ESWL. Intraoperative bleeding may result from trauma renal parenchyma or injury to
the perinephric vessels [11]. It has been reported that the
size of stones and stone complexity are important factors
for severe vessel injury besides, the number of calyceal
punctures is one of the predictive factors of intraoperative
bleeding in PCNL [12]. Moreover, the use of a rigid nephroscope may injure the renal parenchyma, resulting in
increased bleeding [13]. In our patient, probably, the big
size stone, the unsuccessful attempt to puncture the lower
calyx, and the use of a rigid nephroscope to reach the calyces occupied by the stone favored the renal hematoma.
Given that there was no clinical or analytical repercussion,
with a decrease in hemoglobin, conservative management
with ultrasound follow-up was done.
Conclusions
Surgical management of renal stone disease in patients with urinary diversion requires detailed evaluation and individualized consideration depending on stone location and burden, diversion type, and surgeon’s experience.
Declarations
Authors’ contributions
All of the authors have participated in the article. Irache Abáigar Pedraza: research and writing. Santiago Moreno Pérez de la Cruz: review. Andrés López de Alda: review.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and informed consent
Not applicable.
Consent for publication
Written informed consent for publication was obtained.
References
1. Abreu LA, Lara C, Dionísio MA, Pelosi AD, & Figueiredo FA. Endoscopic management of ureteral calculus in a patient with ureterosigmoidostomy diversion. Int Braz J Urol, 2013, 39(4): 593-596. [Crossref]
2. Simon J. Operation for directing the orifices of ureters into the rectum; temporary success, subsequent failure; autopsy. Lancet 1852: 568-570.
3. Goodwin WE, & Scardino PT. Ureterosigmoidostomy. J Urol, 1977, 118(1 Pt 2): 169-174. [Crossref]
4. Vasdev N, Moon A, & Thorpe AC. Metabolic complications of urinary intestinal diversion. Indian J Urol, 2013, 29(4): 310-315. [Crossref]
5. Nemoy NJ, & Staney TA. Surgical, bacteriological, and biochemical management of “infection stones”. JAMA, 1971, 215(9): 1470-1476.
6. Okhunov Z, Duty B, Smith AD, & Okeke Z. Management of urolithiasis in patients after urinary diversions. BJU Int, 2011, 108(3): 330-336. [Crossref]
7. Palmer BF, Emmett M. Renal and metabolic complications following urinary diversion. UpToDate. 2022 Jan [cited 2022 Jan 19]. Available from: https://www.uptodate.com/contents/renal-and-metabolic-complicationsfollowing-urinary-diversion Subscription required.
8. Pérez-Fentes D. [Techniques for percutaneous access during percutaneous nephrolithotomy.]. Arch Esp Urol, 2017, 70(1): 155-172.
9. Sfoungaristos S, Mykoniatis I, Poulios E, Paikos D, & Hatzichristou D. Percutaneous Nephrolithotomy in a Patient with Mainz Pouch II Urinary Diversion: A Case Report. Prague Med Rep, 2016, 117(4): 198-203. [Crossref]
10. Poudyal S. Current insights on haemorrhagic complications in percutaneous nephrolithotomy. Asian J Urol, 2022, 9(1): 81-93. [Crossref]
11. Palka J, Farooq Z, & Anderson BG. Safety of retrograde pyelography for infected ureteral stones. Can J Urol, 2020, 27(1): 10130-10134.
12. Turna B, Nazli O, Demiryoguran S, Mammadov R, & Cal C. Percutaneous nephrolithotomy: variables that influence hemorrhage. Urology, 2007, 69(4): 603-607. [Crossref]
13. Gadzhiev N, Malkhasyan V, Akopyan G, Petrov S, Jefferson F, & Okhunov Z. Percutaneous nephrolithotomy for staghorn calculi: Troubleshooting and managing complications. Asian J Urol, 2020, 7(2): 139-148. [Crossref]