Open Access | Commentary
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Endotoxin-induced acute lung injury in old C57BL/6J mice is a translationally relevant geroscience model
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA, USA.
Email: wladiges@uw.edu
Received: 14 March 2025 / Accepted: 17 March 2025 / Published: 28 March 2025
DOI: 10.31491/APT.2025.03.170
Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are clinically important inflammatory lung conditions that contribute to substantial morbidity and mortality in humans, notably in the elderly population. Direct lung infection is a common cause of ALI and can be modeled by experimental administration of lipopolysaccharide (LPS) bacterial endotoxin. Acute pulmonary pathology observations are presented using an intratracheal LPS-induced ALI experimental model in aged C57BL/6J mice. Collectively the histopathologic findings in LPS treated mice demonstrate several key characteristic features of ALI, supporting the translational relevance of this model for investigating the pathogenesis of lung injury in older adults.
Keywords
Acute lung injury, bacterial endotoxin, LPS, aging, mouse model, lung pathology, C57BL/6J, greroscience
The geroscience approach to aging assumes that all
diseases that affect primarily older adults have a
common and major underlying cause of declining
function and resilience that is part of the aging
process. This has been established for chronic diseases,
but is now a reality for acute infections since increasing
age is associated with decreased resilience to pathologic
effects of infectious disease agents. Acute lung
injury (ALI) and acute respiratory distress syndrome (ARDS) are
considered to occur along a continuum, where initial lung injury
results from direct (e.g. pneumonia, aspiration of acid) or
indirect (e.g. pancreatitis, sepsis) conditions, and ARDS represents a
manifestation of severe ALI [1]. The incidence of severe ALI appears to be
increasing, especially with the recent pandemic of COVID-19, with an
increasing mortality rate.
Lipopolysaccharide (LPS) bacterial endotoxin exposure is one of
several approaches to induce experimental ALI [2]. LPS can be
administered to the lungs either intranasally or intratracheally
resulting in direct lung injury. In support of the use of LPS for
modeling human ALI, it has been shown that marmosets, a small
nonhuman primate species, develop ALI in response to aerosol
administration of LPS [3]. The underlying pathophysiology of
ALI/ARDS involves injury to both the alveolar epithelium and
vascular endothelium. Histologically, characteristic features
that may be present in various degrees of acute disease include
neutrophilic alveolitis, deposition of hyaline membranes,
vascular congestion, hemorrhage and formation of microthrombi [4].
An issue in many of the LPS animal model studies is that younger
animals are used [5], thus raising the concern of whether observations
and conclusions are translationally relevant. Therefore, more studies
need to be done in older aged animals to validate whether the pulmonary
pathology simulates the pathology seen in the lungs of older patients,
especially since older individuals experience greater disease severity.
To address this gap, we conducted a preliminary study in male and
female C57BL/6J mice, 24 months of age, obtained from the National
Institute on Aging Aged Rodent Colony and housed in a specific pathogen
free facility at the University of Washington. Mice were acclimated for
three weeks, then given intratracheal LPS (E. coli 055:B5; Sigma) at a
concentration of 800 µg in 50 µL of saline, or 50 µL of saline (control).
After 48 hours, mice were euthanized by cervical dislocation, and lungs
perfused with 10% neutral buffered formalin, fixed for 48 hours, followed
by processing into paraffin blocks and staining of slides with hematoxylin
and eosin. Slides were read by a board-certified veterinary pathologist (J Klug).
Mice given LPS developed moderate to severe lung pathology, characterized by a
predominance of intra-alveolar inflammatory cell infiltrates composed of
neutrophils and lesser mononuclear cells, as well as interstitial inflammation,
vascular congestion and hemorrhage as shown by representative images in Figure 1.
Our preliminary observations in old (24 months) C57BL/6 mice are similar to that
reported in younger (18 months) C57BL/6 mice [6]. Therefore, intratracheal
instillation of LPS into the lungs of old mice induces characteristic
pulmonary pathology features of ALI including neutrophilic alveolitis,
vascular congestion, and hemorrhage. The advantage of using old C57BL/6
mice is that they have significant comorbidities, thus providing a more
translationally relevant model to study pathology of acute injury conditions
of the lungs seen in older people. Further geroscience studies are warranted
to explore age-related differences in disease progression, immune responses,
and potential therapeutic interventions.
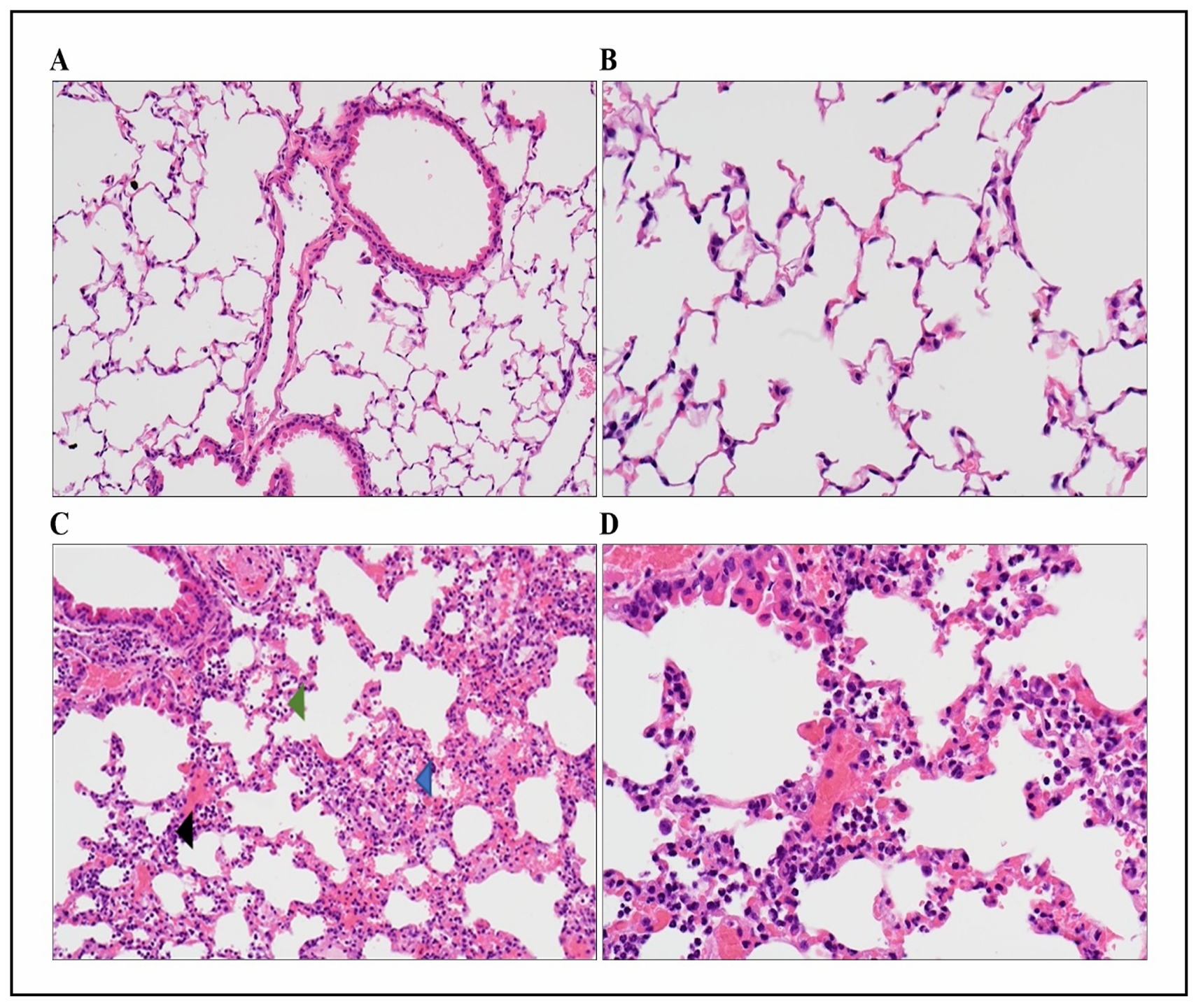
Figure 1. Representative images of H&Estained lung sections from saline (A, B) and LPS (C, D) treated mice. The mice treated with LPS (C, D) show inflammation within alveoli (green arrow), interstitium (black arrow), as well as hemorrhage (blue arrow). Magnification-200x (A and C), 400x (B and D).
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Warren Ladiges is a member of the editorial board of Aging Pathobiology and Therapeutics. The authors declare that they have no conflicts and were not involved in the journal’s review or decision regarding this manuscript.
Acknowledgements
Supported by National Institute on Aging R01 grants AG057381 and AG067193 (Ladiges, PI).
References
1. Mokrá D. Acute lung injury - from pathophysiology to treatment. Physiol Res, 2020, 69(Suppl 3): s353-s366. [Crossref]
2. Chen H, Bai C, & Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med, 2010, 4(6): 773-783. [Crossref]
3. Seehase S, Lauenstein HD, Schlumbohm C, Switalla S, Neuhaus V, Förster C, et al. LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing. PLoS One, 2012, 7(8): e43709. [Crossref]
4. D'Alessio FR. Mouse models of acute lung Injury and ARDS. Methods Mol Biol, 2018, 1809: 341-350. [Crossref]
5. Guo Z, Li Q, Han Y, Liang Y, Xu Z, & Ren T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators Inflamm, 2012, 2012: 540794. [Crossref]
6. Kling KM, Lopez-Rodriguez E, Pfarrer C, Mühlfeld C, & Brandenberger C. Aging exacerbates acute lung injury-induced changes of the air-blood barrier, lung function, and inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol, 2017, 312(1): 1-12. [Crossref]