Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Inflammaging and microbiota: the intersection of aging, inflammation, and gut health
* Corresponding author: Harish Rangareddy
Mailing address: Department of Biochemistry, Haveri Institute of Medical Sciences, India.
Email: harishreddy1349@gmail.com
Received: 03 December 2024 / Revised: 25 December 2024/ Accepted: 14 February 2025 / Published: 27 March 2025
DOI: 10.31491/APT.2025.06.172
Abstract
Inflammaging, characterized by chronic, low-grade inflammation associated with aging, is a key contributor to age-related diseases, including cardiovascular disorders, neurodegenerative conditions, and metabolic syndromes. The intricate interplay between inflammaging and the human microbiota—a diverse ecosystem of microorganisms regulating immune, metabolic, and neurological functions—is crucial in understanding these processes. Aging leads to significant shifts in microbiota composition, resulting in dysbiosis, which fosters pro-inflammatory states and systemic inflammation. Age-related changes in the gut microbiota and microbial metabolites, such as short-chain fatty acids and secondary bile acids, influence inflammation through pathways like Toll-like receptor signaling and cytokine production. Microbial dysbiosis impacts immune responses and gut barrier integrity, contributing to inflammaging and its associated pathologies. Interventions targeting gut health, including dietary modifications, probiotics, prebiotics, and fecal microbiota transplantation, offer potential strategies to mitigate these effects. Advances in bioinformatics and microbiota research enable the development of targeted treatments aimed at improving longevity and reducing chronic inflammation. Intestinal epithelial cells play a central role as physical and antimicrobial barriers, while also mediating microbiota-host immune signaling. Aging-related changes to intestinal epithelial cells, microbiota composition, and immune function disrupt immune homeostasis and exacerbate inflammaging. Environmental factors, including diet and medications, further influence gut microbiota and immune function, either preventing or promoting inflammaging. Lifestyle and pharmacological interventions are suggested to promote healthy aging and reduce the adverse effects of chronic inflammation.
Keywords
Aging, inflammation, dysbiosis, fecal microbiota transplantation
Introduction
Aging is a complex, multifactorial process marked by the progressive deterioration
of physiological functions [1]. One of the hallmarks of aging is the body's increasing
inability to maintain homeostasis, particularly in immune system regulation [2, 3].
Inflammaging, the chronic, low-grade inflammation associated with aging, has emerged
as a crucial mechanism underlying age-related pathologies, including cardiovascular
disease, cancer, and neurodegenerative disorders [4-6]. While the exact causes are not
fully understood, it is believed that a combination of immunosenescence (the decline
in immune function with age), cellular senescence (the process by which cells lose
the ability to divide), and environmental factors (such as diet and lifestyle) play
significant roles [7]. Chronic low-grade inflammation, often referred to as "inflammaging",
plays a critical role in the aging process. While acute inflammation is essential for
tissue repair and immune function, its persistent, dysregulated state accelerates aging
and exacerbates the onset of age-related diseases. This paradoxical relationship arises
from the sustained activation of immune cells such as macrophages and neutrophils, which
secrete pro-inflammatory cytokines that contribute to systemic tissue damage [8, 9].
Inflammaging is characterized by elevated levels of inflammatory biomarkers such as
cytokines (e.g., IL-6, IL-1β), C-reactive protein (CRP), and tumor necrosis
factor-alpha (TNF-α), which are associated with frailty, cognitive decline,
and mortality [10]. Unlike acute inflammation, which serves as a protective
response to injury or infection, chronic inflammation contributes to tissue damage,
impaired organ function, and susceptibility to disease [11]. A combination of
immunosenescence (the decline in immune function with age), cellular senescence
(the process by which cells lose the ability to divide), and environmental factors
such as diet and lifestyle contribute to this persistent inflammatory state [12].
The gut microbiota—a vast and dynamic community of microorganisms residing in the
gastrointestinal tract—plays a pivotal role in maintaining immune homeostasis and
modulating systemic inflammation. Age-associated changes in microbiota composition,
such as reduced diversity, decreased beneficial species, and an increased prevalence
of pro-inflammatory microorganisms, lead to dysbiosis. This imbalance is implicated in
the exacerbation of inflammaging through mechanisms like increased gut permeability,
translocation of microbial products, and alterations in bioactive metabolites, such as
short-chain fatty acids (SCFAs) [12] as shown in Figure 1.
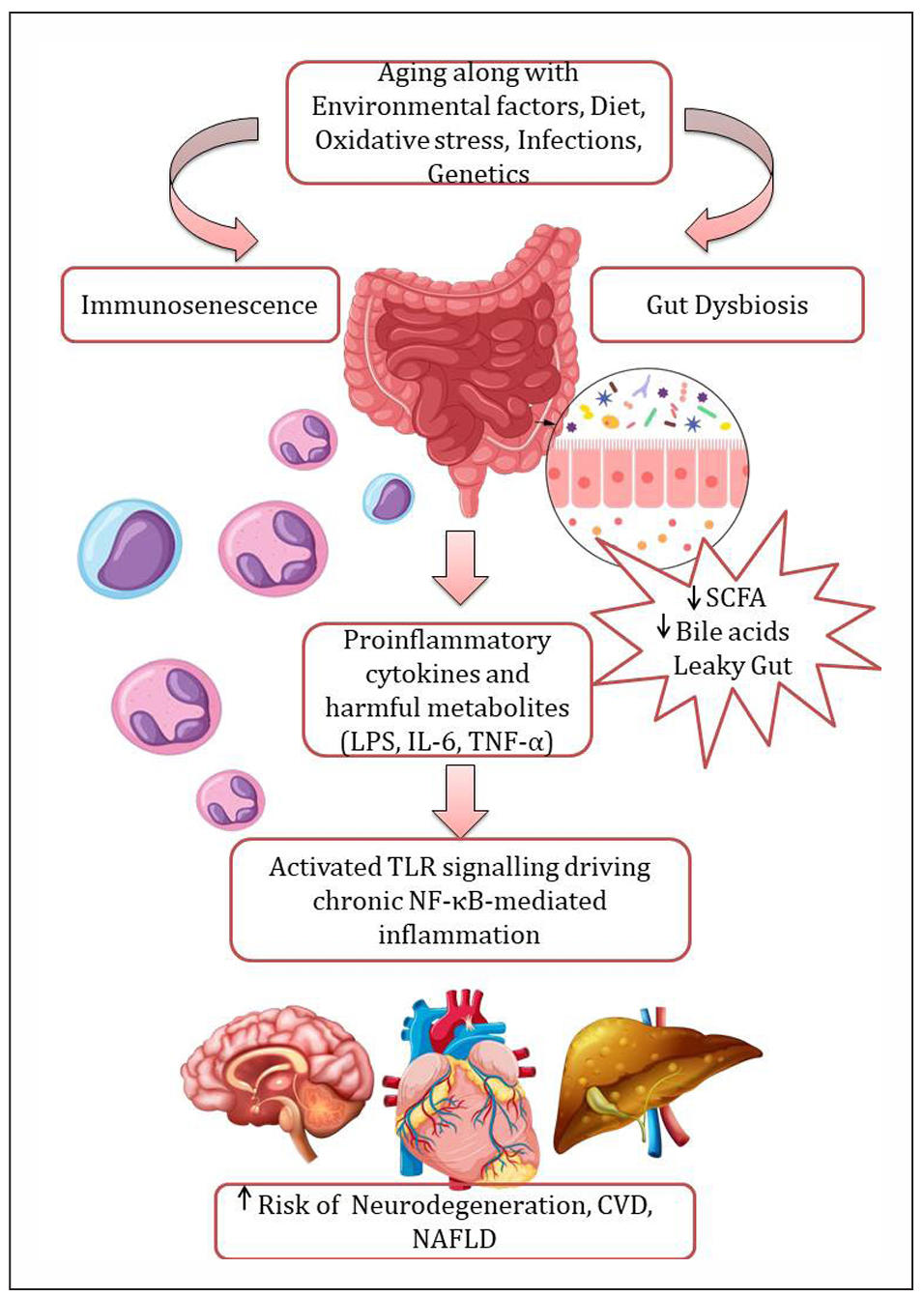
Figure 1. Gut dysbiosis and inflammaging. Aging, along with multiple factors—including environmental influences, dietary changes, oxidative stress, infections, and genetic susceptibility—contributes to gut microbiota dysbiosis. This dysbiosis is characterized by a reduction in beneficial bacteria and an overgrowth of pathobionts, leading to decreased bioavailability of microbial metabolites with immunoregulatory properties, such as short-chain fatty acids (SCFAs) and secondary bile acids, thereby exacerbating gut barrier dysfunction. Increased intestinal permeability results in elevated systemic levels of lipopolysaccharides (LPS), which activate Toll-like receptor 4 (TLR4) and drive nuclear factor kappa B (NF-κB)-mediated chronic inflammation. These alterations contribute to an increased risk of neurodegenerative disorders, cardiovascular diseases (CVD), and nonalcoholic fatty liver disease (NAFLD). Created by author using assets from http://www.freepik.com/.
Age-related immune dysregulation further compounds the issue, as the immune
system undergoes characteristic changes with advancing age. Inflammaging
fosters susceptibility to metabolic, cardiovascular, and neurodegenerative
diseases, while immunosenescence impairs the immune system's ability to respond
to antigens, reducing vaccine efficacy and increasing infection risk. These
alterations, including reduced B cell diversity, thymic involution, and dysfunctional
innate immune responses, highlight the multifaceted impact of aging on immune
function [7, 12].
In addition to its biomedical implications, aging and its associated inflammatory
states have significant societal consequences. The elderly, despite their wealth of
knowledge and generational insights, often face social isolation and ageism, recognized
as a global challenge by the WHO. Ageism is linked to worsened physical and mental
health, increased loneliness, financial insecurity, and even premature death.
Addressing the intersection of biological and societal factors is crucial for
achieving "healthy aging", encompassing both a disease-free lifespan and enhanced
quality of life through societal integration [13].
This article aims to elucidate the intricate relationship between inflammaging
and gut microbiota, focusing on their roles in age-related diseases. Additionally,
it explores potential interventions targeting microbiota to mitigate chronic
inflammation and enhance both the healthspan and societal integration of
aging individuals.
Overview of inflammaging
Key features of inflammaging include elevated pro-inflammatory cytokines like IL-6 and TNF-α, oxidative stress driven by the accumulation of reactive oxygen species (ROS), and immune dysregulation marked by declining adaptive immunity and heightened innate immune activation [10]. Cellular senescence also plays a pivotal role, as senescent cells adopt a pro-inflammatory phenotype known as the senescence-associated secretory phenotype (SASP), which perpetuates tissue dysfunction [14].
Interconnected sources of inflammaging
Inflammaging results from a complex interplay of various biological processes. One key factor is the presence of senescent cells, which secrete inflammatory cytokines, chemokines, and proteases, thereby creating a pro-inflammatory tissue environment [14]. Another contributing factor is oxidative stress, where mitochondrial dysfunction leads to the overproduction of reactive oxygen species (ROS), promoting cellular damage and triggering inflammation [2]. Age-related changes in the gut microbiota also play a role in inflammaging, as dysbiosis increases microbial translocation, which introduces lipopolysaccharides (LPS) into the circulation, thereby activating inflammatory pathways [15]. Additionally, immune system dysfunction contributes to inflammaging through immunosenescence, the decline in adaptive immune responses, coupled with heightened activation of the innate immune system, both of which foster chronic inflammation [14]. Finally, circulating mitochondrial DNA (cmtDNA) and damage-associated molecular patterns (DAMPs) act as inflammatory triggers, further exacerbating the inflammatory response [16]. Non-inflammatory contributors such as epigenetic modifications (e.g., DNA methylation, histone alterations) and molecular regulators like microRNAs and altered glycosylation patterns also amplify inflammatory pathways [17]. These interconnected mechanisms establish a self-sustaining loop of chronic inflammation, underscoring the role of inflammaging in driving age-associated dysfunction and disease.
Gut microbiota and aging
During early childhood, the diversity of the gut microbiota is characterized
by the predominance of species such as Akkermansia muciniphila, Bacteroides,
Veillonella, Clostridium coccoides, and Clostridium botulinum. By around the
age of three, the gut microbiota stabilizes into a composition similar to that
of adults, dominated by three primary phyla: Firmicutes, Bacteroidetes, and
Actinobacteria [18]. As individuals age, changes in diet and the immune system
can significantly influence gut microbiota composition. In older adults, there
is often a decline in Bifidobacterium and an increase in Clostridium and Proteobacteria.
The reduction in Bifidobacterium, an anaerobic bacterium known for its role in
immune system stimulation, is associated with heightened inflammation and a decline
in overall gut health [19].
The human microbiota, a diverse community of microorganisms residing in and on our
body, plays an indispensable role in maintaining health. It influences a variety of
physiological functions, including metabolic regulation, immune modulation, and barrier
integrity. The gut microbiota produces SCFAs through the fermentation of dietary fibers,
which in turn regulate key metabolic pathways, insulin sensitivity, and lipid metabolism.
Among these, butyrate serves as a primary energy source for the colonic lining and
exhibits potent anti-inflammatory and anticancer effects, contributing to overall gut
and systemic health [20].
The gut microbiota also plays a crucial role in shaping adaptive immune responses,
particularly in the development and differentiation of CD4+ and CD8+ T cells.
For instance, Lactobacillus species stimulate and activate regulatory T cells (Tregs),
while Clostridium perfringens (Gram-positive) supports the proliferation and
differentiation of both Tregs and Th17 cells. This interaction promotes the
production of interleukin-17 (IL-17) by intestinal Th17 cells, highlighting
the microbiota's influence on immune modulation [21].
Mechanisms linking microbiota and inflammaging
Inflammaging, is a hallmark of aging and is influenced by the gut microbiota. The activation
of Toll-like receptors (TLRs), particularly TLR4, is a key player in this process. TLRs are
type 1 transmembrane proteins found on epithelial and lamina propria cells, playing a
pivotal role in the innate immune system. These receptors enable host cells to identify
and respond to microbial pathogens by recognizing a diverse array of pathogen-associated
molecular patterns (PAMPs) and DAMP [22].
In humans, ten TLRs have been identified, categorized based on their cellular
localization. TLRs 1, 2, 4, 5, 6, and 10 are positioned on the cell surface and are
primarily responsible for detecting extracellular pathogens. In contrast, TLRs 3, 7, 8,
and 9 are intracellularly located, where they specialize in recognizing viral particles
and other nucleic acid-based signatures. This division underscores the specialized roles
of TLRs in mounting effective immune responses against diverse microbial threats [23].
TLR signaling pathways are categorized into two types: MyD88-dependent and MyD88-independent
(TRIF-mediated) pathways. All TLRs, except for TLR3, utilize the MyD88-dependent pathway.
TLR3 exclusively signals via the TRIF pathway, while TLR4 uniquely employs both MyD88 and
TRIF pathways. Upon activation, TLRs recruit adaptor proteins such as MyD88, initiating
downstream signaling cascades. This leads to the activation of transcription factors
like nuclear factor-kappaB (NF-κB), interferon regulatory factors (IRF3 and IRF7),
and mitogen-activated protein kinases (MAPKs). These factors drive the production of
inflammatory cytokines, facilitating inflammation, immune regulation, cell survival,
proliferation, and even cancer development. TLR4 exemplifies this dual pathway mechanism,
engaging both TRIF and MyD88 adaptors to orchestrate a comprehensive immune response [24].
These signaling cascades underscore the complexity of TLR-mediated immune responses,
linking innate immunity to broader physiological and pathological processes. TLR4 receptors
recognize bacterial components like lipopolysaccharides (LPS), and when the gut barrier
is compromised due to dysbiosis, these microbial products can enter the bloodstream,
triggering systemic inflammation [25] as shown in Table 1.
Table 1.
Overview of TLR activation, signaling pathways, and gut modulation in inflammaging.
| 1.TLR activation and localization | |
|---|---|
| Surface TLRs (e.g., TLR4, TLR5) | Detect extracellular microbial components, such as LPS from gram-negative bacteria. |
| Intracellular TLRs (e.g., TLR3, TLR7) | Recognize viral nucleic acids and intracellular microbial products. |
| 2. Key pathways in TLR signaling | |
| a. MyD88-dependent pathway | |
| Step 1: Receptor activation | TLRs recruit MyD88 adaptor protein via Toll/IL-1 receptor (TIR) domains. MyD88 recruits TIRAP to stabilize the complex. |
| Step 2: Downstream signaling cascade | Activation of IRAK family kinases (e.g. , IRAK4 phosphorylates IRAK1). TRAF6 (TNF receptor-associated factor 6) is recruited and facilitates K63-linked polyubiquitination. |
| Step 3: Activation of NF-κB and MAPKs | TAK1 phosphorylates the IKK complex (IKKα, IKKβ, NEMO). IκBα degradation releases NF-κB, which translocates to the nucleus to induce cytokines (IL-6, TNF-α, IL-1β). MAPKs (ERK, JNK, p38) amplify responses. |
| b. TRIF-dependent pathway | |
| Step 1: Adaptor recruitment | For TLR4, LPS activation triggers MyD88-dependent signaling first. TRIF is later recruited via TRAM. |
| Step 2: Downstream activation | TRIF interacts with TRAF3 to activate TBK1 and IKKε, which phosphorylate IRF3. IRF3 induces Type I interferons (e.g., IFN-β). TRIF also links to NF-κB activation via TRAF6. |
| 3. Gut modulation and its impact on TLR signaling | |
| LPS | Dysbiosis increases circulating LPS, activating TLR4 and driving chronic NF-κB-mediated inflammation. |
| Role of SCFAs (e.g., Butyrate) | SCFAs stabilize IκBα, inhibiting NF-κB activation. Promote Treg cell differentiation, reducing pro-inflammatory cytokines. |
| Probiotics and prebiotics | Akkermansia muciniphila and Lactobacillus plantarum improve mucosal integrity, reducing LPS translocation and systemic inflammation. Dietary fibers enhance SCFA production. |
LPS, a major component of Gram-negative bacterial cell walls, serve as potent inducers
of inflammatory responses. Acting through TLRs and the NF-κB signaling pathway, LPS
triggers the production of inflammatory mediators and activates the innate immune
system [26]. The gut microbiota is a primary source of LPS. Under normal circumstances,
a functional intestinal barrier prevents harm by maintaining low circulating levels of
LPS, as observed in healthy individuals [27, 28].
However, in pathological conditions, the intestinal barrier may lose its integrity,
leading to increased permeability and heightened translocation of LPS produced by gut
bacteria into the bloodstream. Historically, a leaky gut was thought to arise solely
as a consequence of specific diseases. Recent studies, however, suggest that increased
intestinal permeability may play a causative role in the development of certain pathological
conditions, rather than being merely a secondary effect [29]. This paradigm shift highlights
the importance of intestinal barrier integrity in maintaining systemic health.
Elevated levels of circulating LPS and pro-inflammatory cytokines (such as IL-6 and TNF-α)
serve as biomarkers for inflammaging. Monitoring these biomarkers can help clinicians
evaluate the inflammatory status of elderly patients and guide interventions, such as
the use of omega-3 fatty acids or curcumin, which have anti-inflammatory properties.
Restoring gut barrier integrity through dietary interventions or probiotics (e.g.,
Lactobacillus plantarum or Akkermansia muciniphila) may help reduce systemic inflammation
in aging populations [30].
Increasing SCFA production through dietary fiber supplementation or the use of
specific probiotics is being studied as a clinical strategy to reduce chronic inflammation.
High-fiber diets and SCFA-producing probiotics have demonstrated benefits in treating
inflammatory conditions like ulcerative colitis, in experimental model [31]. Abdin AA et al.
evaluated Lactobacillus acidophilus in an oxazolone-induced colitis model in rats. Results
showed significant reductions in inflammatory markers (CRP, TNF-α, IL-6) and disease
activity index (DAI) with L. acidophilus alone or combined with olsalazine, with the
combination proving most effective [31]. These findings suggest L. acidophilus as a
promising adjunct therapy for UC, warranting further clinical validation in humans.
Interventions that enhance SCFA production, such as the use of butyrate-producing
bacterial strains or dietary fibers, may help restore gut barrier function and reduce
microbial translocation, further reducing systemic inflammation [32]. Probiotics like
Akkermansia muciniphila, which enhance mucosal barrier function, are being evaluated
for their potential to prevent gut barrier dysfunction, reduce microbial translocation,
and lower systemic inflammation in the elderly [33]. Experimental studies have shown
that Akkermansia muciniphila treatment alleviates mucosal inflammation by enhancing
gut barrier function, reducing inflammatory cytokines, and improving microbial community
balance [34, 35]. These findings highlight A. muciniphila as a promising probiotic
candidate for managing colitis.
Gut dysbiosis and its role in age-associated diseases
Gut dysbiosis, the imbalance of the gut microbiota, is a contributing factor in
age-related diseases such as metabolic syndrome, cardiovascular diseases,
and cancer [36]. Elderly individuals often show a reduction in beneficial
bacteria like Bifidobacteria and Lactobacilli, while harmful bacteria like
Enterobacteriaceae are more prevalent [36]. This phenomenon extends to various
dietary components, such as non-digestible carbohydrates and dietary proteins
that reach the colon. These substances can significantly influence bacterial
composition and the microbial production of SCFAs alongside potentially harmful
metabolites like hydrogen sulfide (H2S), p-cresol, phenol, and ammonia (NH3).
Non-absorbed dietary iron, including iron supplements, also impacts the microbiota,
while dietary phenolic compounds may serve as prebiotics [37]. The effects of dietary
lipids on the gut microbiota remain poorly understood; however, animal studies have
shown that high-fat diets (exceeding physiological norms) can induce gut dysbiosis.
Interestingly, research by Hildebrandt et al. demonstrated that rats fed a high-fat
diet developed gut dysbiosis independently of their obese phenotype. Significant
alterations in gut microbiota were observed following a switch to a high-fat diet,
including a reduction in Bacteroidetes and an increase in both Firmicutes and
Proteobacteria. These changes were consistent across different genotypes, regardless
of the presence or absence of obesity. This suggests that the high-fat diet itself,
rather than the obese state, is primarily responsible for the observed shifts in gut
microbial composition [38].
Food additives, such as low-calorie sweeteners and emulsifiers, have been shown to
disrupt the gut microbiota, increasing its potential virulence and contributing to
metabolic disorders in both animals and humans [39]. Non-caloric artificial
sweeteners (NAS) have been shown to contribute to glucose intolerance by inducing
significant compositional and functional changes in the intestinal microbiota.
These adverse metabolic effects driven by NAS consumption can be reversed through
antibiotic treatment and are fully transferable to germ-free mice via fecal microbiota
transplantation from NAS-consuming mice or microbiota anaerobically incubated with NAS.
Microbial metabolic pathways altered by NAS correlates with increased host susceptibility
to metabolic diseases [40]. In mice, low concentrations of commonly used emulsifiers,
carboxymethylcellulose and polysorbate-80, were found to induce low-grade inflammation
and metabolic syndrome, as well as exacerbate colitis in genetically predisposed
hosts [41]. These effects were linked to microbiota alterations, including encroachment
on the intestinal mucosa, changes in species composition, and heightened pro-inflammatory
activity. Chaissang B et al. using germ-free mice and fecal transplants demonstrated
that these microbiota alterations were both necessary and sufficient to trigger
inflammation and metabolic syndrome [41].
The gut microbiota are highly responsive to external influences, including drugs,
diet, and environmental pollutants. Factors such as antibiotics, heavy metals,
persistent organic pollutants, pesticides, nanomaterials, and food additives can
significantly alter the composition and functionality of the gut microbial community,
potentially disrupting its balance and contributing to health disorders [42].
The key factors influencing these shifts in microbial composition and diversity
during aging, the mechanisms involved, and their potential impacts are depicted
in Table 2.
Table 2.
Shifts in microbial composition and diversity during aging, the mechanisms involved, and their potential impacts.
| Key factor | Observations | Causes | Implications |
|---|---|---|---|
| Reduction in beneficial bacteria | Decline in Bifidobacteria and Lactobacilli. | Immunosenescence weakens microbial homeostasis. | Increased prevalence of potentially harmful bacteria like Enterobacteriaceae. |
| Increase in Enterobacteriaceae species. | Reduced dietary fiber and prebiotic-rich food intake. | Reduced microbial diversity and beneficial metabolite production. | |
| Decreased gut motility slows intestinal transit time, encouraging pathogen overgrowth. | |||
| Impact of diet on microbial composition | Non-digestible carbohydrates foster SCFA production (butyrate, propionate, acetate). | Lack of undigested carbohydrates limits bacterial fermentation. | SCFAs improve gut barrier integrity and reduce inflammation. |
| Undigested proteins produce harmful compounds (H2S, p-cresol, phenol, NH3). | Excess dietary protein leads to toxic metabolite production. | Toxic compounds contribute to inflammation and systemic toxicity. | |
| High-fat diets induce dysbiosis, reducing Bacteroidetes and increasing Firmicutes. | High dietary fat, independent of obesity, alters microbial balance. | Dysbiosis exacerbates metabolic inflammation. | |
| Phenolic compounds from fruits and teas support beneficial bacteria. | Lack of phenolic-rich diets diminishes prebiotic fermentation. | Reduced microbial support for health. | |
| Iron supplements promote pathogenic bacterial growth. | Excess iron shifts microbial equilibrium. | Altered microbiota composition and inflammation. | |
| Food additives and gut microbiota | NAS consumption induces glucose intolerance by altering microbiota metabolic pathways. | Dysbiosis induced by NAS is transferable via microbial transplantation. | Metabolic disorders and systemic inflammation. |
| Emulsifiers disrupt the mucosal barrier and promote low-grade inflammation. | Additives alter microbial composition and increase bacterial encroachment. | Exacerbated colitis, metabolic syndrome, and barrier dysfunction. | |
| External influences on microbiota | Antibiotic overuse disrupts microbial diversity. | Environmental pollutants (heavy metals, pesticides) affect microbiota functionality. | Increased prevalence of opportunistic pathogens and dysbiosis. |
| PPIs and NSAIDs alter microbial composition. | Drug-microbiota interactions reduce diversity and increase disease risk. | Heightened susceptibility to inflammation and gut-related disorders. | |
| Loss of microbial diversity | Aging reduces overall microbial diversity. | Chronic inflammation alters gut environment. | Correlation with frailty, inflammation, and poor health outcomes. |
| Decreased SCFA production reduces beneficial metabolites. | Physical inactivity decreases microbial richness. | Reduced regulatory T-cell differentiation and barrier integrity. | |
| Metabolic and immune consequences | Pro-inflammatory state ("leaky gut") increases systemic inflammation. | Microbial metabolites (e.g., LPS) enter circulation. | Inflammaging and age-associated diseases. |
| Reduced SCFAs impair gut barrier function and anti-inflammatory pathways. | Loss of beneficial bacteria limits SCFA production. | Weakened gut barrier and increased infection susceptibility. |
Cardiovascular disease
Intestinal bacteria generate various bioactive metabolites, which can be absorbed
into the enterohepatic circulation and subsequently enter the systemic circulation,
thereby influencing host physiology either directly or indirectly [43]. Trimethylamine
N-oxide (TMAO) produced by the microbial metabolism of dietary choline and carnitine,
has been linked to an increased risk of cardiovascular diseases (CVDs) like atherosclerosis,
myocardial infarction, and stroke [44]. Elevated TMAO levels promote endothelial dysfunction
and atherosclerotic plaque formation, contributing to the development of these conditions.
TMAO is also implicated in increased all-cause mortality [45].
Dietary nutrient exposure influences host physiology through both metabolism-dependent
and metabolism-independent mechanisms mediated by the gut microbiota. Metabolism-dependent
pathways include: (1) microbial fermentation of dietary carbohydrates to produce SCFAs,
which enhance host energy expenditure, inhibit histone deacetylase (HDAC) activity,
and activate G-protein-coupled receptor (GPCR) signaling; (2) microbial conversion of
primary bile acids into secondary bile acids, promoting brown adipose tissue (BAT)
activation, energy expenditure, insulin sensitivity, and reduced inflammation; and (3)
microbial transformation of choline and L-carnitine into trimethylamine (TMA), which the
host flavin monooxygenase (FMO) system converts to TMAO, a compound linked to heightened
CVD risk through altered cholesterol transport and macrophage activation [46].
Metabolism-independent effects arise from gut hyperpermeability, enabling translocation
of bacterial components such as LPS and peptidoglycans into the bloodstream. These
circulating microbial-derived molecules activate macrophages, contributing to impaired
reverse cholesterol transport, insulin resistance, hyperlipidemia, and vascular inflammation.
Together, the metabolism-dependent and independent actions of the gut microbiota form a
complex endocrine network that modulates the risk of atherosclerotic CVD, including
myocardial infarction, stroke, and associated mortality [46].
Recent clinical research has focused on leveraging the microbiome to assess cardiovascular
risk. TMAO is emerging as a valuable biomarker that could help clinicians identify
individuals at higher risk of developing CVDs [47, 48]. When combined with other markers
viz., carnitine, creatinine, choline and betaine TMAO levels can aid in the early
detection and prognosis of cardiovascular diseases [48]. Additionally, interventions
targeting the gut microbiota, such as dietary modifications and the use of specific
probiotics, have shown promise in reducing the production of TMAO and improving lipid
profiles [49].
Dietary polyphenols can modulate the gut microbiota, influencing host metabolism through
their interaction with microbial communities. The gut microbiota, in turn, metabolizes
polyphenols into bioactive, low-molecular-weight phenolic compounds that contribute to
the host’s regulatory and metabolic pathways. These compounds play a significant role
in shaping the composition and functionality of the intestinal microbiota. Polyphenols
selectively promote the growth of beneficial microorganisms, such as Lactobacillus and
Bifidobacterium, which have the potential to ameliorate inflammation [50].
In a study by Koeth RA et al., the potential of gut microbial modulation as a strategy
to reduce TMAO production, thereby mitigating CVD risk was highlighted. The research
revealed that individuals with a gut microbiota dominated by Prevotella exhibited
higher circulating TMAO levels compared to those with a Bacteroides-enriched enterotype.
Furthermore, omnivorous participants with elevated plasma TMAO concentrations had an
increased abundance of Peptostreptococcaceae and Clostridium and a reduced prevalence
of Lachnospira compared to vegan or vegetarian individuals, who demonstrated lower
TMAO levels [51]. Moreover, postbiotics, which are metabolites produced by probiotics,
are being explored as an innovative therapeutic strategy for modulating cardiovascular
inflammation, with promising results emerging from early clinical trials [52].
Neurodegenerative diseases: Alzheimer’s and Parkinson’s
Microbial dysbiosis not only contributes to systemic inflammation, but also
affects the brain through the gut-brain axis. This communication pathway
links the gut microbiota with the central nervous system (CNS), influencing
neuroinflammation, cognitive function, and the progression of neurodegenerative
diseases like Alzheimer’s and Parkinson’s. Alterations in gut microbiota
composition can activate microglia, the brain’s immune cells, contributing to
neuroinflammation and cognitive decline [53]. Dysbiosis—the imbalance of the gut
microbiota—along with increased gut permeability, contributes to systemic inflammation
and neuroinflammation, two key drivers of neurodegenerative disease progression.
This connection is further complicated by the gut-brain axis, a bidirectional communication
system linking the central nervous system and the gastrointestinal tract [54].
The gut microbiota metabolize dietary elements such as macronutrients, micronutrients,
fiber, and polyphenols into various bioactive compounds, including short-chain fatty
acids, trimethylamines, amino acid derivatives, and vitamins. These metabolites,
along with the dietary components, play critical roles in metabolic and signaling
processes, influencing host homeostasis, including the integrity of the blood-brain
barrier (BBB) and overall brain function [55]. SCFAs can influence brain neurotransmission
by regulating the expression and functionality of neurotransmitters and their receptors.
Among SCFAs, butyrate is particularly noteworthy for its significant impact on neuronal
activity. It has been shown to modulate key neurotransmitters such as glutamate,
the brain's primary excitatory neurotransmitter involved in synaptic plasticity
and memory, and GABA, the principal inhibitory neurotransmitter that maintains the
balance between neuronal excitation and inhibition [56]. Butyrate’s effects on the
glutamatergic system include regulating glutamate receptor expression and neurotransmitter
release, thereby influencing neuronal excitability and synaptic signaling [57]. Similarly,
its role in GABA-mediated transmission underscores its importance in neural stability and
cognitive function.
Furthermore, butyrate inhibits HDACs, promoting histone acetylation, which relaxes
chromatin structure and enhances gene transcription, including the upregulation of
brain-derived neurotrophic factor (BDNF). Increased BDNF expression supports neurogenesis,
the generation of new neurons from neural stem cells, and enhances synaptic
plasticity [58, 59]. These processes contribute to improved learning and memory
capabilities and offer promising therapeutic potential for addressing neurodegenerative
disorders.
Preclinical studies have shown that gut dysbiosis can accelerate beta-amyloid deposition
in the brain and worsen cognitive impairment in Alzheimer’s disease (AD) [60, 61]. Guillemin
GJ et al. explored the impact of quinolinic acid, a tryptophan-derived metabolite, on AD.
The results revealed that elevated levels of quinolinic acid exacerbated neuroinflammation
by increasing microglial activation and astrogliosis, hallmarks of brain inflammation [61].
This study highlights quinolinic acid as a key factor in AD progression and underscores
its potential as a target for therapeutic intervention.
Probiotic treatment in mice has been shown to enhance spatial memory and reduce hippocampal
plaque accumulation [62]. Supplementation also improved synaptic plasticity and restored
long-term potentiation in amyloid-beta (Aβ)-treated mice [63]. Interventions targeting
the gut microbiota, such as prebiotics and probiotics, may improve cognitive function
and slow the progression of neurodegenerative diseases in older adults [64]. In humans,
a clinical trial reported that probiotics improved cognitive function in Alzheimer's
disease (AD) patients and positively influenced plasma biomarkers, including malondialdehyde
and serum triglycerides [65].
Patients with Parkinson’s disease (PD) often experience intestinal dysbiosis, marked by
a decrease in SCFA-producing bacteria and an increase in proinflammatory
microbial species [66]. This imbalance can disrupt the production of metabolites that
regulate inflammation both systemically and in the brain. A reduction in butyrate
levels may play a role in the chronic activation of microglia, a hallmark of PD pathology
that contributes to neurodegeneration. This sustained microglial activation is thought
to aggravate neuronal damage, accelerating disease progression [67].
Chang et al. investigated metabolic profile changes, focusing on kynurenine metabolism
in the plasma of PD patients. Their findings suggest that these metabolic alterations
may influence disease progression by modulating neuroinflammatory pathways [68].
Shao et al. highlighted advancements in metabolomics, emphasizing that preclinical
studies have revealed notable alterations in metabolites such as lactate and SCFAs.
These metabolites are of particular interest due to their potential contribution
to mitochondrial dysfunction and neuroinflammation, both key features of PD [69].
In clinical settings, therapeutic modulation of the gut-brain axis is gaining
traction as a potential strategy for managing neurodegenerative diseases. Clinical
trial investigating the use of probiotics supplementation containing: Bifidobacterium
bifidum, Lactobacillus casei, Lactobacillus fermentum and Lactobacillus acidophilus
have shown potential in slowing cognitive decline in patients with mild cognitive
impairment, a precursor to AD, suggesting that gut microbiota modulation could delay
AD progression [65]. Similarly, analysis of mucosal and stool samples from individuals
with PD revealed significant down-regulation of several genes in the stool microbiota,
alongside substantial alterations in microbial composition. Specifically, an increase
in bacteria such as Proteobacteria, Betaproteobacteria, Coprococcus, Blautia, Akkermansia,
Oscillospira, Roseburia and Bacteroides was observed. Conversely, there was a notable
decrease in beneficial bacterial groups like Faecalibacterium, Firmicutes, and members
of the class Clostridia. These microbial imbalances may play a role in PD pathophysiology,
potentially influencing gut-brain signaling and contributing to disease progression [70].
Metabolic disorders
The gut microbiota plays a crucial role in regulating metabolism,
and dysbiosis has been implicated in several metabolic disorders,
including type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver
disease (NAFLD), and obesity [71]. One of the key mechanisms by which
the microbiota influences metabolic health is through the process of
metabolic endotoxemia [72]. This occurs when microbial products such
as LPS translocate from the gut into the bloodstream, triggering chronic
low-grade inflammation and insulin resistance.
In clinical practice, microbiota-based therapies are being explored as
adjuncts to conventional treatments for metabolic disorders. Several
studies explored multi-strain probiotic formulations, such as
Sabico et al. which showed significant improvements in HOMA-IR after
6 months of probiotic supplementation [73]. Other studies, such as
Mafi et al., who utilized Lactobacillus, Bifidobacterium and Streptococcus and,
Razmpoosh et al., using Lactobacillus with Bifidobacterium reported
improvements in cardiometabolic risk factors and fasting plasma
glucose [74, 75]. Perraudeau et al. noted that a five-strain probiotic
formulation reduced postprandial glucose levels but did not significantly
affect weight or HOMA-IR [76]. Jiang et al. found that probiotic
supplementation improved glycemic control in T2DM patients with diabetic
nephropathy, underlining the potential of probiotics in managing
diabetes-related complications [77]. In another study, Toejing et al. found
that Lactobacillus paracasei HII01 supplementation reduced inflammatory
markers and hyperglycemia, potentially by modulating the gut microbiota and
treating endotoxemia, which suggests a promising role as an adjunctive
therapy [78]. Kumar et al. explored the impact of probiotics as an adjunct
therapy to metformin, finding reductions in fasting blood glucose (FBG),
postprandial glucose, and HbA1c levels compared to metformin alone, although
the overall efficacy of probiotics in this combination treatment was not
strongly substantiated [79].
Other studies also assessed the effects of different probiotic strains,
with varying results. Chen et al. reported that the combination of metformin
with a blend of multiple probiotics resulted in a more pronounced hypoglycemic
effect, likely through modulation of the gut microbiota and bile acid
metabolism [80]. Hasanpour et al. highlighted that a combination of
soymilk and probiotics improved cardiovascular risk factors in T2DM patients,
though no significant effects were observed on FBG or HOMA-IR [81].
Microbiota modulation as a strategy to combat inflammaging
The gut microbiota represents a promising therapeutic target for mitigating inflammaging, as its composition and functionality profoundly influence host immune responses and metabolic health. Several microbiota modulation strategies, including dietary interventions, probiotics, prebiotics, synbiotics, and fecal microbiota transplantation (FMT), have been explored for their potential to combat inflammaging. Below, we discuss these strategies and their underlying mechanisms.
Dietary interventions
Dietary fibers, primarily composed of non-digestible carbohydrates,
significantly influence the gut microbiota by serving as substrates
for microbial fermentation. The molecular mechanisms underlying the
beneficial effects of high-fiber diets on gut microbiota composition
and systemic inflammation involve several interconnected pathways [82, 83].
Dietary fiber, though resistant to digestion in the upper gastrointestinal
tract, undergoes fermentation in the colon by specific bacterial taxa,
such as Bifidobacterium and Lactobacillus species. These microbes possess
specialized enzymes, including glycoside hydrolases and polysaccharide
lyases, which break down complex carbohydrates into fermentable monosaccharides.
This process yields SCFAs, including acetate, propionate, and butyrate,
which play pivotal roles in maintaining gut health and modulating inflammation.
These commensal microbes compete with pathogenic species through mechanisms
like antimicrobial peptide production and acidification of the colonic
environment via lactic acid and SCFA production [84].
The immune system also benefits significantly from fiber-rich diets.
SCFA-mediated signaling suppresses systemic inflammation by reducing
the levels of pro-inflammatory cytokines like interleukin-6 (IL-6) and
tumor necrosis factor-alpha (TNF-α). These effects are achieved through
inhibition of inflammatory transcription factors such as NF-κB in immune
cells. SCFAs also serve as epigenetic regulators, modulating histone
acetylation and methylation patterns to influence the transcription of
genes involved in inflammatory responses [46, 58]. High-fiber diets
affect microbial tryptophan metabolism, leading to the production of
indole derivatives that activate the aryl hydrocarbon receptor (AhR).
This receptor plays a crucial role in modulating intestinal immunity
and inflammation, highlighting the broader implications of fiber
fermentation in systemic health [85].
Enhanced microbial production of butyrate has also been linked to
improved insulin sensitivity and lipid metabolism, highlighting its
potential in mitigating systemic metabolic dysfunction [80]. These
interconnected mechanisms underscore the critical role of dietary
fiber and its fermentation products in maintaining overall health
and preventing disease.
Probiotics
Probiotic supplementation, particularly with specific strains such as Lactobacillus rhamnosus, Lactobacillus casei, and Bifidobacterium longum, has gained prominence as a therapeutic strategy for modulating gut microbiota composition and alleviating inflammation in aging populations. These effects are mediated through intricate molecular mechanisms involving immune modulation, enhancement of the intestinal barrier, and alterations in microbial metabolite production.
Immune modulation by probiotic strains
Probiotic strains play a critical role in modulating the immune system by interacting with the gut-associated lymphoid tissue (GALT) and influencing both innate and adaptive immune responses. This interaction is mediated through specific molecular pathways, including pattern recognition receptors (PRRs). Probiotics, such as Lactobacillus rhamnosus, engage TLRs and NOD-like receptors (NLRs) on intestinal epithelial and immune cells [86]. For instance, binding to TLR-2 triggers MyD88-dependent NF-κB signaling pathways, resulting in the controlled production of anti-inflammatory cytokines like IL-10, while suppressing pro-inflammatory cytokines such as IL-6 and TNF-α. Additionally, probiotics influence dendritic cells by inducing tolerogenic phenotypes, which, in turn, promote the differentiation of Tregs. Tregs contribute to immune homeostasis by secreting anti-inflammatory mediators, including IL-10 and TGF-β, thereby reducing chronic inflammation, a hallmark of aging [86].
Enhancement of intestinal barrier function
Probiotic strains also strengthen the integrity of the intestinal barrier, which tends to weaken with age, leading to increased systemic inflammation [87]. One mechanism involves the upregulation of tight junction proteins, such as occludin, claudin, and zonula occludens-1 (ZO-1) [84]. For example, Bifidobacterium longum enhances ZO-1 expression via the PI3K/Akt signaling pathway, thereby decreasing epithelial permeability and preventing the translocation of endotoxins [88]. Furthermore, probiotics stimulate goblet cells to increase mucin production, particularly MUC2, which enhances the protective mucus layer on the epithelial surface and limits microbial invasion. By fortifying the gut barrier, probiotics reduce endotoxemia by minimizing lipopolysaccharide (LPS) translocation, which otherwise triggers systemic inflammation through TLR-4 signaling [84].
Microbial metabolite modulation
Probiotics contribute to the production of beneficial microbial metabolites with anti-inflammatory properties. Notably, they enhance the generation of SCFAs such as acetate, propionate, and butyrate by fostering the growth of SCFA-producing bacteria. These SCFAs act as ligands for G-protein-coupled receptors, including GPR43 and GPR109A, which inhibit NF-κB signaling and suppress the release of pro-inflammatory cytokines [46, 58, 59]. Additionally, probiotics influence tryptophan metabolism, leading to the formation of indole derivatives that activate the aryl hydrocarbon receptor (AhR) pathway. This activation supports mucosal homeostasis and reduces inflammation [85].
Prebiotics
Prebiotics, such as inulin, fructooligosaccharides (FOS), and galactooligosaccharides (GOS), are non-digestible food components that selectively stimulate the growth and activity of beneficial gut microbiota, including Bifidobacterium and Lactobacillus species. Their fermentation in the colon yields metabolites like SCFAs, which exert both local and systemic effects that contribute to anti-inflammatory and metabolic benefits. These mechanisms make prebiotics a promising intervention for addressing dysbiosis and chronic low-grade inflammation associated with aging, known as inflammaging [84].
Synbiotics
Synbiotics combine prebiotics and probiotics to provide synergistic benefits. This approach not only stimulates the growth of beneficial bacteria but also enhances their activity and survival in the gastrointestinal tract. Synbiotics also exhibit potential in reducing gut permeability, a hallmark of chronic inflammation associated with aging [84, 89]. The synergistic mechanism of action and outcomes of synbiotics is shown in Table 3.
Table 3.
The synergistic mechanism of action and outcomes of synbiotics.
| Mechanism | Key actions | Outcomes |
|---|---|---|
| Enhanced growth and activity of beneficial bacteria |
• Prebiotics (inulin, FOS, GOS) selectively fuel probiotic strains like Lactobacillus and Bifidobacterium.
• Amplifies metabolic activity of probiotics, increasing SCFA production. |
Impaired protein folding, decreased chaperone function (HSP70, HSP90), and defective autophagy. |
| Improved gut barrier integrity |
• Probiotics secrete antimicrobial peptides and regulate tight junction
proteins (e.g., occludin, claudin).
• Prebiotics enhance the mucus layer by stimulating goblet cells for mucin production. |
Decreased chaperone activity, proteasome dysfunction and reduced autophagic activity. |
| Anti-inflammatory effects |
• Downregulates NF-κB signaling, reducing pro-inflammatory cytokines
(e.g., TNF-α, IL-6).
• SCFAs act as HDAC inhibitors, promoting anti-inflammatory epigenetic changes. |
Impaired protein quality control (UPS and autophagy), reduced mitochondrial function, and accumulation of protein aggregates. |
| Modulation of metabolic pathways |
• Enhances insulin sensitivity and lipid metabolism via SCFA-mediated
GPR activation.
• Reduces endotoxemia and LPS-induced inflammation by improving gut permeability. |
Improves glycemic control, lipid profiles and metabolic health. |
Fecal microbiota transplantation (FMT)
FMT is an innovative therapeutic approach involving the transfer of stool from a
healthy donor to a recipient to restore microbial diversity and functionality in
the gut. This method, initially established for its efficacy in treating Clostridioides
difficile infections, is now gaining traction as a potential intervention to mitigate
inflammaging [89]. In the context of aging, where dysbiosis is often driven by dietary
alterations, medication use, and age-related physiological changes, FMT offers a
promising avenue for re-establishing microbial homeostasis and alleviating its downstream
effects on health.
The primary mechanism underlying the benefits of FMT lies in its ability to restore
microbial diversity. Aging is frequently accompanied by a decline in beneficial
gut microbial populations, such as Bifidobacterium and Faecalibacterium, coupled
with an overgrowth of pro-inflammatory species. The reintroduction of a balanced
microbial community through FMT promotes ecological stability and re-establishes
a functional microbiota capable of supporting host health. This restoration is
particularly crucial in aging populations, where microbial diversity is a determinant
of gut and systemic health [89].
Another critical benefit of FMT is its ability to enhance gut barrier integrity.
The introduction of beneficial bacteria through FMT stimulates the production of
SCFAs, such as butyrate, which strengthen intestinal tight junctions and enhance
mucus production. Improved gut barrier function reduces intestinal permeability,
a phenomenon that often leads to endotoxin translocation and systemic inflammation
in aging populations. By reinforcing gut barrier integrity, FMT helps mitigate the
cycle of dysbiosis, inflammation, and metabolic disruption [90].
By reinstating beneficial bacteria, FMT suppresses the overgrowth of pathogenic
microbes that release inflammatory triggers like LPS. These transferred microbes
modulate inflammatory pathways, particularly by downregulating NF-κB signaling,
thereby reducing the production of pro-inflammatory cytokines such as TNF-α and IL-6.
The resulting anti-inflammatory effects are crucial for mitigating chronic low-grade
inflammation that contributes to age-associated diseases [89, 91].
Moreover, FMT has shown promise in modulating the gut-liver axis, a critical interface
affected by dysbiosis in aging. Dysbiosis disrupts bile acid metabolism, exacerbating
systemic inflammation and metabolic disturbances. FMT restores the balance of bile
acid-metabolizing bacteria, thereby regulating bile acid signaling pathways involved
in lipid metabolism and inflammatory responses. This modulation of the gut-liver axis
is particularly relevant for addressing metabolic disorders commonly associated with
aging [91].
Emerging evidence also suggests that FMT has beneficial effects on the gut-brain axis,
which is intricately linked to aging and neuroinflammation. Dysbiosis in the elderly
has been associated with cognitive decline and neurodegenerative diseases such as
Alzheimer’s and Parkinson’s. By influencing microbial composition and reducing systemic
inflammation, FMT may help mitigate neuroinflammation and improve cognitive outcomes
in these populations [89].
FMT represents a promising therapeutic strategy to address the systemic inflammation,
metabolic disturbances, and cognitive decline associated with aging. While existing
evidence is encouraging, further research is essential to refine treatment protocols,
optimize donor selection, and ensure long-term safety and efficacy. These advancements
could pave the way for the broader application of FMT in addressing the challenges
posed by inflammaging.
Conclusions
The relationship between microbiota and inflammaging is a rapidly evolving field with significant clinical implications. Microbiota-targeted interventions could reduce inflammation, improve metabolic health, and promote longevity. Integrating microbiome profiling into clinical practice will enable personalized treatments that address the underlying causes of inflammaging and related diseases. The convergence of microbiota science and aging research holds promise for enhancing immune function and reducing chronic disease burdens in older adults. Aging is often associated with microbial dysbiosis, linked to diseases like obesity, metabolic syndrome, cardiovascular disease, and neurodegeneration. Dysbiosis contributes to metabolic disturbances, insulin resistance, and cardiovascular risk through inflammation and harmful microbial metabolites such as TMAO. Restoring microbial balance through diet or probiotics shows promise in improving metabolic health and reducing cardiovascular risk. Furthermore, therapies like FMT are being explored for neurodegenerative diseases, aiming to restore microbial balance, reduce neuroinflammation, and slow disease progression in aging individuals.
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Not applicable.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, & Rocha-Santos T. A synopsis on aging-theories, mechanisms and future prospects. Ageing res rev, 2016, 29: 90-112. [Crossref]
2. Maldonado E, Morales-Pison S, Urbina F, & Solari A. Aging hallmarks and the role of oxidative stress. Antioxidants, 2023, 12(3): 651-661. [Crossref]
3. Guarente L. Aging research-where do we stand and where are we going? Cell, 2014, 159(1): 15-19. [Crossref]
4. Ruparelia N, Chai JT, Fisher EA, & Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol, 2017, 14(5): 314-324. [Crossref]
5. Leonardi GC, Accardi G, Monastero R, Nicoletti F, & Libra M. Ageing: from inflammation to cancer. Immun Ageing, 2018, 15: 1-10. [Crossref]
6. Fabbri E, An Y, Zoli M, Simonsick EM, Guralnik JM, Bandinelli S, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci, 2015, 70(1): 63-70. [Crossref]
7. Ferrucci L, & Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol, 2018, 15(9): 505-522. [Crossref]
8. Straub RH, & Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health, 2016(1): 37-51. [Crossref]
9. Andonian BJ, Hippensteel JA, Abuabara K, Boyle EM, Colbert JF, Devinney MJ, et al. Inflammation and aging-related disease: a transdisciplinary inflammaging framework. Geroscience, 2024: 01364. [Crossref]
10. Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, et al. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp, 2016, 64(2): 111-126. [Crossref]
11. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2018, 9(6): 7204-7218. [Crossref]
12. Caldarelli M, Rio P, Marrone A, Giambra V, Gasbarrini A, Gambassi G, et al. Inflammaging: the next challenge-exploring the role of gut microbiota, environmental factors, and sex differences. Biomedicines, 2024, 12(8): 1716-1726. [Crossref]
13. Hohman LS, & Osborne LC. A gut-centric view of aging: do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging cell, 2022, 21(9): e13700. [Crossref]
14. Cuollo L, Antonangeli F, Santoni A, & Soriani A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology, 2020, 9(12): 485-495. [Crossref]
15. Zhao M, Chu J, Feng S, Guo C, Xue B, He K, et al. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother, 2023, 164: 114985. [Crossref]
16. Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, et al. Source of chronic inflammation in aging. Front Cardiovasc Med, 2018, 5: 12-22. [Crossref]
17. Indellicato R, & Trinchera M. Epigenetic regulation of glycosylation in cancer and other diseases. Int J Mol Sci, 2021, 22(6): 2980-2990. [Crossref]
18. Amabebe E, Robert FO, Agbalalah T, & Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr, 2020, 123(10): 1127-1137. [Crossref]
19. Guigoz Y, Doré J, & Schiffrin EJ. The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care, 2008, 11(1): 13-20. [Crossref]
20. Soto-Martin EC, Warnke I, Farquharson FM, Christodoulou M, Horgan G, Derrien M, et al. Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. mBio, 2020, 11(4): 00886. [Crossref]
21. Song B, Li P, Yan S, Liu Y, Gao M, Lv H, et al. Effects of dietary astragalus polysaccharide supplementation on the Th17/treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front Immunol, 2022, 13: 781934. [Crossref]
22. Kawai T, & Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity, 2011, 34(5): 637-650. [Crossref]
23. Takeda K, & Akira S. Toll-like receptors. Curr Protoc Immunol, 2015, 109: 14.12.11-14.12.10. [Crossref]
24. Fang Y, Yan C, Zhao Q, Zhao B, Liao Y, Chen Y, et al. The association between gut microbiota, toll-like receptors, and colorectal cancer. Clin Med Insights Oncol, 2022, 16: 11795549221130549. [Crossref]
25. Akira S, Uematsu S, & Takeuchi O. Pathogen recognition and innate immunity. Cell, 2006, 124(4): 783-801. [Crossref]
26. Muzio M, Polntarutti N, Bosisio D, Prahladan MK, & Mantovani A. Toll like receptor family (TLT) and signalling pathway. Eur Cytokine Netw, 2000, 11(3): 489-490.
27. Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr, 2010, 91(4): 940-949. [Crossref]
28. Erridge C, Attina T, Spickett CM, & Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr, 2007, 86(5): 1286-1292. [Crossref]
29. Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One, 2017, 12(3): e0172914. [Crossref]
30. Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther, 2022, 7(1): 135-145. [Crossref]
31. Abdin AA, & Saeid EM. An experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without "olsalazine". J Crohns Colitis, 2008, 2(4): 296-303. [Crossref]
32. Vital M, Howe AC, & Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio, 2014, 5(2): e00889. [Crossref]
33. Rodrigues VF, Elias-Oliveira J, Pereira ÍS, Pereira JA, Barbosa SC, Machado MSG, et al. Akkermansia muciniphila and gut immune system: a good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front Immunol, 2022, 13: 934695. [Crossref]
34. Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol, 2019, 10: 2259-2269. [Crossref]
35. Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (dss)-induced acute colitis by NLRP3 activation. Microbiol Spectr, 2021, 9(2): e0073021. [Crossref]
36. Carding S, Verbeke K, Vipond DT, Corfe BM, & Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis, 2015, 26: 26191. [Crossref]
37. Morales P, Fujio S, Navarrete P, Ugalde JA, Magne F, Carrasco-Pozo C, et al. Impact of dietary lipids on colonic function and microbiota: an experimental approach involving orlistat-induced fat malabsorption in human volunteers. Clin Transl Gastroenterol, 2016, 7(4): e161. [Crossref]
38. Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology, 2009, 137(5): 1716-1724.e1711-1712. [Crossref]
39. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients, 2020, 12(5): 1474-1484. [Crossref]
40. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature, 2014, 514(7521): 181-186. [Crossref]
41. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 2015, 519(7541): 92-96. [Crossref]
42. Jin Y, Wu S, Zeng Z, & Fu Z. Effects of environmental pollutants on gut microbiota. Environ Pollut, 2017, 222: 1-9. [Crossref]
43. Fan Y, & Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol, 2021, 19(1): 55-71. [Crossref]
44. Zhen J, Zhou Z, He M, Han HX, Lv EH, Wen PB, et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol (Lausanne), 2023, 14: 1085041. [Crossref]
45. Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL, et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep, 2017, 7(1): 13781. [Crossref]
46. Brown JM, & Hazen SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med, 2015, 66: 343-359. [Crossref]
47. Zhong Z, Liu J, Zhang Q, Zhong W, Li B, Li C, et al. Targeted metabolomic analysis of plasma metabolites in patients with coronary heart disease in southern China. Medicine, 2019, 98(7): e14309. [Crossref]
48. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J, 2014, 35(14): 904-910. [Crossref]
49. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med, 2018, 24(9): 1407-1417. [Crossref]
50. Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-n-oxide (TMAO)-induced atherosclerosis by regulating tmao synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio, 2016, 7(2): e02210-02215. [Crossref]
51. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med, 2013, 19(5): 576-585. [Crossref]
52. Anhê FF, Jensen BAH, Perazza LR, Tchernof A, Schertzer JD, & Marette A. Bacterial postbiotics as promising tools to mitigate cardiometabolic diseases. J Lipid Atheroscler, 2021, 10(2): 123-129. [Crossref]
53. Ullah H, Arbab S, Tian Y, Liu CQ, Chen Y, Qijie L, et al. The gut microbiota-brain axis in neurological disorder. Front Neurosci, 2023, 17: 1225875. [Crossref]
54. Rao M, & Gershon MD. Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci, 2018, 19(9): 552-565. [Crossref]
55. Parker A, Fonseca S, & Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes, 2020, 11(2): 135-157. [Crossref]
56. Jiang Y, Li K, Li X, Xu L, & Yang Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem Biol Interact, 2021, 341: 109452. [Crossref]
57. Ge X, Zheng M, Hu M, Fang X, Geng D, Liu S, et al. Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J Clin Invest, 2023, 133(4): 154612. [Crossref]
58. Fawad JA, Luzader DH, Hanson GF, Moutinho TJ, Jr., McKinney CA, Mitchell PG, et al. Histone deacetylase inhibition by gut microbe-generated short-chain fatty acids entrains intestinal epithelial circadian rhythms. Gastroenterology, 2022, 163(5): 1377-1390.e1311. [Crossref]
59. Ye Q, Zeng X, Wang S, Zeng X, Yang G, Ye C, et al. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells. Faseb j, 2021, 35(2): e21316. [Crossref]
60. Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, et al. Altered gut microbiota in a mouse model of Alzheimer's disease. J Alzheimers Dis, 2017, 60(4): 1241-1257. [Crossref]
61. Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, & Brew BJ. Quinolinic acid in the pathogenesis of Alzheimer's disease. Adv Exp Med Biol, 2003, 527: 167-176. [Crossref]
62. Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M, et al. Exercise and probiotics attenuate the development of Alzheimer's disease in transgenic mice: role of microbiome. Exp Gerontol, 2019, 115: 122-131. [Crossref]
63. Rezaei Asl Z, Sepehri G, & Salami M. Probiotic treatment improves the impaired spatial cognitive performance and restores synaptic plasticity in an animal model of Alzheimer's disease. Behav Brain Res, 2019, 376: 112183. [Crossref]
64. Shokryazdan P, Faseleh Jahromi M, Navidshad B, & Liang JB. Effects of prebiotics on immune system and cytokine expression. Med Microbiol Immunol, 2017, 206(1): 1-9. [Crossref]
65. Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci, 2016, 8: 256-266. [Crossref]
66. Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, et al. Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov Disord, 2019, 34(3): 396-405. [Crossref]
67. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell, 2016, 167(6): 1469-1480.e1412. [Crossref]
68. Chang KH, Cheng ML, Tang HY, Huang CY, Wu YR, & Chen CM. Alternations of metabolic profile and kynurenine metabolism in the plasma of Parkinson's disease. Mol Neurobiol, 2018, 55(8): 6319-6328. [Crossref]
69. Shao Y, & Le W. Recent advances and perspectives of metabolomics-based investigations in Parkinson's disease. Mol Neurodegener, 2019, 14(1): 3-13. [Crossref]
70. Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord, 2015, 30(10): 1351-1360. [Crossref]
71. Dabke K, Hendrick G, & Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest, 2019, 129(10): 4050-4057. [Crossref]
72. Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, & Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut, 2001, 48(2): 206-211. [Crossref]
73. Sabico S, Al-Mashharawi A, Al-Daghri NM, Wani K, Amer OE, Hussain DS, et al. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr, 2019, 38(4): 1561-1569. [Crossref]
74. Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, & Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct, 2018, 9(9): 4763-4770. [Crossref]
75. Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, & Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr, 2019, 13(1): 175-182. [Crossref]
76. Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care, 2020, 8(1): 1319-1329. [Crossref]
77. Jiang H, Zhang Y, Xu D, & Wang Q. Probiotics ameliorates glycemic control of patients with diabetic nephropathy: a randomized clinical study. J Clin Lab Anal, 2021, 35(4): e23650. [Crossref]
78. Toejing P, Khampithum N, Sirilun S, Chaiyasut C, & Lailerd N. Influence of Lactobacillus paracasei HII01 supplementation on glycemia and inflammatory biomarkers in type 2 diabetes: a randomized clinical trial. Foods, 2021, 10(7): 1455-1465. [Crossref]
79. Kumar VM, Ahmed Z, & Rahman SA. Probiotics efficacy and safety as add-on therapy to metformin in type 2 diabetes mellitus. Indian Journal of Public Health Research & Development, 2022, 13(4): 317-321. [Crossref]
80. Chen Y, Shen X, Ma T, Yu X, Kwok LY, Li Y, et al. Adjunctive probio-X treatment enhances the therapeutic effect of a conventional drug in managing type 2 diabetes mellitus by promoting short-chain fatty acid-producing bacteria and bile acid pathways. mSystems, 2023, 8(1): e0130022. [Crossref]
81. Hasanpour A, Babajafari S, Mazloomi SM, & Shams M. The effects of soymilk plus probiotics supplementation on cardiovascular risk factors in patients with type 2 diabetes mellitus: a randomized clinical trial. BMC Endocr Disord, 2023, 23(1): 36-46. [Crossref]
82. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell, 2021, 184(16): 4137-4153.e4114. [Crossref]
83. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Isme j, 2011, 5(2): 220-230. [Crossref]
84. Roy S, & Dhaneshwar S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: current perspectives. World J Gastroenterol, 2023, 29(14): 2078-2100. [Crossref]
85. Zhao C, Bao L, Qiu M, Feng L, Chen L, Liu Z, et al. Dietary tryptophan-mediated aryl hydrocarbon receptor activation by the gut microbiota alleviates Escherichia coli-induced endometritis in mice. Microbiol Spectr, 2022, 10(4): e0081122. [Crossref]
86. Mazziotta C, Tognon M, Martini F, Torreggiani E, & Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells, 2023, 12(1): 184-194. [Crossref]
87. Mack DR. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients, 2011, 3(2): 245-264. [Crossref]
88. Abdulqadir R, Engers J, & Al-Sadi R. Role of Bifidobacterium in modulating the intestinal epithelial tight junction barrier: current knowledge and perspectives. Curr Dev Nutr, 2023, 7(12): 102026. [Crossref]
89. Baldi S, Mundula T, Nannini G, & Amedei A. Microbiota shaping - the effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: a systematic review. World J Gastroenterol, 2021, 27(39): 6715-6732. [Crossref]
90. Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol, 2018, 3(1): 17-24. [Crossref]
91. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab, 2017, 26(4): 611-619.e616. [Crossref]