Open Access | Research
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Hecogenin, a plant-derived small molecule, as a potential therapeutic intervention for neurodegenerative disorders: insights from a zebrafish model
# These authors contributed equally to the manuscript.
* Corresponding author: Senthilkumar Palanisamy
Mailing address: Department of Genetic Engineering, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, Chengalpattu, 603203, India.
Email: mpsenthilkumar@gmail.com
Received: 08 September 2025 / Revised: 10 October 2025 / Accepted: 11 November 2025 / Published: 30 December 2025
DOI: 10.31491/APT.2025.12.194
Abstract
Background: Alzheimer’s disease is a neurodegenerative disorder of the brain and the most prevalent form of dementia, affecting approximately 55 million people worldwide. It is characterized by the presence of tau tangles and amyloid-beta plaques. Medicinal plants have been used since ancient times for the treatment of the disease. A plant steroidal saponin compound hecogenin, found in Sida cordifolia, exhibits pharmacological properties like anti-inflammatory and gastroprotective agent, but its neuroprotective potential remains unexplored. The study investigated the neuroprotective properties of hecogenin against aluminium chloride induced toxicity in zebrafish, compared to the standard donepezil.
Methods: In the present study, 6 months old zebrafish (both male and female) were grouped into control, treatment of hecogenin (5 mg/L and 2.5 mg/L), donepezil (1 mg/L) and aluminium chloride treated zebrafish (n = 10 in each group with both the sexes). The neuroprotective effect of hecogenin was compared to the standard with biochemical parameters (oxidative stress markers), behavioral analysis (novel tank diving test and T-maze) and histopathological analysis (H&E and Thioflavin S staining).
Results: The results revealed that hecogenin reduced the oxidative stress biomarkers levels like SOD, MDA, protein carbonyl, GSH and AChE when compared to the standard. T-maze and novel tank diving test revealed that hecogenin was found to exhibit anxiolytic effect by improved cognitive behavior. The histopathological staining of the zebrafish brains with H&E and thioflavin revealed the reduction of amyloid plaques in the hecogenin treated zebrafish model compared to that of the standard drug.
Conclusion: Hecogenin exhibits neuroprotectivity and anxiolytic effects in an aluminium chloride induced AD in zebrafish model. The results indicate that hecogenin is a potential plant derived therapeutic alternative for Alzheimer’s disease management.
Keywords
Alzheimer’s disease, Sida cordifolia, amyloid plaques, anxiolytic effect, neurodegeneration, neuroprotection
Introduction
Neurological disorder characterized by the shrinkage of cholinergic neurons is known as Alzheimer’s disease. Cholinergic neurons are crucial for memory and retention [1-3]. Neuropathology is visualized by the phosphorylation of microtubule-associated tau protein and formation of amyloid plaques [4-6]. The pathological conditions include impaired ubiquitin proteasome system, oxidative stress and defect in mitochondrial dynamics [7, 8]. Approximately 69 million people over the age of 65 are currently affected by Alzheimer’s disease and the numbers are projected to rise to about 13.8 million by 2060. Zebrafish is being used in neuroscience as a powerful tool to study the disorders of the central nervous system due to its genome size, visible behavior, meteoric developmental stages and fertilization, access to high throughput screen ing, accurate genetic manipulation, and cost effectiveness. The zebrafish brain closely resembles the human brain in terms of neurochemical and neuroanatomical structures. There is also strong evidence supporting the existence of behavioral and physiological similarities among them [9]. Scientific investigations have examined the potential of various Ayurvedic medicinal plants and their derivatives in treatment of the disease. While the precise mechanism of action is not fully understood, extensive phytochemical research has revealed a diverse array of therapeutic compounds from medicinal plants. These compounds include polyphenols, alkaloids, tannins, flavonoids, sterols, lignans and triterpenes which demonstrate a wide array of pharmacological activities [10].
A steroidal saponin, hecogenin was found to exhibit therapeutic potential such as anticancer [11], anti-inflammatory [12], anti-ulcer [13], antimicrobial [14], hepato-protectant [15] and antiproliferative [16]. Previous in silico studies suggested that the compound hecogenin derived from Sida cordifolia has been found to exhibit neuroprotective activity against BACE-1, an enzyme involved in the production of amyloid beta plaques plays a vital role in Alzheimer’s pathogenesis [17]. Though various pharmacological activities have been demonstrated by hecogenin, the neuroprotective activity of hecogenin has not been understood. The study is aimed to understand the neuroprotective effect of the compound hecogenin in the zebrafish model by inducing aluminium chloride compared to the standard drug donepezil.
Materials and methods
Materials
Hecogenin (https://www.caymanchem.com/prod-uct/21372/hecogenin ) was purchased from Cayman chemical Michigan, USA.
Animals
A total of 50 zebrafish adults aged 6 months were obtained. The fish were divided into the following groups: the control group, aluminium chloride treated group, 5 mg/L hecogenin treated group, 2.5 mg/L hecogenin treated group and 1 mg/L donepezil (standard) treated group. The fishes were kept in 30 × 30 × 30 cm tanks in light/ dark cycles and fed twice a day with commercial food. The fishes were kept in aluminium chloride for 26 days to induce Alzheimer’s disease followed by training them for behavioral analysis and later euthanized in ice cold water for further biochemical and histopathological analysis [18]. All zebrafish experiments were carried out as per the institutional animal ethics committee guidelines.
Behavioral assessment
Novel tank diving test (NTT)
NTT analysis was carried out to evaluate the anxious response and locomotory activity of zebrafish as described by Cachat et al [19]. A 1.5-liter trapezoidal tank was vir-tually divided into two horizontal lines as top and bottom sections. The zebrafishes were assessed for 6 minutes to evaluate the locomotory activity by measuring the average velocity and distance travelled.
T-Maze test
T maze test is a color-biased test described by Maddula et al [20, 21]. The T-maze test tank was made up using an acrylic glass material in a T-shaped design (50 cm × 10 cm × 10 cm), two short arms had dimensions of 20 cm in length and 10 cm in width and height. In the training period the fishes were placed in the long arm and after 1 minute they were allowed to swim towards the small arm and observed for 4 minutes. The tank was divided into two colored zones-red and green. If the zebrafish entered the red zone, they were disturbed with a glass rod and if they entered the green zone they were given food pellets. The zebrafishes were trained for 3 days and during the test period the same process was followed as during the training period. The number of entries into the red and green zone was visualized using a camera [22].
Histopathological studies of zebrafish brain
The zebrafishes from each group were euthanized by placing them in cold water followed by fixing them in 4% formaldehyde and decalcified using 0.35 M EDTA at pH 8 and embedded in paraffin. The brain tissues were sectioned using a microtome. The brain tissues were deparaffinized and stained with Hematoxylin and Eosin (H&E) stain [23]. The zebrafishes were placed in ice-cold water for 10 minutes and euthanized followed by dissection of the brain tissues from the zebrafishes. The brain tissues were fixed using 4% formaldehyde followed by sectioning using a microtome. These tissue sections were washed with 0.01 molar phosphate buffered solution for 30 minutes at room temperature, followed by the incubation for 8 minutes in dark at room temperature with 0.3% Thioflavin S. The sections were washed in dark after the incubation for three times. The sections were analyzed using fluorescence microscopy using Image-Pro Plus version 5.1 [24].
Brain homogenate preparation
The zebrafishes were placed in ice-cold water for 10 minutes and euthanized followed by dissection of the brain tissues from the zebrafishes. The tissues were homogenized in 2 milliliters of 20 millimolar sodium phosphate buffer with pH 7.4, that contained 140 millimolar KCl. The homogenates were centrifuged for 10 minutes at 4ºC at 750 × g and the cell debris pellet was discarded and the supernatant was transferred in a fresh 1.5 milliliter tube and stored at 4ºC. The homogenate was used for further biochemical parameters [25, 26].
Biochemical parameters assessment
Measurement of superoxide dismutase activity
The activity of superoxide dismutase was determined by Winterbourn et al. protocol [27], where 1.5 mL of each reach mixture contained 75 mM NBT, 200 µL of supernatant, 2 µM riboflavin, 100 mM TRIS/HCL and 6mM EDTA. The absorbance of the blue-colored complex formed was evaluated using a spectrophotometer at 560 nm. SOD units are determined as the amount necessary to reduce the rate of NBT reduction by 50% with enzyme activity reported as units/mg protein.
Measurement of protein carbonyl activity
Brain protein oxidation was determined using the concentration of protein carbonyl groups by modified protocol of Luo et al. [28] and Oliver et al [29]. Approximately 2 mg of protein from the supernatant was divided into two portions, and 10 % TCA was added to precipitate both the aliquots. Equal volume of 2N HCl was added to one sample and another sample was treated with DNPH with 2 N HCl, following this the samples were incubated at 25ºC for 5 minutes and mixed. The protein carbonyl levels were evaluated in terms of nmol/ mg protein.
Measurement of reduced glutathione level
The GSH levels in the brain homogenate was determined using Fukuzawa and Tokumura methods [30]. The reaction mixture contained 130 microliters of 0.04% DTNB, 200 microliters of brain supernatant and 1.1 mL of 0.25 molar sodium phosphate buffer (pH 7.4). To the reaction mixture 1.5 milliliter of distilled water was added and the absorbance was measured spectrophotometrically at 412 nm. The values were expressed as µg GSH/g protein.
Measurement of MDA level
Lipid peroxidation was quantitatively measured according to the method of Ohkawa et al [31]. The assay estimated the concentration of malondialdehyde (MDA), the endproduct of lipid peroxidation, in the brain homogenate. The reaction mixture consisted of 1 mL of 26 mM Thio barbituric acid in 0.1 M hydrochloric acid and 1 mL of 50% trichloroacetic acid in 0.1 M hydrochloric acid. To this mixture, 200 µL of brain homogenate supernatant was added for the reaction. The solution was vortexed and then subjected to incubation at 95ºC for 20 minutes after which the samples were centrifuged at 960 × g for 10 minutes. The resulting supernatant was assessed at 532 nm and the quantified results were reported as nmol/mg of protein.
Measurement of acetylcholinesterase activity
Ellman’s method was used to estimate the acetylcholinesterase activity [32], where the 600 microliter reaction mixture contained 5 millimolar acetylcholine chloride, 1 millimolar DTNB and 0.26 molar phosphate buffer with pH 7.4 [33]. The absorbance was read at 412 nm with the enzyme activity expressed as nmol of ACT/min per/mg of protein.
Statistical analysis
The results obtained were expressed as the mean ± standard error of the mean using GraphPad Prism 9.0 [34].
Results
Behavioral assessment
NTT
The anxiety-related behavior of zebrafish was evaluated using the NTT. In this assay, control fish initially remain at the bottom of the tank when introduced to a new environment due to natural anxiety and gradually explore the upper zones as they acclimate. In contrast, aluminium chloride (AlCl₃)-treated zebrafish exhibited abnormal hyperactivity, spending significantly more time in the top zone (~250 s) and less time at the bottom (~80 s) resulting in an increased top/bottom ratio (~4) compared to the control group (~0.5), indicating loss of normal cautious behavior. Treatment with hecogenin restored typical behavioral responses similar to those observed in the donepezil-treated group, with a reduced top/bottom ratio (~0.5) and balanced exploration. Improvements were evident in parameters such as the top/bottom time ratio, distance travelled in the top zone (~13–20 m), total time spent at the top (~120 s), and number of entries into the top zone (~40–50 entries) compared to the AlCl3-treated group (Figure 1). Collectively these findings indicate that aluminium chloride disrupts normal anxiety related behavior in zebra fish while hecogenin treatment effectively reinstates balanced exploratory behavior closely aligning to the donepezil treated group reflecting its potential neuroprotective role.
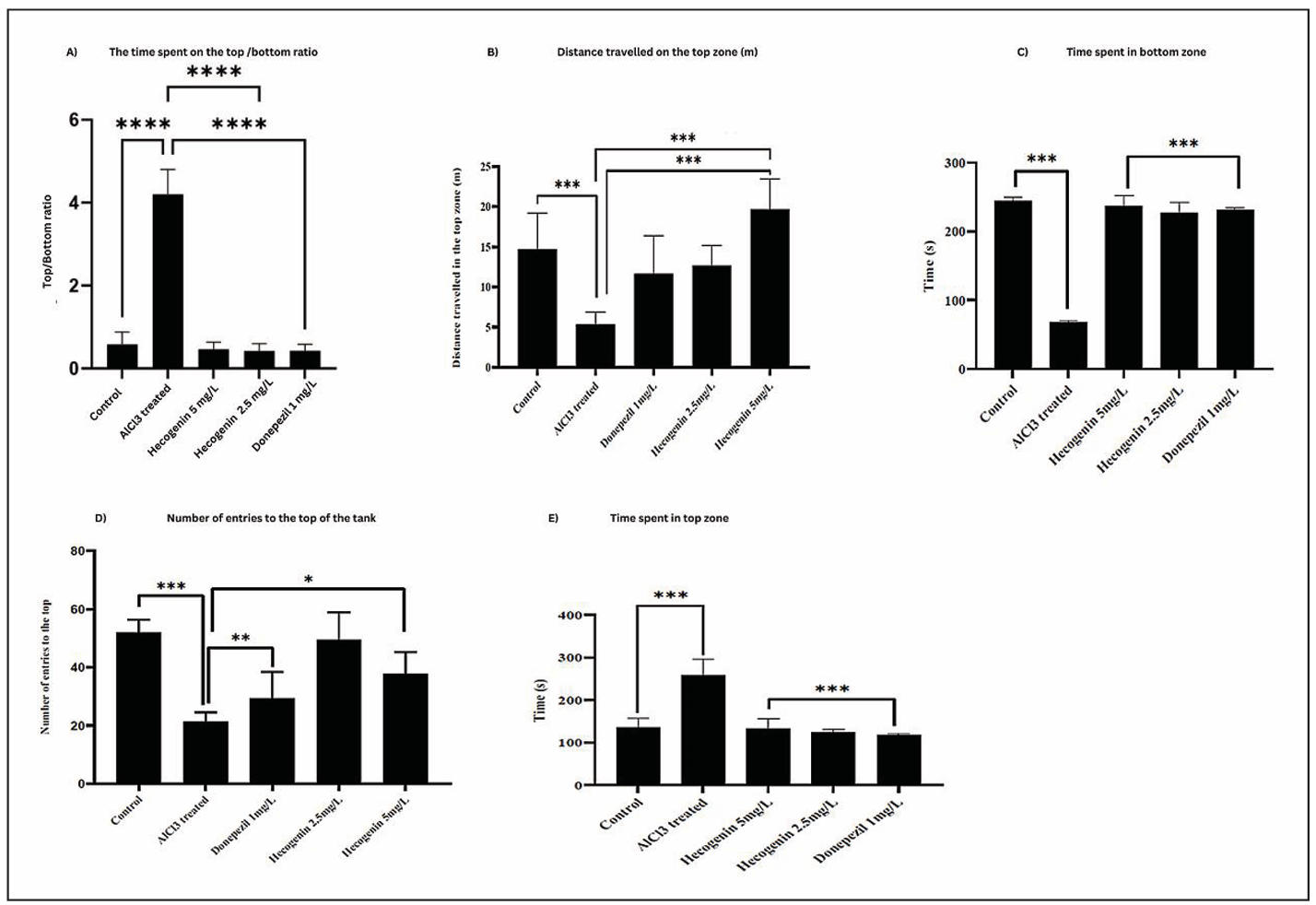
Figure 1. NTT analysis of control, aluminium chloride treated, aluminium chloride + hecogenin (5 and 2.5 mg/L) and aluminium chloride + Donepezil. The effects of Hecogenin (2.5 mg/L and 5 mg/L) and Donepezil (1 mg/L) on anxiety-like behavioral and cognitive performance were evaluated using the novel tank diving test. (A) Time spent in the top/bottom (B) Distance travelled in the top. (C) Time spent in the bottom. (D) Number of entries to the top zone. (E) Time spent at the top.
T-Maze Test
The T-maze test was employed to assess spatial learning and memory in zebrafish across the control, aluminium chloride (AlCl₃)-treated, AlCl₃ + hecogenin (2.5 and 5 mg/L), and AlCl₃ + donepezil groups. The AlCl₃-exposed group demonstrated significant cognitive impairment, characterized by reduced distance travelled in the green (target) zone (~1.4 m), increased time spent in the red (~28 s) and vertical zones (~42 s), and prolonged latency (~226 s) to reach the target arm. In contrast, hecogenin-treated fish showed marked improvement in all these behavioral parameters, spending more time and covering greater distance in the green zone (~4.4 m) while exhibiting shorter latency times (~163 s). These improvements were comparable to those observed with donepezil, treated groups (Figure 2). Overall T-Maze findings indicate that aluminium chloride exposure severely impaired spatial memory and learning ability in zebrafish while hecogenin treatment particularly at 5 mg/L significantly restored cognitive performance showing shorter latency period and greater preference for green zone closely paralleling the improvements observed in donepezil treated groups.
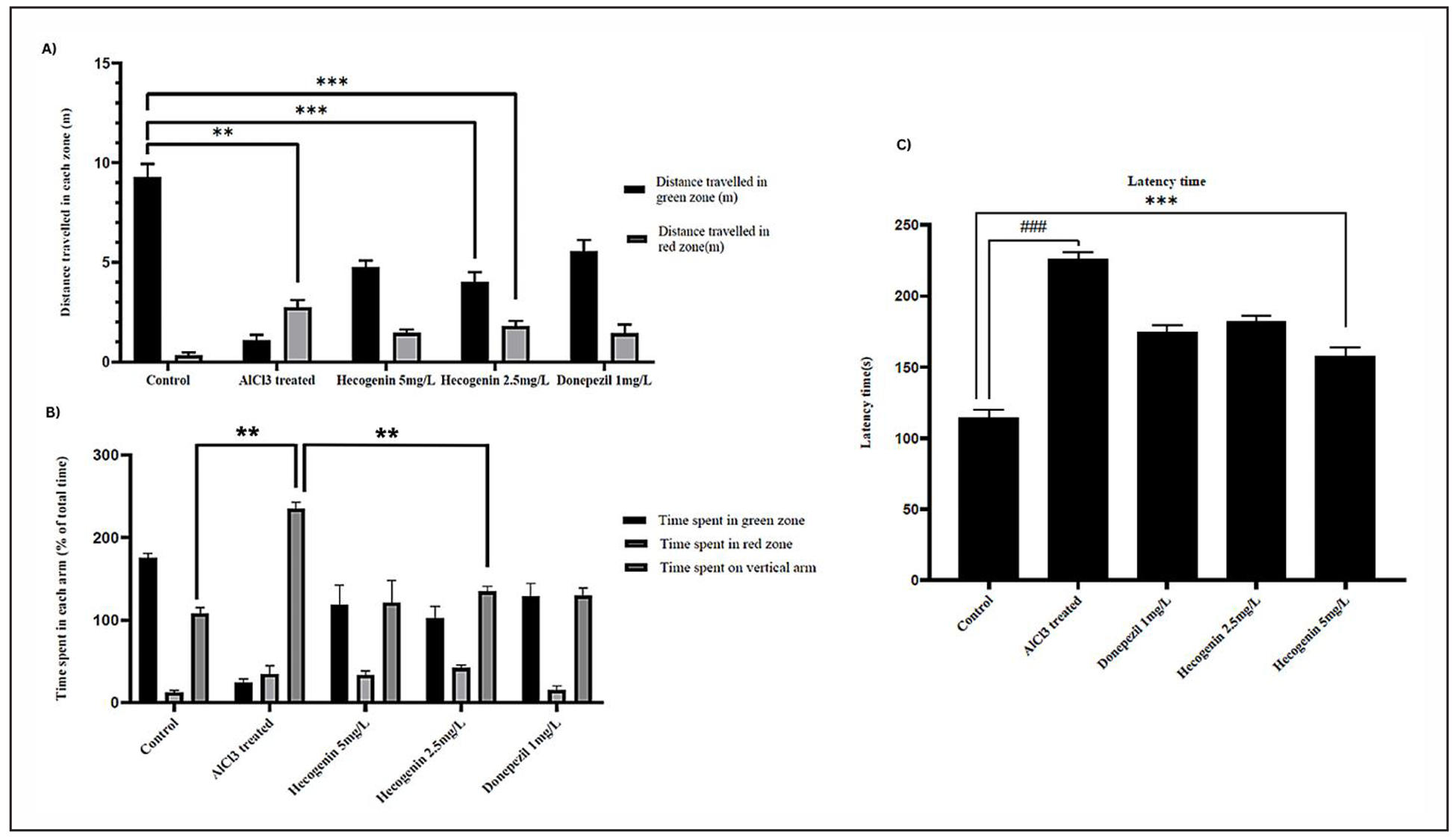
Figure 2. T maze analysis of control, aluminium chloride treated, aluminium chloride + hecogenin (5 and 2.5 mg/L) and aluminium chloride + Donepezil. (A) Distance travelled in green and red zones (m). (B) Time spent in green, red, and vertical zones (% of total time). (C) Latency time (s) across different groups.
Histopathological studies of zebrafish brain
Histopathological analysis of zebrafish brain sections in 20X revealed distinct morphological differences among the groups. The control group showed well-organized neuronal layers with intact architecture, indicating normal brain morphology. The Donepezil-treated group displayed preserved neuronal structure with minimal shrinkage, confirming its neuroprotective effect. The Hecogenin 5 mg/L group exhibited compact neuronal arrangement, showing strong protection comparable to Donepezil. The Hecogenin 2.5 mg/L group showed mild disorganization, suggesting partial, dose-dependent protection. Disorganized cellular layer with a loss of density was observed in the neuronal cells of aluminium chloride induced zebrafishes compared to the control zebrafishes (Figure 3). The overall histopathological analysis showed normal neuronal architecture in control and Donepezil-treated zebrafish brains, while AICL-induced brains exhibited marked neuronal loss and degeneration. Hecogenin-treated groups, especially at 5 mg/L, showed restored neuronal density and tissue integrity, indicating neuroprotective effects.
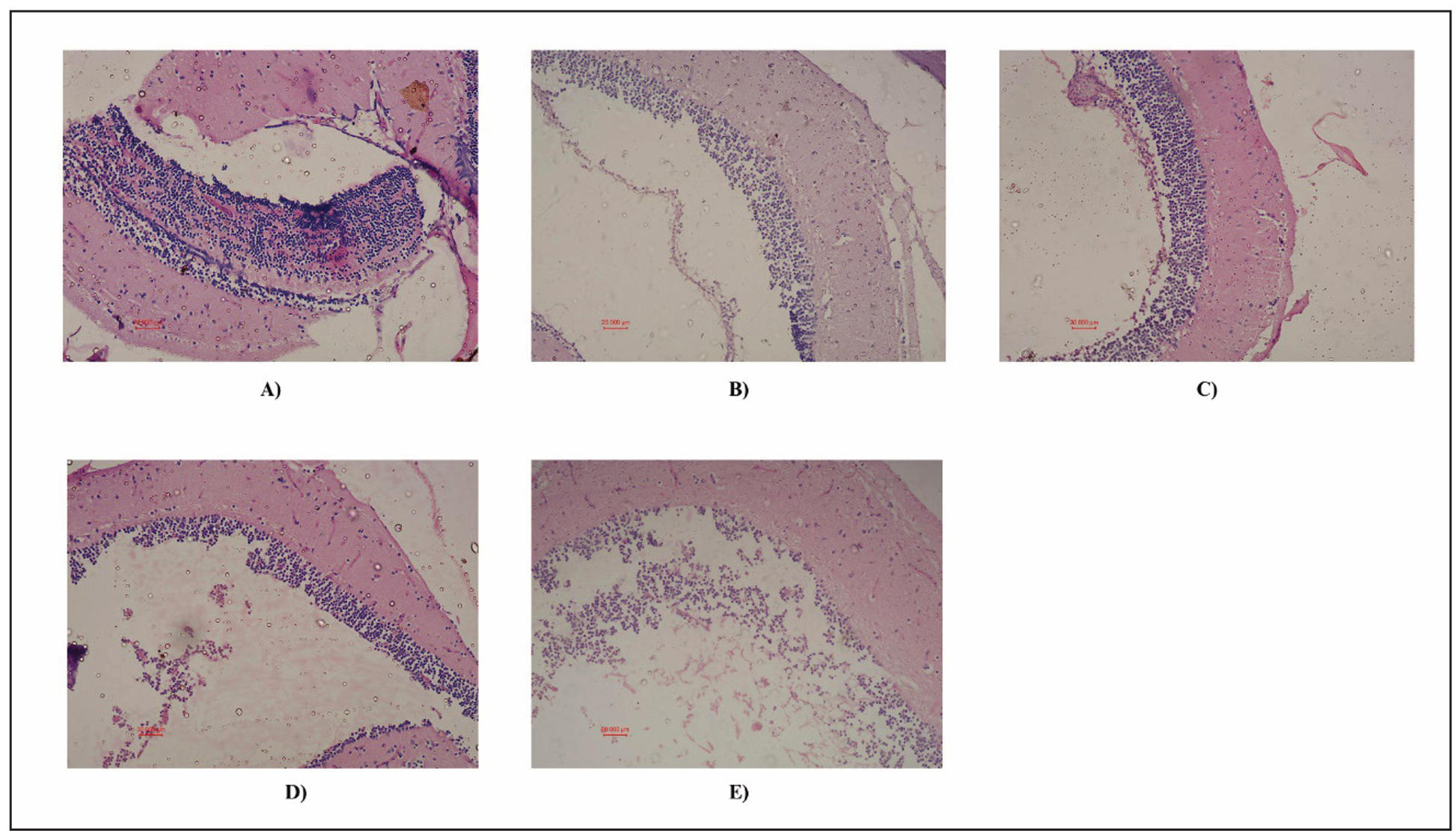
Figure 3. Histopathological analysis of zebrafish brain using H&E staining. Representative brain sections are shown. (A) Control (normal zebrafish brain), (B) Positive control (Donepezil treated), (C) Hecogenin 1a (5 mg/L), (D) Hecogenin 1b (2.5 mg/L), (E) Untreated (AlCl3 induced disease group).
Amyloid-β plaques were absent in the brain tissues of the control zebrafish, significant amyloid-β plaques were found in aluminium chloride induced zebrafish with neurodegenerative changes. Zebrafish treated with donepezil post aluminium chloride exposure showed a marked reduction in the number and size of amyloid-β plaques compared to the aluminium chloride group. Donepezil treatment was associated with less neurodegeneration suggesting a neuroprotective effect against aluminium chloride induced toxicity. Treatment with hecogenin also results in a significant decrease in amyloid-β plaques formation compared to diseased groups. Hecogenin (5 mg/L) treated zebrafishes exhibited improved brain morphology and reduced signs of neurodegeneration, indicating its potential therapeutic efficacy (Figure 4). Overall, these findings demonstrate that aluminium chloride exposure promotes amyloid-β plaque accumulation and neurodegeneration in zebrafish brains, while hecogenin treatment markedly reduces plaque formation and restores neuronal morphology, displaying a neuroprotective efficacy comparable to donepezil.
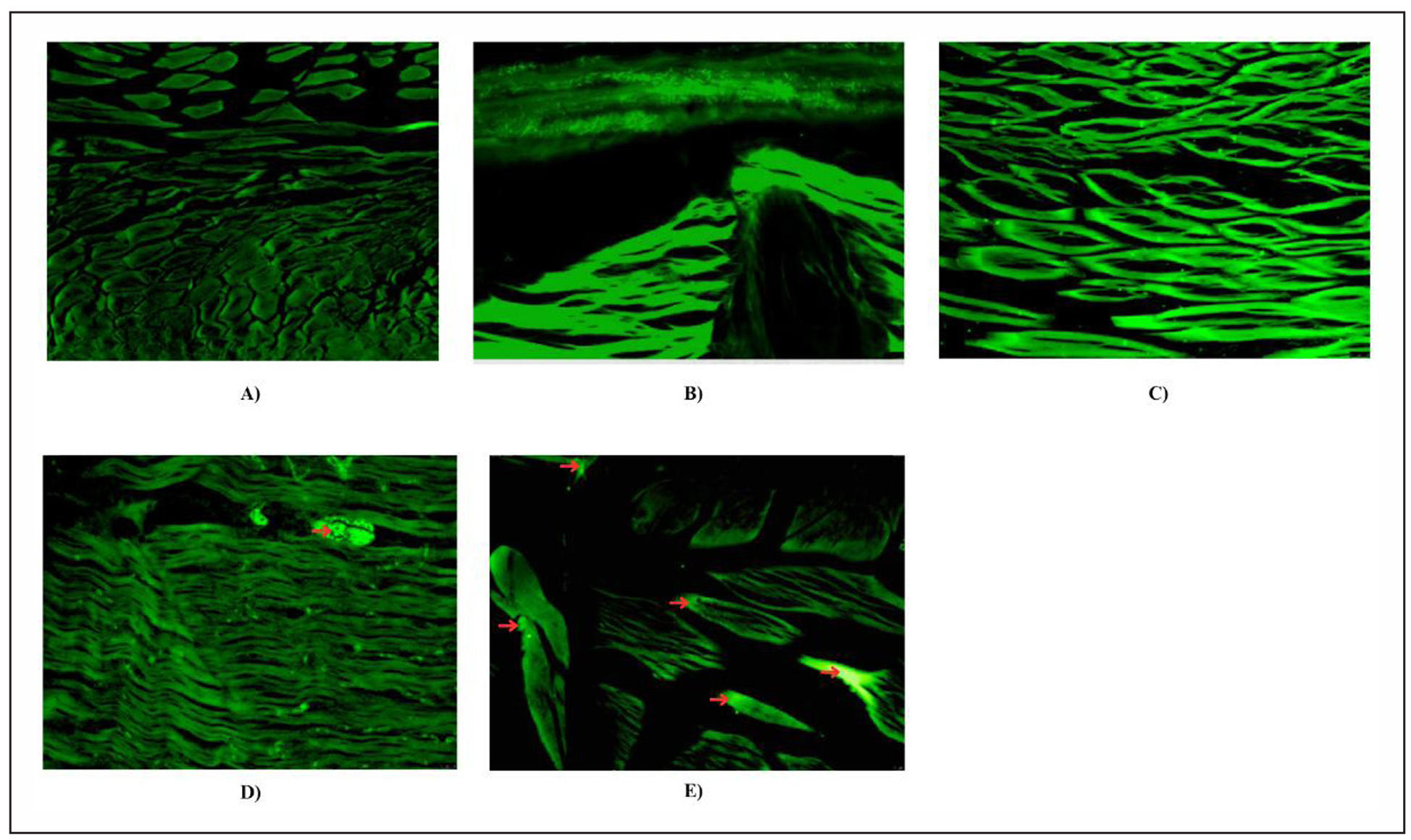
Figure 4. Histopathological analysis of zebrafish brain using Thioflavin S staining. Representative brain sections are shown. (A) Control (normal zebrafish brain), (B) Positive control (Donepezil treated), (C) Hecogenin 1a (5 mg/L), (D) Hecogenin 1b (2.5 mg/L), (E) Untreated (AlCl3 induced disease group). The amyloid-β plaques were marked using red color arrows. The intense green fluorescence corresponding to severe amyloid aggregation and marked tissue disorganization.
Measurement of acetylcholinesterase activity
Administration of aluminium chloride in zebrafish significantly increased the levels of brain acetylcholinesterase in comparison to the control group. Treatment with hecogenin (5 mg/L and 2.5 mg/L) significantly decreased the levels of acetylcholinesterase in the brain as compared to disease induced zebrafish (Figure 5). The result suggest that Hecogenin effectively counteracts the cholinergic dysfunction caused by aluminium chloride, thereby maintaining neurotransmission balance. The reduction in AChE activity indicates a neuroprotective effect comparable to the standard drug Donepezil, emphasizing Hecogenin’s potential as a natural cholinesterase inhibitor.
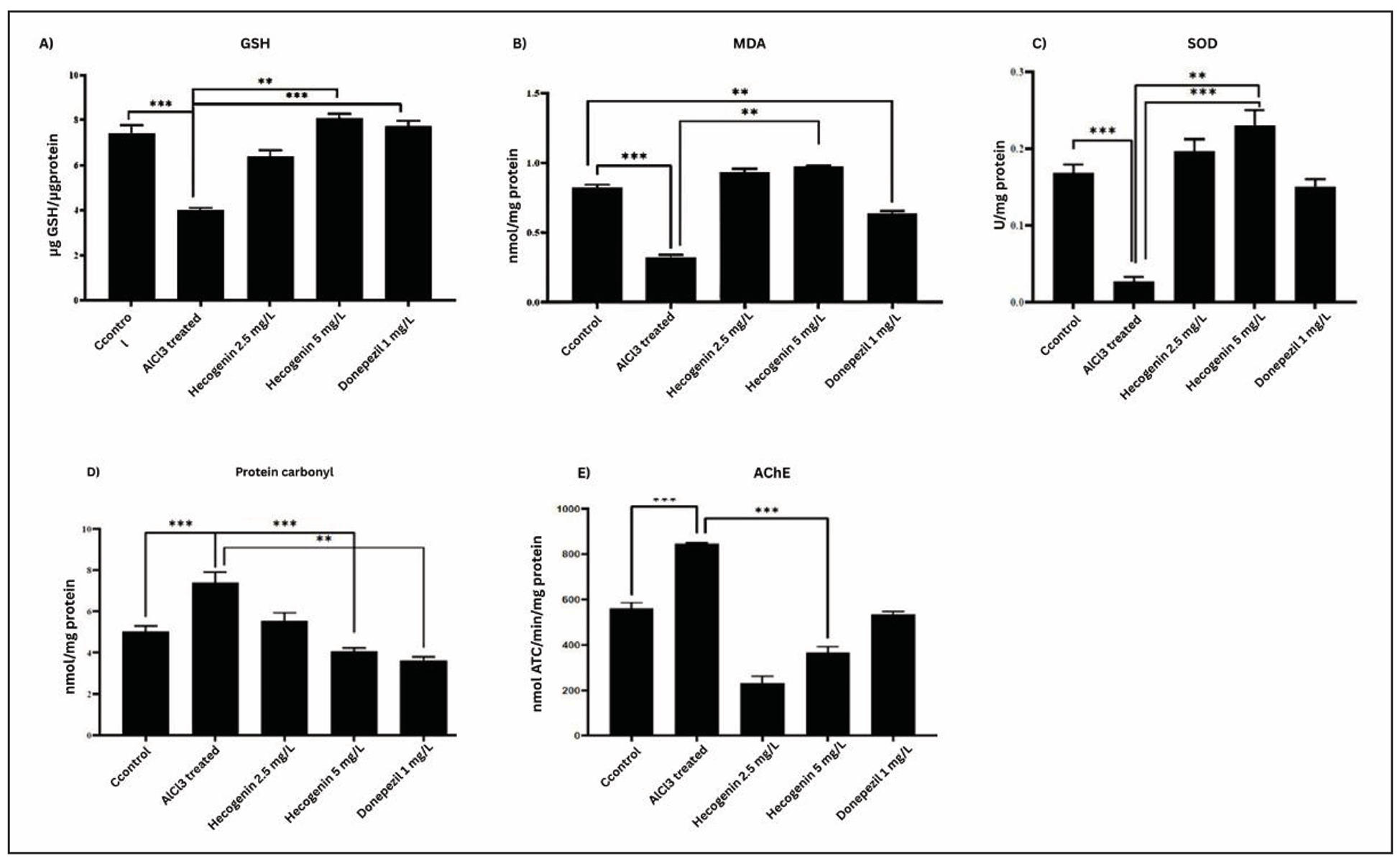
Figure 5. Biochemical assay of zebrafish brain homogenate using GSH (A), MDA (B), SOD (C), Protein carbonyl (D), and AChE (E).
Measurement of MDA level
Increased levels of lipid peroxidation were observed in the brain tissue of aluminium chloride induced zebrafishes compared to that of the control group, which indicates oxidative stress levels. Significant decreases in lipid peroxidation levels were observed in hecogenin (5 mg/L and 2.5 mg/L) treated zebrafishes compared to the diseased group (Figure 5). This reduction in MDA levels reflects Hecogenin’s strong antioxidant potential, suggesting its ability to stabilize neuronal membranes and mitigate aluminium chloride induced oxidative damage, thereby restoring redox balance in the brain.
Measurement of reduced glutathione level
Reduced glutathione is an endogenous antioxidant that reacts with reactive oxygen species to decrease the levels of hydrogen peroxide and lipid peroxidation levels. Significant decrease in the reduction of glutathione was observed in aluminium chloride induced fishes compared to the standard. Elevated levels of reduced glutathione were detected in hecogenin (5 mg/L and 2.5 mg/L) treated zebrafishes compared to the diseased group (Figure 5). This restoration of GSH levels indicates that Hecogenin enhances the antioxidant defense mechanism, effectively counteracting oxidative stress and maintaining cellular redox homeostasis in neuronal tissues.
Measurement of superoxide dismutase activity
Superoxide dismutase catalyzes the oxidation of superoxide ions to hydrogen peroxide and catalase converts the hydrogen peroxide into water. The present study revealed that aluminium chloride significantly decreased the levels of superoxide dismutase activity and treatment with hecogenin (5 mg/L and 2.5 mg/L) increased the levels of superoxide dismutase in zebrafishes compared to the diseased group. Hecogenin treatment was found to be significant when compared to the standard drug (Figure 5). Increase SOD activity shows antioxidant potential of Hecogenin, suggesting its ability to mitigate oxidative damage by improving the enzymatic defense system against reactive oxygen species.
Measurement of protein carbonyl activity
Reduced levels of protein carbonyl and lipid peroxidation was observed in hecogenin (5 mg/L and 2.5 mg/L) treated groups compared to the diseased group. The levels of protein carbonyl were found to be increased in aluminium chloride induced zebrafish due to lipid peroxidation. Donepezil was found to reduce protein carbonyl levels and lipid peroxidation in diseased zebrafish (Figure 5). This reduction in protein carbonyl content following hecogenin treatment indicates its protective role against oxidative protein damage, reflecting improved redox balance and decreased oxidative stress in neural tissues.
Discussion
The learning and memory of zebrafish was evaluated by behavioral testing, which includes, T-maze and colorbiased appetite test. Memory impairments are caused from the accumulation of amyloid plaques, leading to a steady decline in brain function and decreased acetylcholine levels [35]. Hippocampus plays a vital role in learning and remembering information. Damages to the neurons results in difficulties in learning and short-term memory of the brain. T-maze test affects the memory and the learning of zebrafish, where the fishes are sensitive to red and green comparatively to other colors [36]. In the current study it was observed that hecogenin showed higher recognition towards green color compared to the red, like the donepezil treated group. In the aluminium chloride induced group, it was observed that the fishes have least effectiveness in spatial learning and recognition compared to other groups.
Anxiety behaviors can be assessed based on the time spent at the top and latency to reach the top in zebrafish models. Elevated anxiety levels in zebrafish were reflected by an increased latency to enter the upper half of the tank. The fish exhibited prolonged hesitation to explore the upper region, indicating stress or response to external stimuli. Conversely, a decreased latency to explore the upper half signifies reduced anxiety and improved exploratory behavioral [37, 38]. In novel tank diving test analysis of aluminium chloride induced diseased models prolonged latency was observed in the control group. In zebrafishes treated with hecogenin, reduced latency was observed indicating reduction in the anxiety levels of the zebrafish when compared to the diseased group. Similarly in the donepezil induced group, anxiety levels were found to be decreased compared to hecogenin, it was less. It was observed that diseased zebrafish models exhibited a reduced number of entries into the upper half zone, likely due to aversive stimuli and increased perseverance under stress [39].
Oxidative stress significantly contributes to Alzheimer's disease with the central nervous system being particularly susceptible due to its high metabolic activity, abundance of redox-active metals, elevated oxygen demand and the presence of oxidizable lipids [40]. In the brain tissues of Alzheimer’s disease patients, amyloid plaques cause reactive oxygen species (ROS) to be constantly produced. This excessive ROS leads to neuronal damage which is one of the pathogenesis in Alzheimer's disease [41]. In our study, hecogenin effectively restored the antioxidant defense system by reducing oxidative stress, as evidenced by improvements in biomarkers including protein carbonyl content, superoxide dismutase (SOD), malondialdehyde (MDA), reduced glutathione (GSH), and acetylcholinesterase (AChE) activity. Similarly compounds such as safranal [42] and linalool from aromatic plants revealed that these biomarkers were restored to normal on its treatment after the disease induction [43].
The histopathological analysis of zebrafish treated with hecogenin, aluminium chloride, donepezil and control sample elucidates distinct effects and potential therapeutic implications of these compounds. The control samples showed no noticeable alterations in brain tissues, whereas hecogenin-treated diseased fish exhibited marked anti-inflammatory and anti-apoptotic effects. This was evidenced by the reduction in inflammatory markers, indicating the potential of hecogenin as a protective agent against cellular stress and inflammation. In aluminium chloride induced zebrafish models neurotoxic and hepatotoxic effects were visualized using H&E and thioflavin staining. Histopathological examination of aluminium chloride treated zebrafish brains revealed neuronal disorganization and reduced cellular density, consistent with neurodegenerative changes. Treatment with donepezil also exhibited notable neuroprotective properties highlighting its potential efficacy in mitigating aluminium chloride induced neurotoxicity which acts as a potential cholinesterase inhibitor by enhancing cholinergic transmission in the neuronal cells.
Conclusions
The study provides significant evidence supporting the potential anti-Alzheimer’s effects of hecogenin in aluminium chloride induced zebrafish models (Figure 6). The study highlighted the pharmacological application of hecogenin in aluminium chloride induced memory dysfunction by reducing the neurological damage, cholinergic deficits and oxidative stress markers. Histopathological examinations revealed that hecogenin treatment attenuated neuronal damage and preserved the structural integrity in the zebrafish brain. Biochemically, hecogenin significantly reduced oxidative stress markers and improved cholinergic function. Further studies can be focused on elucidating the molecular mechanism pathways through which hecogenin exerts its neuroprotective effects and exploring its efficacy and clinical trials.
Figure 6. Graphical abstract.
Declarations
Author Contributions
Senthilkumar Palanisamy conceived and designed the study and supervised data analysis. Anith Kumar Rajendran oversaw the experiments and drafted the manuscript. Shametha Francis Xavier, Sanjaai K. M, and Deepthi Padmanabhan performed the experiments and contributed to manuscript preparation. All authors contributed the manuscript at various stages.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Not applicable.
Ethical approval and consent to participate
Not applicable.
Consent for Publication
Not applicable.
References
1. Davies P, & Maloney A. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet, 1976, 2(8000): 1403-1415. [Crossref]
2. Chen Z, Huang J, Yang S, & Hong F. Role of cholinergic signaling in Alzheimer's disease. Molecules, 2022, 27(6): 1816-1826. [Crossref]
3. Francis P, Palmer A, Snape M, & Wilcock G. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry, 1999, 66(2): 137-147. [Crossref]
4. Bramblett G, Goedert M, Jakes R, Merrick S, Trojanowski J, & Lee V. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron, 1993, 10(6): 1089-1099. [Crossref]
5. Masters C, Simms G, Weinman N, Multhaup G, McDonald B, & Beyreuther K. Amyloid plaque core protein in Alzheimer disease and down syndrome. Proc Natl Acad Sci USA, 1985, 82(12): 4245-4249. [Crossref]
6. Chen Y, & Yu Y. Tau and neuroinflammation in Alzheimer's disease: interplay mechanisms and clinical translation. J Neuroinflammation, 2023, 20(1): 165-174. [Crossref]
7. Ciechanover A, & Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron, 2003, 40(2): 427-446. [Crossref]
8. Nixon R, Wegiel J, Kumar A, Yu W, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol, 2005, 64(2): 113-122. [Crossref]
9. Saleem S, & Kannan R. Zebrafish: an emerging real-time model system to study Alzheimer's disease and neurospecific drug discovery. Cell Death Discov, 2018, 4: 45-58. [Crossref]
10. Bordoloi S, Pathak K, Devi M, Saikia R, Das J, Kashyap V, et al. Some promising medicinal plants used in Alzheimer’s disease: an ethnopharmacological perspective. Discover Applied Sciences, 2024, 6(5): 215-229. [Crossref]
11. Corbiere C, Liagre B, Bianchi A, Bordji K, Dauça M, Netter P, et al. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int J Oncol, 2003, 22(4): 899-905.
12. Ingawale D, & Patel S. Hecogenin exhibits anti-arthritic activity in rats through suppression of pro-inflammatory cytokines in complete Freund's adjuvant-induced arthritis. Immunopharmacol Immunotoxicol, 2018, 40(1): 59-71. [Crossref]
13. Santos Cerqueira G, dos Santos e Silva G, Rios Vasconcelos E, Fragoso de Freitas AP, Arcanjo Moura B, Silveira Macedo D, et al. Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur J Pharmacol, 2012, 683(1-3): 260-269. [Crossref]
14. Zhang J, Xu Z, Cao Y, Chen H, Yan L, An M, et al. Antifungal activities and action mechanisms of compounds from Tribulus terrestris L. J Ethnopharmacol, 2006, 103(1): 76-84. [Crossref]
15. Cruz M, Barroso S, Navoni J, Teles M, Barbosa-Filho J, de Oliveira Rocha H, et al. Effect of Hecogenin on DNA instability. Toxicol Rep, 2016, 3: 539-543. [Crossref]
16. Fernández-Herrera M, López-Muñoz H, HernándezVázquez J, Sánchez-Sánchez L, Escobar-Sánchez M, Pinto B, et al. Synthesis and selective anticancer activity of steroidal glycoconjugates. Eur J Med Chem, 2012, 54: 721-727. [Crossref]
17. Padmanabhan D, Natarajan P, & Palanisamy S. Integrated metabolite and transcriptome profiling-mediated gene mining of Sida cordifolia reveals medicinally important genes. Genes, 2022, 13(10): 1909-1921. [Crossref]
18. Capatina L, Boiangiu R, Dumitru G, Napoli E, Ruberto G, Hritcu L, et al. Rosmarinus officinalis essential oil improves scopolamine-induced neurobehavioral changes via restoration of cholinergic function and brain antioxidant status in zebrafish (Danio rerio). Antioxidants, 2020, 9(1): 62-75. [Crossref]
19. Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung K, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc, 2010, 5(11): 1786-1799. [Crossref]
20. Maddula K, Kumar V, & Anusha J. Assessment of aqueous extract of ocimum sanctum leaves in memory enhancement and preventing memory impairmentactivities in zebra fish model. J basic clinic pharmacy, 2017, 8: 185-192.
21. Amoah V, Atawuchugi P, Jibira Y, Tandoh A, Ossei P, Sam G, et al. Lantana camara leaf extract ameliorates memory deficit and the neuroinflammation associated with scopolamine-induced Alzheimer's-like cognitive impairment in zebrafish and mice. Pharm Biol, 2023, 61(1): 825-838. [Crossref]
22. Pusceddu M, Hernandez-Baixauli J, Puiggrós F, Arola L, Caimari A, Del Bas J, et al. Mediterranean natural extracts improved cognitive behavior in zebrafish and healthy rats and ameliorated lps-induced cognitive impairment in a sex dependent manner. Behav Brain Funct, 2022, 18(1): 5-17. [Crossref]
23. Ellis J, & Yin C. Histological analyses of acute alcoholic liver injury in zebrafish. J Vis Exp, 2017, (123): 55630. [Crossref]
24. Chao F, Jiang L, Zhang Y, Zhou C, Zhang L, Tang J, et al. Stereological investigation of the effects of treadmill running exercise on the hippocampal neurons in middleaged APP/PS1 transgenic mice. J Alzheimers Dis, 2018, 63(2): 689-703. [Crossref]
25. Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, & Lissi E. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys, 2001, 388(2): 261-266. [Crossref]
26. Agostini J, Toé H, Vieira K, Baldin S, Costa N, Cruz C, et al. Cholinergic system and oxidative stress changes in the brain of a zebrafish model chronically exposed to ethanol. Neurotox Res, 2018, 33(4): 749-758. [Crossref]
27. Winterbourn C, Hawkins R, Brian M, & Carrell R. The estimation of red cell superoxide dismutase activity. J Lab Clin Med, 1975, 85(2): 337-341.
28. Luo S, & Wehr N. Protein carbonylation: avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep, 2009, 14(4): 159-166. [Crossref]
29. Oliver C, Ahn B, Moerman E, Goldstein S, & Stadtman E. Age-related changes in oxidized proteins. J Biol Chem, 1987, 262(12): 5488-5491.
30. Fukuzawa K, & Tokumura A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J Nutr Sci Vitaminol, 1976, 22(5): 405-407. [Crossref]
31. Ohkawa H, Ohishi N, & Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 1979, 95(2): 351-358. [Crossref]
32. Worek F, Eyer P, & Thiermann H. Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal, 2012, 4(3-4): 282-291. [Crossref]
33. Ellman G, Courtney K, Andres V, Jr., & Feather-Stone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol, 1961, 7: 88-95. [Crossref]
34. Swift M. GraphPad Prism, data analysis, and scientific graphing. Journal of Chemical Information and Computer Sciences, 1997, 37(2): 411-412. [Crossref]
35. Kim Y, Lee K, Park A, & Min T. Adding preferred color to a conventional reward method improves the memory of zebrafish in the T-maze behavior model. Animal Cells and Systems, 2017, 21(6): 374-381. [Crossref]
36. Perathoner S, Cordero-Maldonado M, & Crawford A. Potential of zebrafish as a model for exploring the role of the amygdala in emotional memory and motivational behavior. J Neurosci Res, 2016, 94(6): 445-462. [Crossref]
37. Kysil E, Meshalkina D, Frick E, Echevarria D, Rosemberg D, Maximino C, et al. Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish, 2017, 14(3): 197-208. [Crossref]
38. Egan R, Bergner C, Hart P, Cachat J, Canavello P, Elegante M, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res, 2009, 205(1): 38-44. [Crossref]
39. Raduan S, Ahmed Q, Kasmuri A, Rusmili M, Sulaiman W, Shaikh M, et al. Neurotoxicity of aluminium chloride and okadaic acid in zebrafish: insights into Alzheimer's disease models through anxiety and locomotion testing, and acute toxicity assessment with Litsea garciae bark's methanolic extract. Journal of King Saud UniversityScience, 2023, 35(7): 102807. [Crossref]
40. Wang J, Eslinger P, Doty R, Zimmerman E, Grunfeld R, Sun X, et al. Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res, 2010, 1357: 184-194. [Crossref]
41. Ozben T, & Ozben S. Neuro-inflammation and antiinflammatory treatment options for Alzheimer's disease. Clin Biochem, 2019, 72: 87-89. [Crossref]
42. Baluchnejadmojarad T, Mohamadi-Zarch S, & RoghaniM. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β-induced rat model of Alzheimer's disease: underlying mechanisms. Metab Brain Dis, 2019, 34(6): 1747-1759. [Crossref]
43. Yuan C, Shin M, Park Y, Choi B, Jang S, Lim C, et al. Linalool alleviates Aβ42-induced neurodegeneration via suppressing ROS production and inflammation in fly and rat models of Alzheimer's disease. Oxid Med Cell Longev, 2021, 2021: 8887716. [Crossref]