Open Access | RESEARCH
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Myrmecodia platytyrea tuber aqueous extract exacerbates neuroinflammation
* Corresponding author: Mizaton Hazizul Hasan
Mailing address: Group of Affinity, Safety, and Efficacy Studies
(OASES), Faculty of Pharmacy, Universiti Teknologi MARA
(UiTM) Cawangan Selangor, Bandar Puncak Alam, Selangor,
42300, Malaysia.
Email: mizaton_hazizul@uitm.edu.my
Received: 03 December 2024/ Revised: 23 December 2024 / Accepted: 10 January 2025 / Published: 28 March 2025
DOI: 10.31491/APT.2025.03.168
Abstract
Background: Myrmecodia platytyrea, a member of Rubiaceae, has been traditionally used to treat inflammation-related diseases, including cancer and rheumatoid arthritis. However, its potential in managing neurodegenerative disorders remains unexplored. This study aimed to investigate the neuroprotective effect of M.platytyrea tuber aqueous extract (MPAE).
Methods: The cytotoxicity effect on astrocytes was assessed using the MTT assay. The effect of MPAE (0.025–0.5 mg/mL) on reactive oxygen species (ROS) and pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) were evaluated in Fe2SO4-, H2O2-, and LPS-stimulated astrocyte cell lines.
Results: MPAE was found to be non-cytotoxic to astrocytes, with no significant protective effect against Fe2SO2-, H2O2-, or LPS-induced stress. Instead, MPAE increased astrocyte cell death, as well as ROS and cytokine levels, in a dose-dependent manner.
Conclusion: Although MPAE is non-cytotoxic to astrocytes, its potential to exacerbate neuroinflammation in vitro raises concerns about its use, particularly among ageing individuals who consume this plant to manage inflammatory conditions. These findings highlight the need for caution and emphasise the importance of further research to evaluate its safety and efficacy before advocating for its medicinal use, especially for neurodegenerative disease therapy.
Keywords
Astrocytes, neuroinflammation, oxidative stress, pro-oxidant, Myrmecodia platytyrea
Introduction
Historically, the substantial contributions of natural products and
their structural analogues to pharmacotherapy cannot be overlooked.
Their role in treating chronic ailments, including infectious diseases
and cancer, is pivotal. However, in the 1990s, the pharmaceutical industry’s
pursuit of these natural products began to wane, attributed to challenges
like isolation, screening, characterisation, and optimisation. In recent years,
there has been renewed interest in these natural products, especially those from
traditional medicinal plants, as potential leads for inflammation-related diseases [1, 2].
The accessibility, affordability, and historical efficacy of medicinal
plants make them particularly attractive, especially in developing countries.
Neurodegenerative diseases, a spectrum of brain disorders encompassing
Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), and amyotrophic
lateral sclerosis, are characterised by a steady decline in cognitive,
physical, and social faculties. Such diseases, deeply rooted in
neuroinflammation and chronic oxidative stress, significantly impair
the quality of life [3, 4]. The brain’s vulnerability to oxidative
stress is heightened due to its high oxygen consumption and enrichment
with polyunsaturated fatty acids [5-7]. This vulnerability underscores
the significance of metabolic coupling between neurons and astrocytes in
combating brain oxidative stress [8, 9]. Astrocytes, principal regulators
of neuroinflammation, play a vital role both during neurogenesis and in
the context of brain pathology [10], maintaining the health and function
of the central nervous system (CNS) [11]. With their multifunctional capabilities,
it’s unsurprising that these cells are linked to the onset and progression of
various neurodegenerative disorders [12, 13]. Recent research highlights
astrocytes’ protective roles against free-radical toxicity and their
responsiveness to inflammatory signals [14, 15], emphasising their suitability
as in vitro models for neurodegenerative diseases [16-18].
Interestingly, due to their diverse properties, natural compounds like
flavonoids, alkaloids, resveratrol, and curcumin, are emerging as
potential multitarget therapeutics for neurodegenerative disorders.
Myrmecodia platytyrea, commonly referred to as ant-plant or Sarang
Semut, is a myrmecophyte renowned in traditional medicine for its
antioxidant, anti-inflammatory, and anticancer properties [19-21].
The tuber of this plant, rich in bioactive components such as flavonoids,
tannins, polyphenols, and stigmasterol which contribute to its therapeutic
potential [19, 22, 23]. Flavonoids, one of the main bioactive
groups in M. platytyrea, are particularly noteworthy for their ability
to cross the blood-brain barrier (BBB) and exert neuroprotective effects.
These compounds demonstrate antioxidant and anti-inflammatory properties,
critical in mitigating oxidative stress and neuroinflammation in the CNS [24-26].
Despite its traditional use in treating inflammation-linked ailments,
research on the specific effects of Myrmecodia sp. decoction on
neuroinflammation and its potential for treating neurodegenerative
diseases remains sparse. This study aims to bridge this knowledge
gap by elucidating the neuroprotective effects of M. platytyrea
tuber aqueous extract (MPAE) on nerve cells, focusing on its
potential to prevent or mitigate neuroinflammatory processes.
Methods
Plant extraction
M. platytyrea tubers were collected from Northern Sulawesi and were identified by Prof. Eko Baroto Walujo, from Herbarium Bogoriense, Research Centre for Biology, Indonesian Institute of Sciences, Bogor, Indonesia (voucher identification numbers BO1647929 and BO0009642). This study utilised a decoction method [27]. Briefly, the dried tuber of M. platytyrea was grounded into powder form and soaked in boiling distilled water (1:9) for 15 min and filtered through 4 different filter paper ranges from Whatman No. 1, 40, 42 and cellulose acetate membrane filter. The solvent in the filtrate was eliminated using a rotary evaporator (Heidolph, Germany) under reduced pressure at 100 mbar, 55°C. The concentrated filtrate was stored in a -80°C freezer for 2 days (Sanyo, Japan) and freeze-dried at a pressure of 0.007 mbar in a freeze-dryer (Labconco, UK) to get the dried powder of aqueous extract. The powdered extract was kept at -20°C (Sanyo, Japan) until further use.
Astrocyte culture
The murine astrocyte cell lines, C8-D1A (ATCC® CRL-2541TM), were purchased from ATCC. The C8-D1A cell line has the morphology of fibrous astrocytes and was isolated from the cerebellum of the mouse (Mus musculus sp.) brain. The complete growth medium for this cell line was Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich) with foetal bovine serum (FBS; Thermofisher, UK) to a final concentration of 10 % in 75 cm3 flask.
Cytotoxicity of MPAE
Once confluent, MPAE was tested on the cells for cytotoxicity study using MTT assay [28]. Cells (100 µL) were seeded into each 96-multiwell plate (Corning, Sigma, USA). After 24 h, the MPAE extracts (0.0001, 0.001, 0.01, 0.1, 1, and 10 mg/mL) were added into the wells and incubated for 24, 48 or 72 h. At the end of each incubation period, 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) solution was added to each well. After 4 h of incubation, the supernatant was removed, and 100 µL of DMSO was added into the wells to dissolve the formazon. MTT reduction was quantified at 550 nm using a microplate reader (Infinite M200, Tecan, Switzerland).
Determination of the effect of MPAE on cell viability activity of Fe2SO4- and H2O2-induced oxidative stress and LPS-induced inflammation on astrocytes
Astrocytes (2 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h. Then, the plated cells were treated with MPAE extract (i.e. 25, 50,125, 250, and 500 μg/mL) for 1 h. After that, the media containing Fe2SO4 (7.5 mM), H2O2 (0.2 mM) and LPS (1 µg/mL), respectively, were added into each well and then incubated at 37 °C, 5 % CO2 for 24 h. Then, the MTT assay was carried out. Cell viability was determined after a 4-h incubation by measuring the absorbance at 550 nm using a microplate reader (Infinite M200 Tecan, Switzerland).
Determination of the effect of MPAE on reactive oxygen species (ROS) level in Fe2SO4- and H2O2-induced oxidative stress
ROS accumulation was measured by the fluorescent probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) [29]. Astrocytes (2 × 104 cells/well) were seeded in a 96-well black plate and incubated for 24 h. Then, the plated cells were treated with various concentrations of MPAE extract (i.e. 25, 50,125, 250, and 500 μg/mL) for 1 h, after which the plate was incubated with Fe2SO4 (7.5 mM) or H2O2 (0.2 mM), respectively for 24 h. Then, the cells were washed with Kreb’s buffer. Then, the measurement started by adding 100 µL of 5 µM of DCF-DA solution into the wells in the dark. After 30 min of incubation at 37ºC, ROS production was measured using a microplate reader at Ex = 485 and Em = 538 nm.
Determination of effect of MP on pro-inflammatory cytokines of LPS-induced astrocytes
Astrocytes (5 × 105 cells/well) were seeded in a 6-well plate for 24 h before being treated with MPAE (25, 50, 125, 250, and 500 μg/mL). After 24 h, LPS (1 µg/mL) was added to the cells, followed by a 24-h incubation [9]. Cells were then collected and centrifuged at 3000 rpm for 10 min at 4ºC. The activity of pro-inflammatory cytokines (i.e., TNF-α, IL-1β, and IL-6) was measured by an ELISA kit (eBioscience, Austria). The assays were carried out according to the manufacturer’s protocol. The absorbances were measured using a microplate reader (Infinite M200, Tecan, Switzerland).
Statistical analysis
Values are represented as mean ± SD of three parallel measurements. Statistical analysis was performed using One-way ANOVA in GraphPad Prism 7 software. Post-hoc comparisons between groups were made using the Bonferroni test. Value P < 0.05 was considered statistically significant.
Results
M. platytyrea tubers (MPAE) were prepared as an aqueous
extract using the decoction method to replicate traditional
medicine practices. The percentage yield of MPAE was 16.05 ± 0.14 %.
The C8-D1A murine astrocyte cell line was the cell model
employed to represent CNS functionalities, as these cells
are key players in both physiological neuronal functions
and the pathological process. Through the MTT assay, the
IC50 of MPAE in astrocytes was 1.54 ± 0.26 mg/mL (Figure 1)
with maximal inhibition of 81.40 ± 2.33% at 10 mg/mL, indicating
that it is not cytotoxic against normal astrocyte cell line.
The criteria used for the classification of cytotoxicity is
as follows: IC50: < 20 µg/mL (high cytotoxicity), IC50: 21-200 µg/mL (moderate cytotoxicity),
IC50: 201-500 µg/mL (weak cytotoxicity), IC50: >501 µg/mL (no cytotoxic activity) [30].
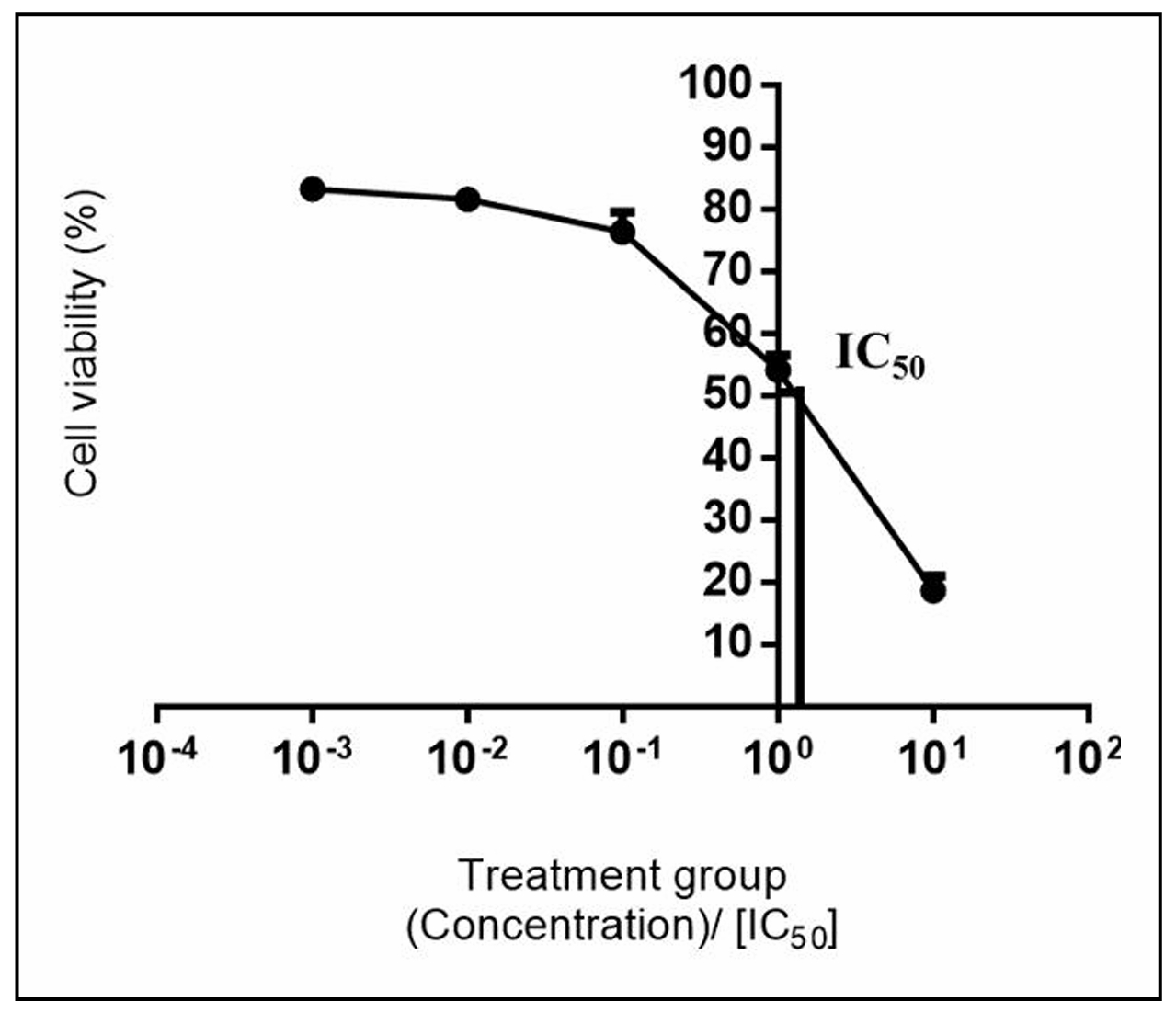
Figure 1. Effect of M. platytyrea tuber aqueous extract on astrocyte viability. Astrocytes (2 × 104 cells/well) were seeded on 96-well plates and incubated with MPAE (0.001, 0.01, 0.1, 1, and 10 mg/mL) for 24 h. Cell viability was determined by MTT assay. Mean ± SD (n = 3). IC50 for MPAE was 1.54 ± 0.26 mg/mL.
The effects of MPAE on astrocyte viability were evaluated under oxidative and inflammatory conditions induced by Fe2SO4, H2O2 or LPS for 24 h (Figure 2). Exposure to Fe2SO4 (7.5 mM), H2O2 (0.2 mM), and LPS (0.1 µg/mL) significantly (P < 0.05) reduced astrocyte viability to 51.39 ± 4.63% (Figure 2A), 52.25 ± 0.6% (Figure 2B), and 70.1 ± 3.4% (Figure 2C), respectively. Pretreatment with MPAE (25, 50, 125, 250, and 500 µg/mL) further exacerbated the reduction in cell viability in a concentration-dependent manner (P < 0.05) for all three inducers. No cytoprotection by MPAE was observed; instead, cell viability decreased further.
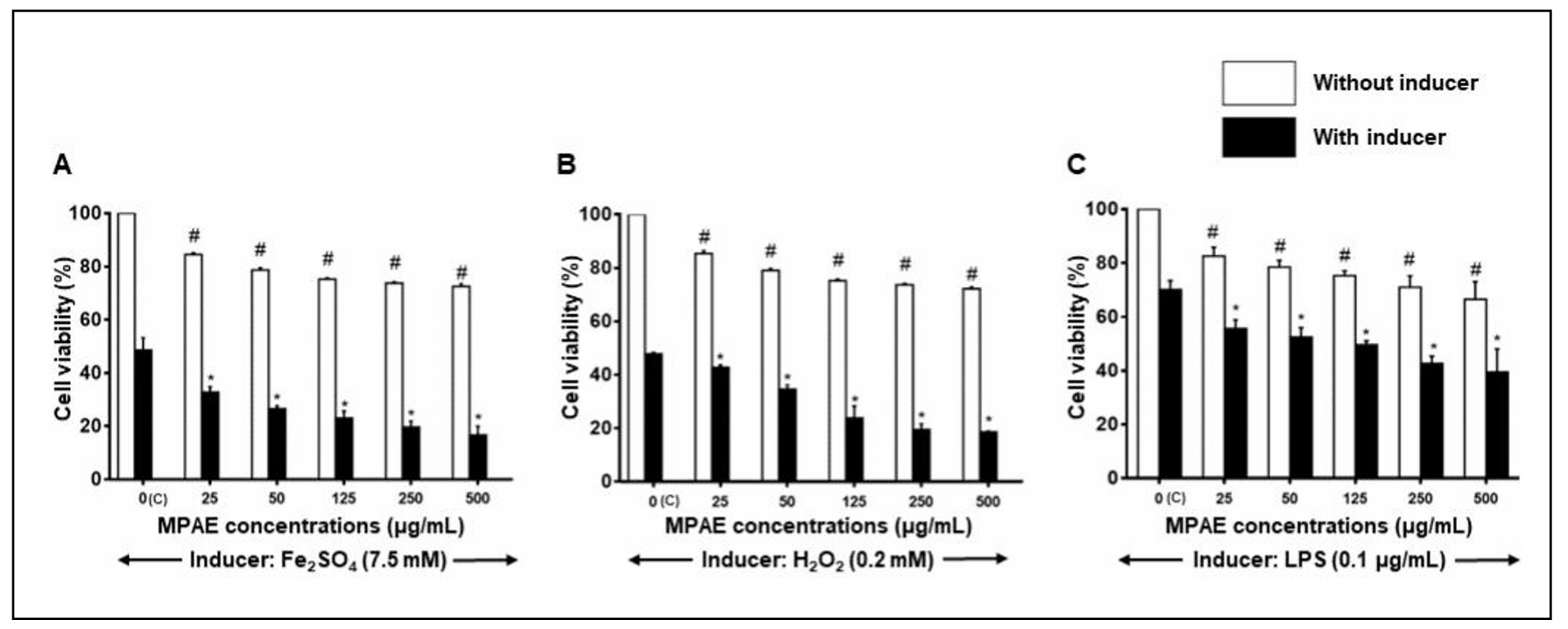
Figure 2. Effects of MPAE on viability of astrocytes induced with (A) Fe2SO2, (B) H2O2 and (C) LPS. Cell viability was determined by MTT assay. Mean ± SD (n = 3). # Significantly different from control cells which were not treated with MPAE or inducer agent. * Significantly different from cells treated with inducer agent only (P < 0.05, One-way ANOVA + Bonferroni test).
ROS accumulation in the astrocytes was measured by DCF-DA assay (Figure 3). Fe2SO4 (7.5 mM) alone induced a significant 4.70 ± 0.83-fold increase in ROS production compared to control cells (P < 0.05, Figure 3A). Pretreatment with MPAE (25–500 µg/mL) did not significantly alter ROS levels in Fe2SO4-treated cells, with fold changes ranging from 4.75 ± 0.33 to 5.7 ± 1.66, compared to cells treated with Fe2SO4 alone (P > 0.05).
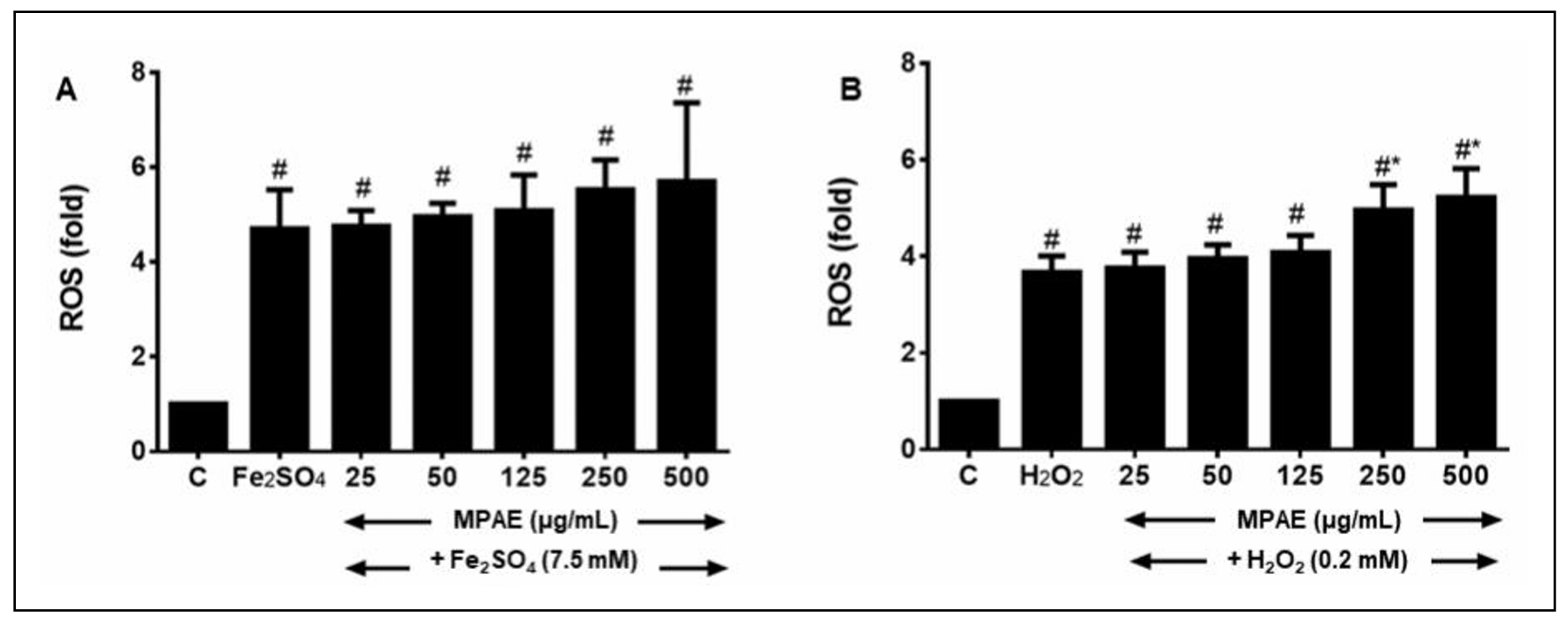
Figure 3. Effect of MPAE on ROS production by astrocytes exposed to (A) Fe2SO2- or (B) H2O2-induced oxidative stress. ROS was determined by DCF-DA assay. Mean ± SD (n = 3). # Significantly different compared to control cells which were not treated with MPAE or inducer agent. *Significantly different from cells treated with inducer agent only. Pretreatment of cells with MPAE did not affect ROS production by Fe2SO2 or H2O2 (P > 0.05, One-way ANOVA + Bonferroni test).
Similarly, H2O2 (0.2 mM) caused a significant 3.7 ± 0.3-fold
increase in ROS production compared to control
cells (P < 0.05, Figure 3B). Pretreatment
with MPAE (25–500 µg/mL) further increased ROS
production, particularly at higher
concentrations (250 and 500 µg/mL),
eliciting 5.0 ± 0.5 and 5.2 ± 0.6-fold increases,
respectively (P < 0.05). This indicates that higher
concentrations of MPAE exacerbated ROS accumulation
in H2O2-treated cells.
LPS (1 µg/mL) significantly
increased TNF-α (45.78 ± 9.61 pg/mL, Figure 4A), IL-1β (94.7 ± 5.8 pg/mL, Figure 4B),
and IL-6 (121.0 ± 18.0 pg/mL, Figure 4C) levels compared to control cells (P < 0.05).
Pretreatment with MPAE further elevated these cytokine levels in a
concentration-dependent manner. Significant increases in TNF-α and IL-6
beyond LPS alone were observed at 250 and 500 µg/mL of MPAE (P < 0.05, Figure 4A and C).
Additionally, higher concentrations of MPAE (125, 250, and 500 µg/mL)
significantly increased IL-1β levels compared to LPS alone (P < 0.05, Figure 4B).

Figure 4. Effect of MPAE on (A) TNF-α, (B) IL-1β and (C) IL-6 levels of astrocytes exposed to LPS-induced inflammation. Cytokines were determined by ELISA assay. Mean ± SD (n = 3). # Significantly different compared to control cells not treated with MPAE or LPS, *Significantly different compared to cells treated with LPS only (P < 0.05, One-way ANOVA + Bonferroni test).
Discussion
Aqueous extraction was prioritised for its safety for consumption,
while organic solvent (i.e., methanol, acetone, chloroform,
dichloromethane, etc.) extracts are highly likely to produce
toxicity [31]. Additionally, aqueous extraction retains all
the compounds of the plant material, while other extraction
methods may retain only some compounds depending on the
polarity of the solvent [32].
An oxidative stress model was created by inducing
astrocytes with iron overload using Fe2SO2. Iron overload
increases synaptic activity, leading to cytotoxic
effects [31, 32] and may occur in AD, PD, and HD [33-35].
A preliminary study by a colleague who looked at the effect
of MPAE on ferrous-ion chelating (FIC) assay showed that
MPAE has good chelating activity with IC50 of 147.62 ± 18.82 µg/mL [36].
More so, tuber extracts of M. platytyrea can impede the
production of ROS and reduce the cytokine levels in HepG2
cells [36]. However, in this present study, MPAE caused an
increase in cell death in a concentration-dependent manner,
which showed no protective effect on the astrocytes
against Fe2SO2 assault.
The second oxidative stress model was induced by H2O2.
Excessive H2O2 mediates cell damage through the direct
oxidation of lipids, proteins, and DNA, or it acts as a
signalling mechanism to trigger the cellular apoptotic
pathway [37, 38]. Cell viability dropped dramatically
when astrocytes were induced by 0.2 mM of H2O2.
Pretreatment with MPAE increased the oxidative stress
response in astrocytes and significantly accelerated
cell death compared to untreated cells. MPAE, which
has a high content of antioxidants, possibly acted as
a pro-oxidant in the presence of H2O2, inducing oxidative
stress either by the generation of ROS or by inhibiting
antioxidants. Free radical scavengers, which are antioxidants,
can be pro-oxidants unless linked to a radical sink.
A radical sink means the antioxidant radical has to "sink"
its unpaired electron in other reactions. Antioxidants
such as ascorbic acid, vitamin E and polyphenols act as
pro-oxidants in the presence of transition metals [39].
For example, resveratrol promotes oxidative DNA damage
in the presence of Cu2+ ions that may pose a problem as
a neuroprotectant [40].
There is a likelihood that the antioxidative action of
MPAE due to the extremely high antioxidant activity was
changed to pro-oxidant action. Surprisingly, some common
and eminent antioxidant flavonoids act as pro-oxidants [41].
Generally, the "double-edge sword" action was triggered in
the presence of the metal ions and contributed by the chemical
structure of the compounds in MPAE [42, 43]. Flavonoid
antioxidant action may be connected to the hydroxyl (OH)
functional group’s electron-donating ability [44, 45].
Polyphenols without OH groups, like flavone and flavanone
have no antioxidant or Fe/Cu-initiated pro-oxidant activity.
The quantity of free OH groups in a flavonoid affects
its Fe/Cu-initiated pro-oxidant activity [34, 46].
Pro-oxidant activity increases with OH groups. O-methylation
and possibly additional O-substitutions of flavonoid OH groups
inactivate antioxidant and pro-oxidant activity [39, 47].
Therefore, pro-oxidant activity in the astrocytes produces ROS,
including H2O2. Excess H2O2 causes oxidative-nitrosative stress,
oxidising lipids, proteins, and DNA, and damaging cells [48, 49].
H2O2 overload increases inducible nitric oxide synthase (iNOS)
expression and cytokine release (TNF-α, IL-1β, IL-1, and IL-6)
and lowers antioxidant defence (SOD, CAT, and GPx), causing
mitochondrial membrane potential dysfunction, morphological
changes, and apoptotic cell death [50, 51]. However, neuronal
injury can be reduced by inhibiting these mediators [50].
A model of neuroinflammation was created by incubating
astrocytes with 0.1 μg/mL LPS, which caused about 26% of
cell death. A few studies have successfully used 0.1 μg/mL of LPS to
induce inflammation in astrocytes but without apparent
toxic effect (~25-30 % cell death) [9, 42, 43].
A previous study also reported that 0.1 μg/mL of LPS activates
astrocytes by increasing cytokine production and the
glial fibrillary acidic protein (GFAP) level that
exerts TLR-4 expression [9].
Pretreatment of LPS-induced astrocytes with MPAE
triggered cytokine release. Inflammation is initiated
by activating pro-inflammatory signalling cascades,
such as mitogen-activated protein kinase (MAPK) and NF-κβ [44].
Activated NFκβ stimulates the production of pro-inflammatory
cytokines (i.e. TNF-α and interleukins), the release of which
triggers inflammation and promotes cell death via necrosis [45].
This was shown by the increased loss of cell viability
in this study in the presence of MPAE. Thus, treatment
with MPAE triggers more oxidative damage and inflammation,
leading to increased cell death in the presence of inducers.
The decoction of M. platytyrea tuber is claimed by the
indigenous people of Papua New Guinea as an anticancer
remedy [20]. A few studies have been conducted to determine
the efficacies of this tuber, which indicated its potential
in treating inflammation-related diseases such as cancer
and diabetes, including pain inhibition [20, 21].
The benefits of the tuber were attributed to the presence
of phenolic compounds (flavonoids, terpenoids, anthocyanins)
and phytosterols (stigmasterol and β-sitosterol), as mentioned
before [19, 22, 23]. These compounds possess strong
antioxidant and anti-inflammatory properties [46-48].
The primary reasons for neurodegeneration appear to
involve abnormal processing of proteins, genetic
disorders, misfolding and aggregation of various
proteins, activating cellular apoptosis, triggering
mitochondrial dysfunction, neuroinflammation, production
of free radicals, and oxidative stress [48]. Since the
identified compounds in the tuber of M. platytyrea have
the potential to block oxidative stress and inhibit
neuroinflammation, there are great possibilities for
this plant to prevent the susceptibility of
neurodegenerative disorders in the ageing society.
Myrmecophytes contain phenolic compounds (i.e. rosmarinic acid,
procyanidin B1 and polymer of procyanidin B1) [50] and
flavonoids (i.e. kaempferol, luteolin, apigenin, and
quercetin). M. platytyrea tubers contain bioactive compounds
such as stigmasterol and morindolide with a high
antioxidant capacity [23]. Yet, in this study, MPAE
worked differently in the astrocytes.
Several studies display controversial results on
antioxidants. The type, dosage and matrix of antioxidants
may be determining factors that affect the balance between
their useful and harmful effects [37, 51]. Pro-oxidant
activity on normal cells damages biomolecules such as DNA,
proteins and lipids and induces lipid peroxidation and
apoptosis as a consequence of cell death [52]. It can
also initiate an intracellular signalling pathway that
increases pro-inflammatory cytokine production, which
leads to inflammation [53]. Due to the high content of
polyphenolics in the MPAE, there is a high probability
that it is a "double-edged sword" with activity, acting
as antioxidants or pro-oxidants depending on the
concentration/doses or presence of metal ions [54].
Hence, the proposed mechanism of action of MPAE in
astrocytes ( Figure 5). However, this study was limited
because microglia were not used in its investigation.
The comparison of findings from both cell types may
provide a comprehensive understanding and treatment of
neuroinflammatory disorders. Furthermore, it is
recommended that the impact of MPAE should be extended
to in vivo studies, which will help better understand
the multifaceted origins of neurodegenerative diseases
such as AD and PD.
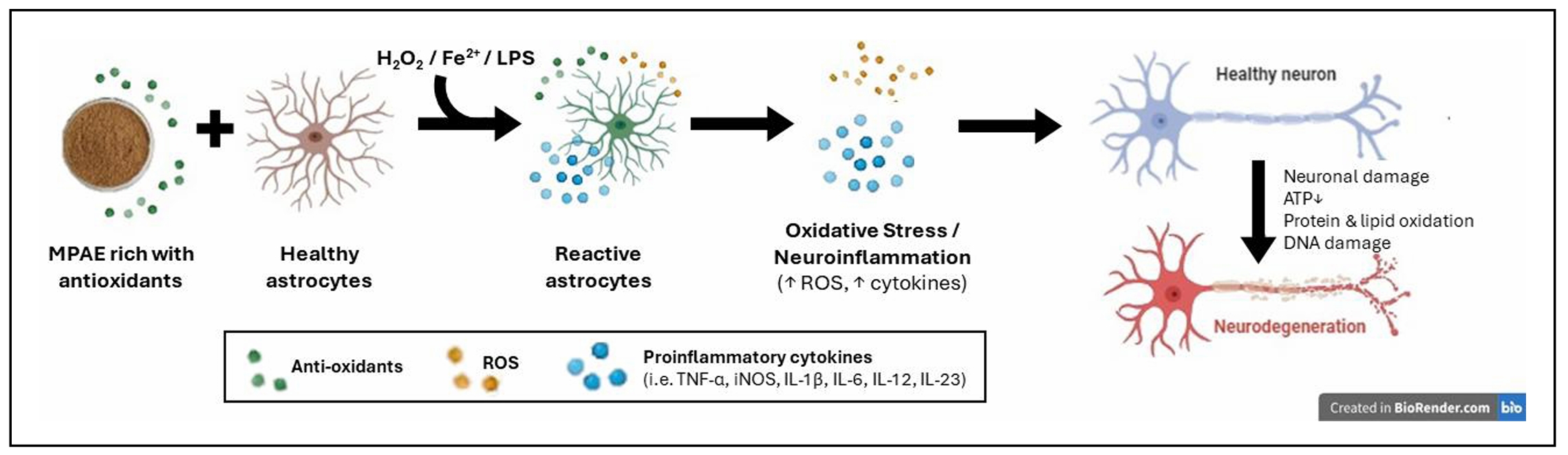
Figure 5. Plausible mechanism of action of MPAE on astrocytes exposed to hydrogen peroxide/iron (II) sulphate/lipopolysaccharide that leads to neuronal damage. Oxidative stress and neuroinflammation were aggravated due to the "double-edged sword" action of MPAE. The rich antioxidant MPAE may contain excessive phenolic antioxidant compounds, which, in the presence of the inducers, cause more damage because of the pro-oxidant activity.
Conclusions
To our knowledge, this is the first study examining the impact of MPAE on neuroinflammation. Pretreatment with MPAE on astrocytes resulted in dose-dependent cytotoxicity with elevated ROS and cytokine levels, potentially attributable to its "double-edged sword" mechanism. While MPAE is non-cytotoxic to astrocytes, its capacity to aggravate neuroinflammation in vitro raises concerns regarding its application, especially in ageing individuals utilising this plant for inflammatory disorders. Before recommending its therapeutic usage, particularly for treating neurodegenerative diseases, these findings demonstrate the necessity of caution and stress the significance of more research to assess its safety and effectiveness. However, further studies must be conducted to confirm which compounds in MPAE exacerbate oxidative stress and inflammation in the astrocytes.
Declarations
Acknowledgements
The authors express sincere appreciation to the Faculty of Pharmacy, University Teknologi MARA for providing state-of-the-art facilities to complete the research. The authors also thank Dr. Thellie Ponto for supplying the plant material, M. platytyrea tubers.
Authors’ contributions
Conceptualisation: SS, MHH. Investigation and Formal Analysis: AN, SS, MHH. Supervision: SS, AHJ, MHH. Visualisation: MFM, AN, MHH. Writing-original draft: AN, SS, MHH. Writing-review and editing: MFM, SS, AN, AHJ, HSJC, MHH. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
The authors appreciate the financial support from the Ministry of Higher Education, Malaysia, for the research grants, grant numbers 600-RMI/FRGS 5/3 (21/2014) and FRGS/1/2022/SKK06/UITM/02/5.
Conflicts of interest
All authors declare that there are no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
1. Atanasov AG, Zotchev SB, Dirsch VM, & Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov, 2021, 20(3): 200-216. [Crossref]
2. Cragg GM, & Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta, 2013, 1830(6): 3670-3695. [Crossref]
3. Zhang W, Xiao D, Mao Q, & Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther, 2023, 8(1): 267-277. [Crossref]
4. Solleiro-Villavicencio H, & Rivas-Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front Cell Neurosci, 2018, 12: 114-124. [Crossref]
5. Cioffi F, Adam RHI, & Broersen K. Molecular mechanisms and genetics of oxidative stress in Alzheimer’s Disease. J Alzheimers Dis, 2019, 72(4): 981-1017. [Crossref]
6. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem, 2006, 97(6): 1634-1658. [Crossref]
7. Suh SW, Gum ET, Hamby AM, Chan PH, & Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest, 2007, 117(4): 910-918. [Crossref]
8. Chen Y, Qin C, Huang J, Tang X, Liu C, Huang K, et al. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif, 2020, 53(3): e12781. [Crossref]
9. Li N, Zhang X, Dong H, Zhang S, Sun J, & Qian Y. Lithium ameliorates LPS-induced astrocytes activation partly via inhibition of toll-like receptor 4 expression. Cell Physiol Biochem, 2016, 38(2): 714-725. [Crossref]
10. Colombo E, & Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol, 2016, 37(9): 608-620. [Crossref]
11. Garland EF, Hartnell IJ, & Boche D. Microglia and astrocyte function and communication: what do we know in humans? Front Neurosci, 2022, 16: 824888. [Crossref]
12. Phatnani H, & Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harb Perspect Biol, 2015, 7(6): a020628. [Crossref]
13. Farina C, Aloisi F, & Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol, 2007, 28(3): 138-145. [Crossref]
14. Linnerbauer M, Wheeler MA, & Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron, 2020, 108(4): 608-622. [Crossref]
15. Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One, 2012, 7(9): e45069. [Crossref]
16. Jiwaji Z, & Hardingham GE. Good, bad, and neglectful: astrocyte changes in neurodegenerative disease. Free Radic Biol Med, 2022, 182: 93-99. [Crossref]
17. Roesslein M, Hirsch C, Kaiser JP, Krug HF, & Wick P. Comparability of in vitro tests for bioactive nanoparticles: a common assay to detect reactive oxygen species as an example. Int J Mol Sci, 2013, 14(12): 24320-24337. [Crossref]
18. Franklin H, Clarke BE, & Patani R. Astrocytes and microglia in neurodegenerative diseases: lessons from human in vitro models. Prog Neurobiol, 2021, 200: 101973. [Crossref]
19. Agatonovic-Kustrin S, Morton DW, Adam A, Mizaton HH, & Zakaria H. High-performance thin-layer chromatographic methods in the evaluation of the antioxidant and anti-hyperglycemic activity of Myrmecodia platytyrea as a promising opportunity in diabetes treatment. J Chromatogr A, 2017, 1530: 192-196. [Crossref]
20. Ju A, Cho Y, Kim BR, Lee S, Le HT, Vuong HL, et al. Anticancer effects of methanol extract of Myrmecodia platytyrea Becc. leaves against human hepatocellular carcinoma cells via inhibition of ERK and STAT3 signaling pathways. Int J Oncol 2018, 52: 201-210. [Crossref]
21. Hasan MH, Zakaria H, Wahab IA, Ponto T, & Adam A. Myrmecodia platytyrea methanol tuber extract ameliorates hyperglycemia in STZ-induced diabetic Sprague-Dawley male rats. Indonesian Journal of Pharmacy, 2021. [Crossref]
22. Agatonovic-Kustrin S, Morton DW, Mizaton HH, & Zakaria H. The relationship between major polyphenolic acids and stigmasterol to antioxidant activity in different extracts of Myrmecodia platytyrea. South African Journal of Botany, 2018, 115: 94-99. [Crossref]
23. Haris NFM, Hasan MKN, Wahab IA, Hasan MH, Ponto T, & Adam A (2016). Compounds from the antioxidant active fraction of M. platytyrea.
24. Ayaz M, Sadiq A, Junaid M, Ullah F, Ovais M, Ullah I, et al. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front Aging Neurosci, 2019, 11: 155-165. [Crossref]
25. Bakoyiannis I, Daskalopoulou A, Pergialiotis V, & Perrea D. Phytochemicals and cognitive health: are flavonoids doing the trick? Biomed Pharmacother, 2019, 109: 1488-1497. [Crossref]
26. de Andrade Teles RB, Diniz TC, Costa Pinto TC, de Oliveira Júnior RG, Gama ESM, de Lavor É M, et al. Flavonoids as therapeutic agents in Alzheimer’s and Parkinson’s diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev, 2018, 2018: 7043213. [Crossref]
27. Nn A. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Medicinal and Aromatic plants, 2015, 4: 1-6. [Crossref]
28. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods, 1983, 65(1-2): 55-63. [Crossref]
29. Figueroa D, Asaduzzaman M, & Young F. Real time monitoring and quantification of reactive oxygen species in breast cancer cell line MCF-7 by 2’,7’-dichlorofluorescin diacetate (DCFDA) assay. J Pharmacol Toxicol Methods, 2018, 94(Pt 1): 26-33. [Crossref]
30. Sajjadi SE, Ghanadian M, Haghighi M, & Mouhebat L. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. Journal of HerbMed Pharmacology, 2015, 4: 15-19.
31. Pandey AK, & Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry, 2014, 2: 115-119.
32. Zhang QW, Lin LG, & Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med, 2018, 13: 20-30. [Crossref]
33. Altamura S, & Muckenthaler MU. Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis.J Alzheimers Dis, 2009, 16(4): 879-895. [Crossref]
34. Jomová K, Hudecova L, Lauro P, Simunkova M, Alwasel SH, Alhazza IM, et al. A switch between antioxidant and prooxidant properties of the phenolic compounds myricetin, morin, 3’,4’-dihydroxyflavone, taxifolin and 4-hydroxy-coumarin in the presence of copper(II) ions: a spectroscopic, absorption titration and DNA damage study. Molecules, 2019, 24(23): 4335-4345. [Crossref]
35. DeTure MA, & Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 2019, 14(1): 32-42. [Crossref]
36. Mizaton HH., Maisarah MZ., Nik H., Mohd K, Ibtisam AW., & Adam A. Anti-inflammatory and anti-hyperlipidaemic activities of Myrmecodia platytyrea tuber: the in vitro studies. Malaysian Journal of Medicine & Health Sciences, 2022: 92-101.
37. Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J, 2016, 15(1): 71-82. [Crossref]
38. Lin HJ, Wang X, Shaffer KM, Sasaki CY, & Ma W. Characterization of H2O2-induced acute apoptosis in cultured neural stem/progenitor cells. FEBS Letters, 2004, 570(1): 102-106. [Crossref]
39. Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int, 2014, 2014: 761264. [Crossref]
40. Granzotto A, & Zatta P. Resveratrol and Alzheimer’s disease: message in a bottle on red wine and cognition. Front Aging Neurosci, 2014, 6: 95-105. [Crossref]
41. Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys, 2008, 476(2): 107-112. [Crossref]
42. Hinojosa AE, Caso JR, García-Bueno B, Leza JC, & Madrigal JL. Dual effects of noradrenaline on astroglial production of chemokines and pro-inflammatory mediators. J Neuroinflammation, 2013, 10: 81-92. [Crossref]
43. Zhou XY, Liu J, Xu ZP, Fu Q, Wang PQ, & Zhang H. Dexmedetomidine inhibits the lipopolysaccharide-stimulated inflammatory response in microglia through the pathway involving TLR4 and NF-κB. Kaohsiung J Med Sci, 2019, 35(12): 750-756. [Crossref]
44. Tong W, Chen X, Song X, Chen Y, Jia R, Zou Y, et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp Ther Med, 2020, 19(3): 1824-1834. [Crossref]
45. Jazvinšćak Jembrek M, Oršolić N, Mandić L, Sadžak A, & Šegota S. Anti-oxidative, anti-inflammatory and anti-apoptotic effects of flavonols: targeting Nrf2, NF-κB and p53 pathways in neurodegeneration. Antioxidants, 2021, 10(10): 1628-1639. [Crossref]
46. Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, & Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev, 2016, 2016: 7432797. [Crossref]
47. Panche AN, Diwan AD, & Chandra SR. Flavonoids: an overview. J Nutr Sci, 2016, 5: e47. [Crossref]
48. Othman RA, & Moghadasian MH. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr Rev, 2011, 69(7): 371-382. [Crossref]
49. Simunkova M, Alwasel SH, Alhazza IM, Jomova K, Kollar V, Rusko M, et al. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol, 2019, 93(9): 2491-2513. [Crossref]
50. Engida AM, Faika S, Nguyen-Thi BT, & Ju YH. Analysis of major antioxidants from extracts of Myrmecodia pendans by UV/visible spectrophotometer, liquid chromatography/tandem mass spectrometry, and high-performance liquid chromatography/UV techniques. J Food Drug Anal, 2015, 23(2): 303-309. [Crossref]
51. Bouayed J, & Bohn T. Exogenous antioxidants--Double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev, 2010, 3(4): 228-237. [Crossref]
52. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev, 2017, 2017: 8416763. [Crossref]
53. Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev, 2016, 2016: 5698931. [Crossref]
54. Eghbaliferiz S, & Iranshahi M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytother Res, 2016, 30(9): 1379-1391. [Crossref]