Open Access | Model
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Catilage organoid model of age-related ostoarthritis to study intervention strategies
#These authors contributed equally to this work.
* Corresponding author: Zhiqiang Shi
Mailing address: Department of Emergency Surgery, The Second Affiliated Hospital of Inner Mongolia Medical University,
Hohhot 010010, Inner Mongolia, People's Republic of China.
Email: 727842992@qq.com
* Corresponding author: Yongxiong He
Mailing address: Department of Orthopedic Surgery, Beijing Tuberculosis & Thoracic Tumor Research Institute,
Beijing Chest Hospital affiliated to Capital Medical University, No. 9 Beiguan street, Tongzhou District,
Beijing 101149, People's Republic of China.
Email: spinedoctor@sina.com
Received: 03 March 2025 / Revised: 21 March 2025 / Accepted: 16 April 2025 / Published: 27 June 2025
DOI: 10.31491/APT.2025.06.178
Abstract
Aging is an inevitable biological process in nature. As aging progresses, physical functions continue to decline, eventually leading to aging-related diseases. It is urgent to find effective ways to prevent and treat aging-related diseases. The use of organoids to study the development of aging-related diseases is based on the fact that they retain the physiological structure and functional characteristics of their origin, compensating for the fact that the complexity of human aging-related diseases cannot be reproduced in animals, cells, yeast, and Caenorhabditis elegans. Osteoarthritis (OA) is a common aging-related musculoskeletal disease, and chondrocyte senescence is one of the major risk factors for OA. Therefore, it is necessary to construct cartilage organoids to model OA. This brief review describes the cell source, culture environment, and intervention methods of cartilage organoids, providing insights for future drug screening and treatment of OA.
Keywords
Aging, osteoarthritis, cartilage organoid
It is widely accepted that aging is an inevitable and normal
physiological phenomenon [1].
Aging generally begins after sexual maturity, and there is a steady
decline in bodily functions as we age, eventually leading to the
onset of a variety of diseases [2].
It is now generally accepted that aging is one of the major risk factors
for the development of aging-related diseases [3].
Nowadays, the global economic burden of aging-related diseases is
increasing due to increasing longevity and aging population, and it is
urgent to develop effective prevention and treatment strategies
[1]. Researchers have mainly established
models of aging-related diseases in animals, cells, yeast, and Caenorhabditis
elegans for their studies. However, it is unclear how well these models reflect
the complexity of human aging [4].
Organoids are multicellular clusters formed by in vitro 3D culture of stem
cells that mimic the physiology and cellular composition of real tissues or
organs [4, 5]. Organoids retain the structural and functional properties of
their origin and are capable of self-renewal and self-organization
[5]. Genetic and pharmacological
manipulations in organoids, which reflect the complex cellular environment
of human biology, provide the opportunity to study organ development and the
early stages of disease pathogenesis. In many aspects of preclinical drug
development, animal and in vitro models are complemented by organoids
(Table 1) [4]. For example,
patient-derived tumor organoids retain the genetic and phenotypic heterogeneity
in the original tumor, which predicts patient response to different drugs and
provides a basis for personalized treatment [6].
Based on the differences between mouse and human brains, brain organoids were used
to mimic brain diseases such as Alzheimer's disease, glioblastoma, Parkinson's
disease, primary microcephaly [7].
Therefore, organoids may be a new promising tool to model changes in the aging
process and to study the onset of aging-related diseases.
Table 1.
Advantages of organoids.
| Organoids | Advantages | References |
|---|---|---|
| Intestinal organoid | To assess drug absorption | [46] |
| Brain organoids | To address the differences between human and mouse brains | [47] |
| Non‐small cell lung cancer organoids | For drug screening biomarker identification | [48] |
| Heart organoids | To assess the toxic effects of drugs on the heart | [49] |
| Liver organoids | To model liver regeneration, to study the toxic effects of drugs on the liver, and to address the shortage of liver donor transplants | [50, 51] |
| Skin organoids | To study the mechanism of human skin regeneration. | [52] |
The Global Burden of Disease in 2017 (GBD 2017) study found that aging-related diseases accounted for 92 out of 293 (31.4%), including neurodegenerative diseases, cancer, cardiovascular diseases, immune system diseases, metabolic diseases, musculoskeletal disorders, etc [8, 9]. The elderly are more susceptible to injuries and degenerative musculoskeletal disorders than the young [9]. Osteoarthritis (OA), which affects more than 80% of people over the age of 65, causes disability in the elderly and is costly to patients and society [10, 11]. Chondrocyte loss and aging contribute to the development of OA [11]. Therefore, it is necessary to generate cartilage organoids to establish OA disease models [12]. This paper reviews the cell sources, culture environments, and management of disease-mimicking cartilage organoids to provide insights for future drug screening and treatment of OA.
Construction of OA
The cell sources, culture environments, and intervention approaches used to construct cartilage organoids are important factors in simulating the disease state of OA.
Cell sources
Organoids are usually established from stem cells or patient-derived induced pluripotent stem cells (iPSCs) [13]. Healthy human chondrocytes can only be obtained under special conditions according to the principle of no harm. For example, cartilage debris from patients can be used as a cellular source for cartilage organoids only after obtaining informed consent from the amputee patient and official ethical approval [14]. Since it is difficult to obtain healthy chondrocytes from human samples, stem cell induction is commonly used to construct cartilage organoids, most commonly using mesenchymal stem cells (MSCs) and iPSCs [14]. Various techniques and methods are available to induce differentiation of MSCs into chondrocytes, adipocytes, and osteoblasts [15, 16], but only iPSCs can differentiate into endothelial cells, osteoclasts, and other immune components. In addition to conventional stem cells, embryonic stem cells (ESCs), pluripotent cell lines, human periosteum-derived cells (hPDCs), and autologous digestive chondrocytes can also be used to construct cartilage organoids (Table 2) [12].
Table 2.
Cell source of cartilage organoids.
| Cell resources | methods | Reference |
|---|---|---|
| MSCs | 0.1 μM dexamethasone, 40 μg/mL L-proline, ITS, 50 μg/mL ascorbic acid 2-phosphate and 10 ng/mL TGF-β3 | [18] |
| iPSCs | 1% L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 1% ITS-X, 50 µg/mL ascorbic acid, 0.1 mM 2-mercaptoethanol, 10 ng/mL human basic fibroblast growth factor, 10 ng/mL TGF-β1, 10 ng/mL bone morphogenetic protein-2 (BMP-2), 10 ng/mL growth differentiation factor 5 (GDF5) | [53] |
| ESCs | 0.1 µM LDN193189, 10 µM SB431542, 3 µM CHIR99021, 10 µM ROCK inhibitor Y-27632, 20 ng/mL brainderived neurotrophic factor, 100 ng/mL fibroblast growth factor 8 (FGF8), 20 ng/mL Shh, 20 ng/mL epidermal growth factor (EGF) | [54] |
| hPDCs | 0.1 μM ascorbate-2 phosphate, 0.1 μM dexamethasone, 40 µg/mL proline, 20 μM ROCK inhibitor Y-27632, ITS+, 100 ng/mL BMP-2, 100 ng/mL GDF5, 10 ng/mL TGF-β1, 1 ng/mL BMP-6, and 0.2 ng/mL basic FGF2 | [55] |
| MPCs | MPCs 0.1 μM dexamethasone, 40 μg/mL L-proline, 10 μg/mL ITS, 50 μg/mL ascorbic acid and 10 ng/mL TGF-β1/TGF-β3 | [17] |
| iMPCs | [19] |
Additionally, progenitor cells were also used to construct cartilage organoids. For example, mesenchymal progenitor cells (MPCs) were cultured to form cartilage organoids by 10 ng/mL transforming growth factor β1 (TGF-β1)/TGF-β3 promoted differentiation in a special serum-free medium containing 1% penicillin-streptomycin, 0.1 μM dexamethasone, 40 μg/mL L-proline, 10 μg/mL ITS pre-mix (insulin, transferrin, selenium, bovine serum albumin and linoleic acid), 50 μg/mL ascorbic acid [17, 18]. MSCs are well-established chondrogenic progenitor cells [14], and iPSC-derived mesenchymal progenitor cells (iMPCs), MSC-like progenitor cells induced by iPSCs [19], can also be used as cells for constructing cartilage organoids.
The culture environment
Stem cells are induced to proliferate, differentiate, and form
organoids in specialized culture environments as required for the
growth and development of tissues and organs. The complex network
structure of the extracellular matrix (ECM), synthesized inside
the cell and secreted outside, could not be formed without its
polysaccharide and protein components, including collagen, elastin,
laminin, fibronectin, glycosaminoglycans, and other macromolecules [20].
In addition, cell adhesion, proliferation, spreading, differentiation,
binding, tissue structure stability, and physiological activities of
cells depend on growth factors, cytokines, and chemokines released from
the ECM [21]. Therefore, stimulating the properties of ECM in biological
tissues is an essential strategy to achieve a better culture environment
for organoids. Several ECM-derived materials, such as Matrigel and hydrogel,
have been used for organoid culture. For example, silk fibroin-based hydrogel
was used to construct cartilage organoids to detect biological and physical
signals [22]. Type I collagen hydrogel was used to construct cartilage
organoids without growth factors [23]. The porcine and human kidney
dECM-derived hydrogels promote the formation of an endogenous vascular
within the time course of kidney organoid differentiation [24]. Growth
factors also play an important role in promoting the proliferation and
differentiation of stem cells into organoids [12]. The common growth
factors are EGF, FGF, and TGF-β. Liver organoids requires EGF, hepatocyte
growth factor, and FGF receptors [25]. EGF and bFGF promote adipose-derived
stem cells differentiated into mature neural cells rather than osteocyte
lineage cells [26]. TGF-β is a potent inducer of stem cell differentiation
to form cartilage organoids, smooth muscle cells, immature cardiomyocytes [27].
Thus, growth factors contribute to the differentiation of stem cells to form
different organoids.
Currently, scaffold-based and scaffold-free are the two main methods for
cartilage organoid culture. scaffold-based is the self-assembly of biomaterials
with multiple holes into multilayered organoid structures along specific spatiotemporal
directions to support cell adhesion and proliferation [12]. Matrix gels and hydrogel
materials are used not only as cell culture media to provide special conditions for
organoids, but also as temporary scaffolds to maintain the stability of organoid
structures [28]. The scaffold-based culture method can help cells proliferate and
differentiate to form organoids more effectively. For example, a 3D culture system
by using collagen gel matrix system increased germ cell viability, meiosis, and
post-meiotic differentiation into presumptively differentiated spermatocytes [29];
whereas a culture system by single-cell suspensions blocked the meiosis in
spermatogenesis and inhibited spermatogenesis [30]. Scaffold-based pancreatic cancer
models reflect in vivo drug efficacy better than scaffold-free pancreatic
cancer models [31]. In scaffold-free culture, organoids are grown by placing cells
in suspension culture or by growing spherical scaffolds [32]. The advantages of
scaffold-free culture include simplicity, reproducibility, massive cell proliferation,
and rapid organoid formation [12]. A study showed that the scaffold-free pancreatic
cancer organoid model formed a mature circular structure after culture for 24 h,
while the scaffold-based model showed stable formation and high viability after
culture for 7 days [31]. A comparison of scaffold-free and scaffold-based
reconstructed human skin models showed that the scaffold-free skin model has
a higher self-renewal capacity than the scaffold-based skin model cells [33].
Figure 1 shows the establishment of
cartilage organoids.
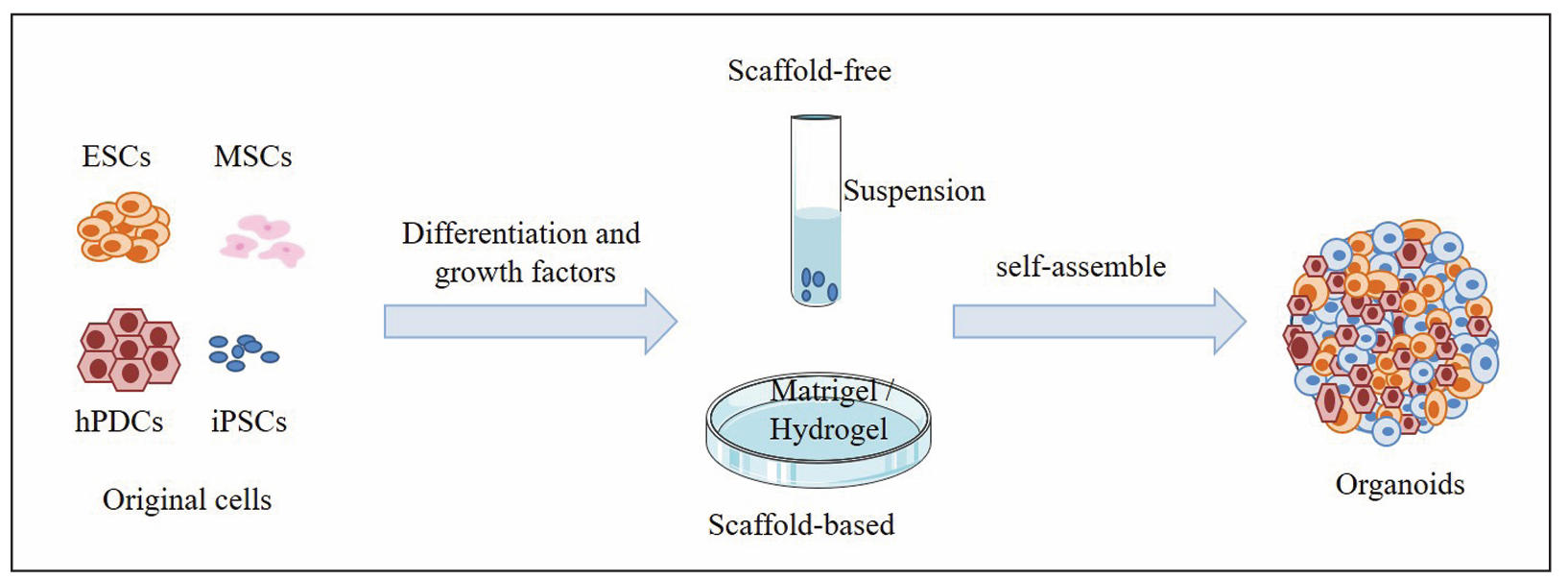
Figure 1. Construction of cartilage organoids.
Simulation of OA
The organoids of full-thickness cartilage tissue removed from smooth-appearing portions of the tibial plateau and femoral condyles of OA patients were smaller in diameter than the ND (non-degenerate) group, but both groups were abundant in proteoglycans and collagen and did not differ in macroscopic appearance in alginate gels [34]. This suggests that OA chondrocytes may have similar characteristics to healthy chondrocytes under the same culture conditions. Therefore, it is necessary to establish additional conditions for the intervention of organoids to better mimic OA. Currently, there are four methods to simulate OA in organoids: cytokines, mechanical stress, microenvironment and genetic modeling.
Mechanical loading
Hyperphysiologic mechanical loading is one of the important factors in the development and progression of OA [13, 35]. Niek G. C. Bloks et al. mechanically loaded spherical neocartilage constructs using a MACH-1 mechanical testing device (Biomomentum, Laval, Canada) with parameters set to a loading rate of 5 Hz, a sinusoidal peak strain of 20%, and a duration of 10 min (Figure 2). For the cylindrical constructs, a customized loading device was used with the same loading parameters. The mechanically loaded cartilage organoids were placed in TGF-β3-free CD medium to prevent their anabolic responses from being perturbed by mechanical loading [35]. The results showed changes in the expression of genes such as CD44, ITGA5, and CAV1, which are associated with anatomical morphogenesis and wound healing response. This confirms that supra-physiological mechanical loading leads to detrimental changes in the phenotypic state of chondrocytes, resulting in OA disease. Therefore, mechanical loading may trigger the onset of OA.
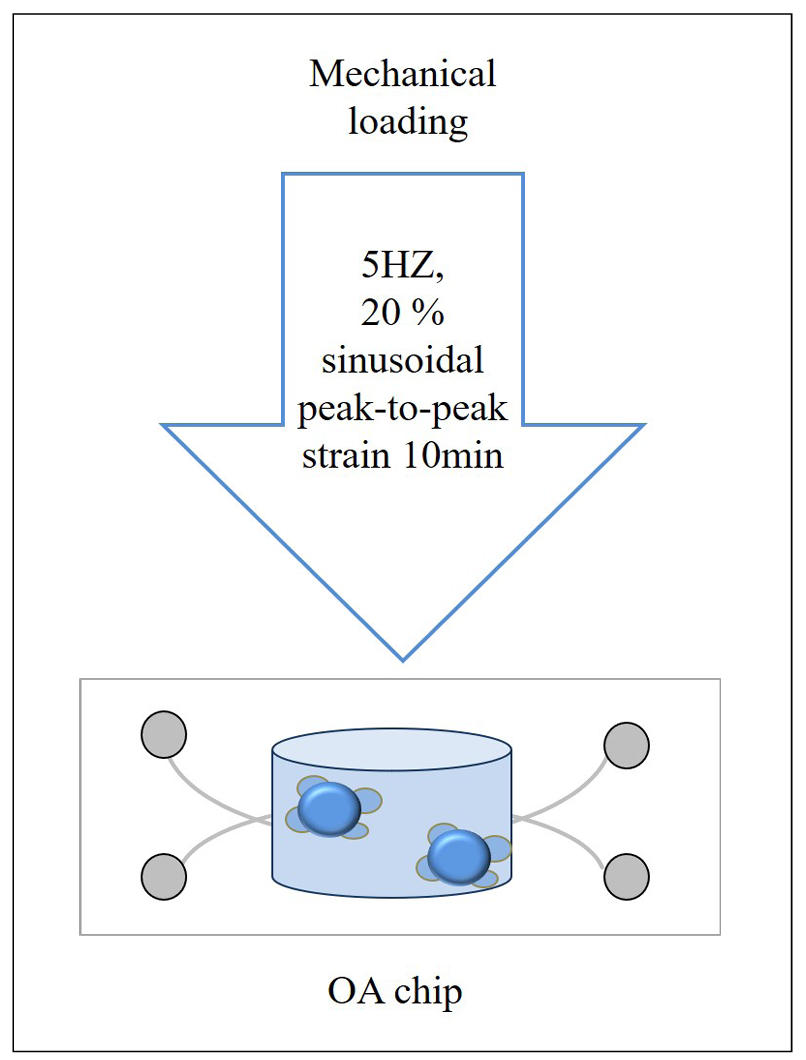
Figure 2. The generation of OA by mechanical loading.
Cytokines
Cytokines are the most commonly used induction modality because they are inexpensive and easy to customize and manipulate. Cytokines play a critical role in the pathogenesis of OA by mediating joint interface remodeling and altering joint hemostasis through chronic inflammation [35]. Diana M. Abraham et al. [36] treated organoids with recombinant IL-1β (5 ng/mL) for 24 h to establish a skeletal inflammation model. It was found that only IL-1β reduced the size of arthrocyte spheroids in a dose-dependent manner [37]. Laura Donges et al. [38] constructed OA cartilage organoids to which low concentrations of pro-inflammatory cytokine mixtures (50 pg/mL TNF-α, 50 pg/mL IL-1β, and 100 pg/mL IL-6, INFL) were added during the hypertrophic culture phase. Figure 3 shows the induction of a cartilage organoid OA disease model with pro-inflammatory cytokines.
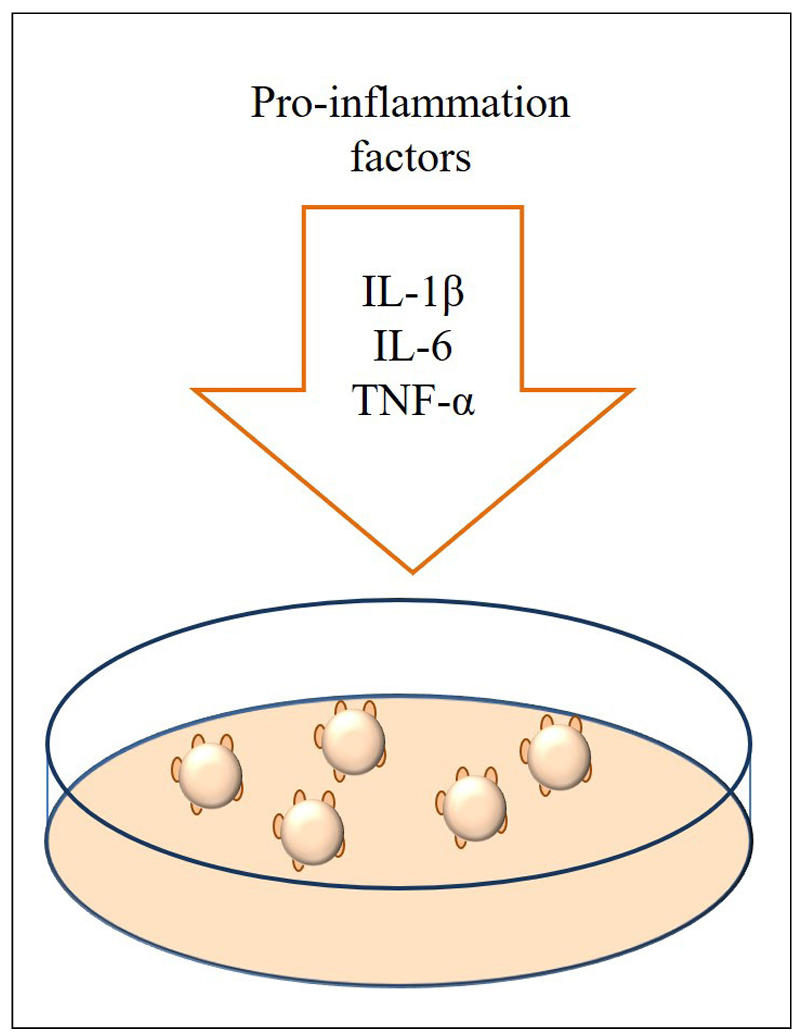
Figure 3. The production of OA by pro-inflammatory cytokines.
Microenvironment
The disadvantage of cartilage monotissue is the inability to reproduce native multi-tissue interactions. However, the reproduction of essential features of OA can be created in bioreactors and microfluidic devices for long-term culture [13]. Researchers have attempted to create a miniature joint system (miniJoint) with osteochondral, adipose, and fibrous analogues for the study of OA [39]. They first created the miniJoint. Adipose tissue (AT), synovial-like fibrous tissue (SFT), and osteochondral units (OC) were added to the miniJoint chamber, and adipogenic medium, fibrogenic medium, and osteogenic medium were used to maintain different tissue phenotypes. In particular, a universal medium (UM) simulated synovial fluid, which flowed to the bottoms of the different tissues and allowed tissue interactions. After 28 days of tissue co-culture, synovial-like fibrous tissues were treated with IL-1β (10 ng/mL) to induce synovial inflammation, and ascorbic acid-2-phosphate (pVC), L-proline (Pro), and TGF-β3 were excluded from the UM. After 3 days, a combined treatment of oligodeoxynucleotides and bone morphogenic protein-7 was added to all media to simulate “systemic” and “local” administration. Finally, after 4 days of therapeutic intervention, various methods were used to evaluate the therapeutic effect (Figure 4). The study demonstrated the ability of miniJoint for the development of disease-modifying drugs for OA [49].
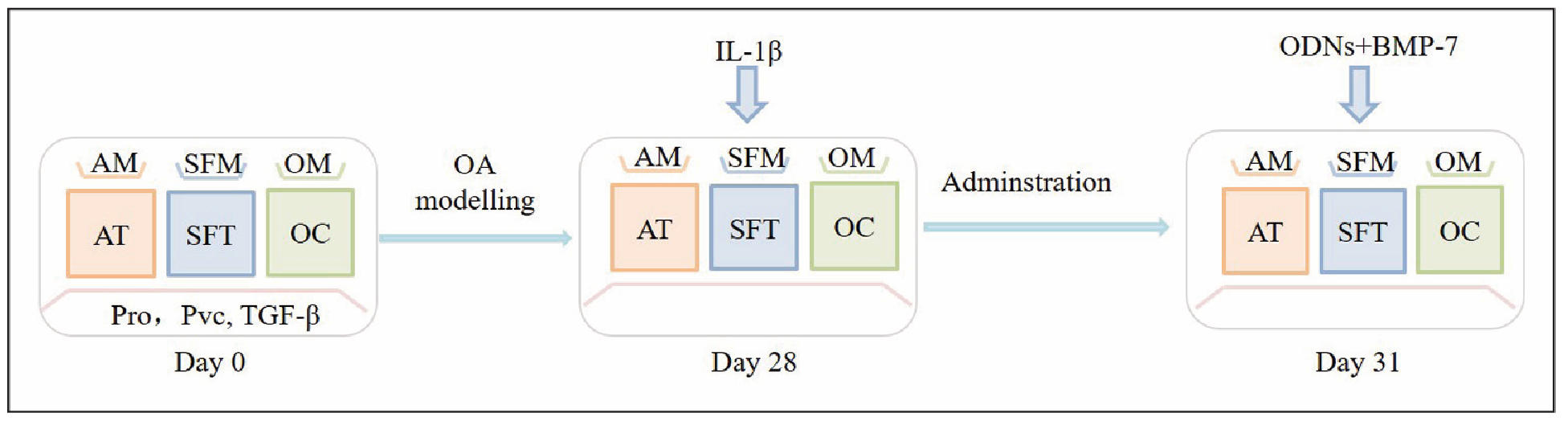
Figure 4. The experimental procedure of creating OA model in the miniJoint.
Genetic modeling
In addition, gene editing techniques such as CRISPR-Cas9 can be used
to introduce gene mutations associated with OA into cartilage organoids
to mimic OA [40]. For example, Niek GC Bloks et al. used
CRISPR-Cas9 establish collagen type VI alpha 3 chain (COL6A3) mutant
hiPSCs cells and then COL6A3 mutant hiPSCs cells were differentiated
to form cartilage organoids to model OA [41].
Furthermore, growth factors (e.g., hypoxia-inducible
factor 2α (HIF-2α)) and chemically induced enzymes (e.g., ECM
catabolic enzymes) can also induce OA. Significantly elevated of HIF-2α
in human and mouse OA chondrocytes enhanced Fas expression and caspase
activity, which promotes chondrocyte apoptosis and OA [42]. Monosodium
iodoacetate induces chondrocyte death resembling the pathological
changes of human OA [43]. But they have only been applied in 2D models
and therefore require further development for application.
The applications of cartilage organoids in OA
There are many OA models available for drug screening and OA
related studies. Vincent P Willard et al. [44] treated
iPSCs-derived cartilage organoids with IL-1α to mimic OA for drug
screening, and found that the nuclear factor kappa-B inhibitor SC-514
was the most effective candidate to protect cartilage organoids,
which effectively reduced cartilage loss, matrix metalloproteinases
production, nitric oxide production, and prostaglandin E2 production.
The cartilage organoids treated with IL-1β were used to test the
protective effect of the adenosine 2a receptor agonist against OA [36].
Implantation of cartilage organoids in the body contributes to cartilage
defect repair and treat OA [45].
Since animal and cellular models cannot reflect the complex mechanisms
of human aging, organoids are used to study aging-related diseases. For OA,
one of the most common diseases of aging, the construction of cartilage
organoid OA models is essential. In this paper, we summarized the cell
sources, culture environments, and intervention methods of cartilage
organoids, which facilitates the development of cartilage organoids
for modeling OA and the development of new methods for treating OA.
Declarations
Acknowledgments
None.
Financial support and sponsorship
None.
Conflicts of interest
The author declares that there are no conflicts.
References
1. Chen S, Gan D, Lin S, Zhong Y, Chen M, Zou X, et al. Metformin in aging and aging-related diseases: clinical applications and relevant mechanisms. Theranostics, 2022, 12(6): 2722-2740. [Crossref]
2. Melzer D, Pilling LC, & Ferrucci L. The genetics of human ageing. Nat Rev Genet, 2020, 21(2): 88-101. [Crossref]
3. Luo J, Mills K, le Cessie S, Noordam R, & van Heemst D. Ageing, age-related diseases and oxidative stress: what to do next? Ageing res rev, 2020, 57: 100982. [Crossref]
4. Torrens-Mas M, Perelló-Reus C, Navas-Enamorado C, Ibargüen-González L, Sanchez-Polo A, Segura-Sampedro JJ, et al. Organoids: an emerging tool to study aging signature across human tissues. Modeling aging with patient-derived organoids. Int J Mol Sci, 2021, 22(19): 10547. [Crossref]
5. Yang H, Sun L, Liu M, & Mao Y. Patient-derived organoids: a promising model for personalized cancer treatment. Gastroenterol Rep (Oxf), 2018, 6(4): 243-245. [Crossref]
6. Gómez-Álvarez M, Agustina-Hernández M, Francés-Herrero E, Rodríguez-Eguren A, Bueno-Fernandez C, & Cervelló I. Addressing key questions in organoid models: who, where, how, and why? Int J Mol Sci, 2023, 24(21): 16014. [Crossref]
7. Yao Q, Cheng S, Pan Q, Yu J, Cao G, Li L, et al. Organoids: development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm (2020), 2024, 5(10): e735. [Crossref]
8. Lublóy Á. Medical crowdfunding in a healthcare system with universal coverage: an exploratory study. BMC Public Health, 2020, 20(1): 1672-1682. [Crossref]
9. Li Z, Zhang Z, Ren Y, Wang Y, Fang J, Yue H, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology, 2021, 22(2): 165-187. [Crossref]
10. Barbour KE, Helmick CG, Boring M, & Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation - United States, 2013-2015. MMWR Morb Mortal Wkly Rep, 2017, 66(9): 246-253. [Crossref]
11. Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther, 2022, 7(1): 391-404. [Crossref]
12. Zeng D, Chen Y, Liao Z, Wei G, Huang X, Liang R, et al. Cartilage organoids and osteoarthritis research: a narrative review. Front Bioeng Biotechnol, 2023, 11: 1278692. [Crossref]
13. Singh YP, Moses JC, Bhardwaj N, & Mandal BB. Overcoming the dependence on animal models for osteoarthritis therapeutics - the promises and prospects of in vitro models. Adv Healthc Mater, 2021, 10(20): e2100961. [Crossref]
14. Hu Y, Zhang H, Wang S, Cao L, Zhou F, Jing Y, et al. Bone/cartilage organoid on-chip: construction strategy and application. Bioact Mater, 2023, 25: 29-41. [Crossref]
15. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, & Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med, 2019, 4: 22-33. [Crossref]
16. Augello A, & De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther, 2010, 21(10): 1226-1238. [Crossref]
17. Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, et al. Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells, 2003, 21(6): 681-693. [Crossref]
18. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, & Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng, 1998, 4(4): 415-428. [Crossref]
19. Lin Z, Li Z, Li EN, Li X, Del Duke CJ, Shen H, et al. osteochondral tissue chip derived from iPSCs: modeling OA pathologies and testing drugs. Front Bioeng Biotechnol, 2019, 7: 411-421. [Crossref]
20. Frantz C, Stewart KM, & Weaver VM. The extracellular matrix at a glance. J Cell Sci, 2010, 123(Pt 24): 4195-4200. [Crossref]
21. Bonnans C, Chou J, & Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol, 2014, 15(12): 786-801. [Crossref]
22. Shen C, Zhou Z, Li R, Yang S, Zhou D, Zhou F, et al. Silk fibroin-based hydrogels for cartilage organoids in osteoarthritis treatment. Theranostics, 2025, 15(2): 560-584. [Crossref]
23. Zhang L, Yuan T, Guo L, & Zhang X. An in vitro study of collagen hydrogel to induce the chondrogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A, 2012, 100(10): 2717-2725. [Crossref]
24. Garreta E, Moya-Rull D, Marco A, Amato G, Ullate-Agote A, Tarantino C, et al. Natural hydrogels support kidney organoid generation and promote in vitro angiogenesis. Adv Mater, 2024, 36(34): e2400306. [Crossref]
25. Nuciforo S, & Heim MH. Organoids to model liver disease. JHEP Rep, 2021, 3(1): 100198. [Crossref]
26. Hu F, Wang X, Liang G, Lv L, Zhu Y, Sun B, et al. Effects of epidermal growth factor and basic fibroblast growth factor on the proliferation and osteogenic and neural differentiation of adipose-derived stem cells. Cell Reprogram, 2013, 15(3): 224-232. [Crossref]
27. Wang MK, Sun HQ, Xiang YC, Jiang F, Su YP, & Zou ZM. Different roles of TGF-β in the multi-lineage differentiation of stem cells. World J Stem Cells, 2012, 4(5): 28-34. [Crossref]
28. Velasco V, Shariati SA, & Esfandyarpour R. Microtechnology-based methods for organoid models. Microsyst Nanoeng, 2020, 6: 76-88. [Crossref]
29. Lee JH, Kim HJ, Kim H, Lee SJ, & Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials, 2006, 27(14): 2845-2853. [Crossref]
30. Yokonishi T, Sato T, Katagiri K, Komeya M, Kubota Y, & Ogawa T. In vitro reconstruction of mouse seminiferous tubules supporting germ cell differentiation. Biol Reprod, 2013, 89(1): 15-25. [Crossref]
31. Xie D, Jia S, Ping D, Wang D, & Cao L. Scaffold-based three-dimensional cell model of pancreatic cancer is more suitable than scaffold-free three-dimensional cell model of pancreatic cancer for drug discovery. Cytotechnology, 2022, 74(6): 657-667. [Crossref]
32. Gunti S, Hoke ATK, Vu KP, & London NR, Jr. Organoid and spheroid tumor models: techniques and applications. Cancers (Basel), 2021, 13(4): 874-885. [Crossref]
33. Kinikoglu B. A comparison of scaffold-free and scaffold-based reconstructed human skin models as alternatives to animal use. Altern Lab Anim, 2017, 45(6): 309-316. [Crossref]
34. Kleuskens MWA, Crispim JF, van Doeselaar M, van Donkelaar CC, Janssen RPA, & Ito K. Neo-cartilage formation using human nondegenerate versus osteoarthritic chondrocyte-derived cartilage organoids in a viscoelastic hydrogel. J Orthop Res, 2023, 41(9): 1902-1915. [Crossref]
35. Bloks NGC, Dicks A, Harissa Z, Nelissen R, Hajmousa G, Ramos YFM, et al. Hyper-physiologic mechanical cues, as an osteoarthritis disease-relevant environmental perturbation, cause a critical shift in set points of methylation at transcriptionally active CpG sites in neo-cartilage organoids. Clin Epigenetics, 2024, 16(1): 64-85. [Crossref]
36. Abraham DM, Herman C, Witek L, Cronstein BN, Flores RL, & Coelho PG. Self-assembling human skeletal organoids for disease modeling and drug testing. J Biomed Mater Res B Appl Biomater, 2022, 110(4): 871-884. [Crossref]
37. Negishi Y, Adili A, de Vega S, Momoeda M, Kaneko H, Cilek MZ, et al. Il-6 reduces spheroid sizes of osteophytic cells derived from osteoarthritis knee joint via induction of apoptosis. Am J Pathol, 2024, 194(1): 135-149. [Crossref]
38. Dönges L, Damle A, Mainardi A, Bock T, Schönenberger M, Martin I, et al. Engineered human osteoarthritic cartilage organoids. Biomaterials, 2024, 308: 122549. [Crossref]
39. Makarczyk MJ, Hines S, Yagi H, Li ZA, Aguglia AM, Zbikowski J, et al. Using microphysiological system for the development of treatments for joint inflammation and associated cartilage loss-a pilot study. Biomolecules, 2023, 13(2): 384-495. [Crossref]
40. Dai K, & Wang J. Human cartilage organoids and beyond. Biomater Transl, 2024, 5(4): 447-450. [Crossref]
41. Bloks NG, Harissa Z, Adkar SS, Dicks AR, Hajmousa G, Steward N, et al. A high-impact COL6A3 mutation alters the response of chondrocytes in neo-cartilage organoids to hyper-physiologic mechanical loading. bioRxiv, 2023: 520461. [Crossref]
42. Ryu JH, Shin Y, Huh YH, Yang S, Chun CH, & Chun JS. Hypoxia-inducible factor-2α regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ, 2012, 19(3): 440-450. [Crossref]
43. Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, et al. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res, 2013, 31(3): 364-369. [Crossref]
44. Willard VP, Diekman BO, Sanchez-Adams J, Christoforou N, Leong KW, & Guilak F. Use of cartilage derived from murine induced pluripotent stem cells for osteoarthritis drug screening. Arthritis Rheumatol, 2014, 66(11): 3062-3072. [Crossref]
45. Kleuskens MWA, Crispim JF, van Donkelaar CC, Janssen RPA, & Ito K. Evaluating initial integration of cell-based chondrogenic constructs in human osteochondral explants. Tissue Eng Part C Methods, 2022, 28(1): 34-44. [Crossref]
46. Tanaka K, Mochizuki T, Baba S, Kawai S, Nakano K, Tachibana T, et al. Robust and reproducible human intestinal organoid-derived monolayer model for analyzing drug absorption. Sci Rep, 2025, 15(1): 11403. [Crossref]
47. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature, 2013, 501(7467): 373-379. [Crossref]
48. Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero M, et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res, 2020, 26(5): 1162-1174. [Crossref]
49. Hofbauer P, Jahnel SM, Papai N, Giesshammer M, Deyett A, Schmidt C, et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell, 2021, 184(12): 3299-3317.e3222. [Crossref]
50. Prior N, Inacio P, & Huch M. Liver organoids: from basic research to therapeutic applications. Gut, 2019, 68(12): 2228-2237. [Crossref]
51. Tadokoro T, Murata S, Kato M, Ueno Y, Tsuchida T, Okumura A, et al. Human iPSC-liver organoid transplantation reduces fibrosis through immunomodulation. Sci Transl Med, 2024, 16(757): eadg0338. [Crossref]
52. Lee J, & Koehler KR. Skin organoids: a new human model for developmental and translational research. Exp Dermatol, 2021, 30(4): 613-620. [Crossref]
53. Tam WL, Freitas Mendes L, Chen X, Lesage R, Van Hoven I, Leysen E, et al. Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Research & Therapy, 2021, 12(1): 513-523. [Crossref]
54. Foltz L, Avabhrath N, Lanchy JM, Levy T, Possemato A, Ariss M, et al. Craniofacial chondrogenesis in organoids from human stem cell-derived neural crest cells. iScience, 2024, 27(4): 109585. [Crossref]
55. Nilsson Hall G, Mendes LF, Gklava C, Geris L, Luyten FP, & Papantoniou I. Developmentally engineered callus organoid bioassemblies exhibit predictive in vivo long bone healing. Adv Sci (Weinh), 2020, 7(2): 1902295. [Crossref]