Open Access | RESEARCH
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License .
Exploring the neuroprotective potential of an iota-carrageenan in in vitro Parkinson's disease model: evaluation of pharmacological safety, antioxidant properties, and mitochondrial function
* Corresponding author: Ricardo Basto Souza
Mailing address: Department of Biochemistry and Molecular Biology,
Federal, University of Ceará, Avenida Humberto Monte, s/n, 60455-760 Fortaleza, Ceará, Brazil.
E-mail: ricardobastosouza@gmail.com
This article belongs to the Special Issue: Evaluating the effects of natural products on cellular and molecular signaling pathways for the management of neurodegenerative diseases
Received: 22 November 2024 / Revised: 23 December 2024 / Accepted: 30 November 2024 / Published: 28 March 2025
DOI: 10.31491/APT.2025.03.168
Abstract
Background: Parkinson's disease (PD) is a neurodegenerative disorder with a worldwide health impact, characterized by well-established roles of reactive oxygen species, mitochondrial dysfunction, and apoptotic biomarkers. Although various treatments are available for PD patients, they often come with adverse effects, and pharmacological efficacy decreases over time. Sulphated polysaccharides are a class of diverse anionic biopolymers reported to have several pharmacological activities. The present study aimed to assess the in vitro neuroprotective potential of the iota-carrageenan (CSf) isolated from the red alga Solieria filiformis.
Methods: After purification process by precipitation method with cetylpyridinium chloride (CPC), CSf was characterized by yield, free-sulphate content, and gel permeation chromatography analysis. The antioxidant potential was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, hydrogen peroxide (H2O2) radical scavenging activity, Reducing power method, and oxygen radical absorbent capacity (ORAC). Cytotoxicity was evaluated using human neuroblastoma (SH-SY5Y) and Balb/c (3T3) mouse fibroblasts cells. The neuroprotection potential was analyzed by 6-hydroxydopamine (6-OHDA)-induced neurotoxicity model on SH-SY5Y cells.
Results: As expected, CSf revealed about 28% of free-sulphate content and an estimated molar mass of 425 kDa. Despite the low antioxidant capacity exhibited by CSf, it showed the ability to scavenge H2O2. Furthermore, CSf protected SH-SY5Y cells against 6-OHDA induced damage by modulating mitochondrial membrane potential, reducing H2O2 generation, and regulating caspase-3 activity. In addition, no cytotoxic effects were recorded on SH-SY5Y and 3T3 cells, in presence of CSf.
Conclusion: The neuropharmacological effects and safety of CSf suggest its potential for the development of novel therapeutic strategies against PD.
Keywords
6-OHDA, 3T3, bopolymer, caspase-3, H2O2, SH-SY5Y, Solieria filiformis, sulphated polysaccharide
Introduction
Parkinson’s disease (PD) is a second most frequent neurodegenerative
disorder, which is characterized mainly by a progressive loss of
catecholaminergic neurons [1]. Because of its multifactorial
pathogenesis, PD origin is still unclear, no effective cure
is currently available, and its treatment remains a challenge
as well [2]. However, in vitro neurodegenerative cellular
models have shown the involvement of oxidative stress,
mitochondrial dysfunction and apoptotic pathway activation
in PD pathogenesis [3, 4]. Among those experimental models,
researchers have frequently used the neurotoxin 6-hydroxydopamine
(6-OHDA), which was initially identified in PD patients,
along with the human neuroblastoma SH-SY5Y cell-line, to
assess neuroprotective potential of drugs [5-8].
The search for natural active polymers may provide new
therapeutic alternatives for the treatment of neurodegenerative
diseases, such as PD [9, 10]. Among those promising biopolymers,
a group of highly complex and heterogeneous polymers present in
the extracellular matrix of marine algae, named sulphated
polysaccharides (SPs), has been accumulating evidences
supporting their neuroprotective activity [11-14]. Carrageenans
represent a generic name of a family of SPs found in marine algae,
and these molecules have been reported to have a range of uses in
the food, cosmetics, and pharmaceutical industries [15-17]. Based
on chemical composition, carrageenans are divided into six basic
forms: Iota (ɩ)-, Kappa (κ)-, Lambda (λ)-, Mu (μ)-, Nu (v)- and
Theta (ө) [18]. The red marine alga Solieria filiformis (Kützing) P. W. Gabrielson
(Gigartinales, Solieraceae) represents a source of ɩ-carrageenan (CSf ) [19].
Its chemical structure has been described in the literature and consists
essentially an iota (ɩ)-type composed of a 3-linked β-D-galactopyranose-4-sulphate
(G4S-units) connected to 4-linked 3,6-anhydro-α-D-galactopyranose-2-sulphate
(DA2S-units) or 3,6-anhydro-α-D-galactopyranose (DA-units) [20-23]. Moreover,
CSf has been reported to possess anti-inflammatory, antiviral, vasorelaxant,
antinociceptive, and gastroprotective activities [19, 21-25] along with the
absence of in vivo toxicity [21]. Nonetheless, the neurological impact of the
CSf and its pharmacological potential is not clear yet. Therefore, this study
aimed to evaluate the neuroprotective potential of the CSf against 6-OHDA-induced neurotoxicity on SH-SY5Y cells.
Methods
Materials
SH-SY5Y and Balb/c 3T3 mouse fibroblast (3T3) cell-lines were obtained from the DSMZ Human and Animal Cell Lines Bank. The cell culture was performed according to the supplier’s handling information. Fetal bovine serum (FBS) was purchased from Gibco (Gaithersburg, MD, USA). JC-1 dye (T3168) was obtained from Molecular Probes (Eugene, OR, USA). Caspase-3 fluorimetric assay kit (Casp3f) was purchased from BioVision (Milpitas, CA, USA). Hydrogen peroxide assay kit (Amplex™ Red, A22188) was purchased from Life Technologies (Carlsbad, CA, USA). The absorbances of antioxidant and cellular assays were measured in Synergy H1 Multi-Mode Microplate Reader (BioTek® Instruments, Winooski, VT, USA). All solutions used in the cellular assays were previously diluted in culture medium without FBS, and sterile filtered (0.2 µm, Whatman™, Little Chalfont, UK). 96-well plates and other chemicals and reagents were obtained from Sigma-Aldrich (Carlsbad, CA, USA).
CSf
Specimen of the red seaweed S. filiformis were collected during winter (August) at the beach of Trairí city (Ceará, Brazil), followed by cleaning process and storage at –20 °C until further use. A voucher specimen (number 35682) was deposited at the Herbarium Prisco Bezerra, Department of Biological Sciences, Federal University of Ceará, Brazil. The isolation of CSf was carried out as previously described by Coura et al. [26]. Briefly, the total extract was submitted to protease digestion by papain (60 °C, 6 h) in 100 mM sodium acetate buffer (pH 5.0) containing EDTA and cysteine (both 5 mM), followed by method of purification through precipitation with cetylpyridinium chloride. After, the following chemical analysis were performed: the yield of carrageenan per gram of alga tissue (dry amount of 5 g) [27], the percentage of free-sulphate [28], and the molecular mass by gel permeation chromatography (GPC) [29]. Additionally, potential presence of protein contaminants was also assessed by Bradford method [30].
Antioxidant potential
The evaluation of the antioxidant potential of the CSf (at 0.1, 0.5, 1.0, and 2.0 mg/mL) was performed and calculated as described previously by Souza et al. [29], by four different methods: DPPH (1,1-diphenyl-2-picrylhydrazyl) assay, hydrogen peroxide (H2O2) radical scavenging activity, reducing power method (RP), and oxygen radical absorbent capacity (ORAC). Ascorbic acid was used as the standard (positive control) in the first three methods. The data were expressed as percentage. Trolox standard (6-hydroxychromane substituted with a carboxy group at position 2 and methyl groups at positions 2, 5, 7, and 8) was used to calculate the equivalence in the ORAC assay, where the oxygen radical absorbance capacity of the CSf was expressed as µmol Trolox equivalents per gram of the sample. Distilled water was used as a negative control in the assays conducted.
Cytotoxic assay
The cytotoxicity of CSf was evaluated on SH-SY5Y and 3T3 cell lines by MTT (3-(3,4-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, as described in Souza et al. [29]. Briefly, the cells were culturing in complete growth medium (CGM): DMEM Han’s F-12 (Biochrom, T481-01) medium with addition of HEPES (3.2%-Panreac, A3268.0100), sodium carbonate (2.2%-Panreac, 131638.1211), FBS (10%, fetal bovine serum-Alfagene, LTID 10270 -106), penicillin G (100 U/mL), amphotericin B (0.25 µg/mL), and streptomycin (100 µg/mL-Sigma, A5955). Every three days, the complete growth medium was refreshed. The cells were subcultured in T25-flasks and maintained in controlled conditions (95% humidity, 5% CO2, 37 °C). The cell suspension was seeded into 96-well plates and incubated until total monolayer was achieved. Then, 1 mg/mL of CSf was added and the plates were incubated for 24 hours. After, the intracellular metabolic activity was assessed with MTT assay (1.2 mM MTT, during 4 h at 37 °C). The formazan products were dissolved in isopropanol (Panreac, 131090.1611), contained HC (0.04 M). The absorbance was measured at 570 nm and reported as a percentage of the non-treated cells (negative control).
Analysis of potential in vitro neuroprotection
The neuroprotective potential of CSf was evaluated according
to Souza et al. [29] by 6-OHDA-induced neurotoxic cellular model on the SH-SY5Y cell line.
6-OHDA-induced cytotoxic model on the SH-SY5Y cell line
Briefly, cells at full confluence were transferred
into a 96-well plate and incubated either under exposure
to 6-OHDA (100 µM) alone or followed by the addition of
CSf (1 to 0.01 mg/mL) for 24 hours. As a negative control,
cells were incubated only with culture medium. The neurotoxic
effects were assessed using the MTT assay, with the results
reported as a percentage relative to the negative control.
Mitochondrial membrane potential (MMP) depolarization assay
To evaluate the neuroprotective effect of CSf on the MMP,
SH-SY5Y cells at total confluence were transferred to a
96-wells plate and incubated for 6 hours either in the
presence of 6-OHDA (100 µM) alone or followed by the
addition of CSf (1 and 0.6 mg/mL). As a negative control,
cells were incubated only with culture medium. MMP was then
analyzed through JC-1 MMP assay, according to the supplier’s
information. The absorbance measurements of JC-1 aggregates (490 nm/590 nm) and its
monomeric form (490 nm/530 nm) were conducted in real-time over 30 minutes.
Results were reported as a percentage of the ratio of JC-1 monomers to
aggregates relative to the negative control.
Caspase-3 assay
To determine the effect of CSf in the caspase-3 activity,
cells at full confluence were transferred to a 96-wells
plate and incubated for 6 hours either in the presence
of 6-OHDA (100 µM) alone or followed by the addition of
CSf (1 and 0.6 mg/mL). As a negative control, cells were
incubated only with culture medium. Then, caspase-3 activity
was measured, according to the supplier’s information.
The absorbance measurements (496 nm/520 nm) were performed
in real-time for 60 min. The results were obtained using a
linear regression model of the fluorescence spectral data
and expressed in arbitrary units (ΔUA) of fluorescence/milligrams of protein/time (min).
H2O2 generation
To verify the effect of CSf in generation of H2O2, cells
at full confluence, were transferred into microplates and
incubated under exposure of 6-OHDA (100 µM) and/or
only CSf (1 and 0.6 mg/mL) for 12 hours. As a negative
control, cells were incubated only with culture medium.
Afterward, quantification of H2O2 levels was performed
using the hydrogen peroxide assay kit (Amplex™ Red),
according to the supplier’s information. The absorbance
measurements (590 nm/530 nm) were performed in realtime during 60 min.
The results were obtained using a linear regression model of the fluorescence spectral data and
expressed as a percentage relative to the negative control.
Data and statistical analyses
Antioxidant and cellular assays results are presented as the SEM (mean ± standard error of mean). Paired Student's ttest was used to compare two groups. For comparisons involving three or more groups, One- or Two-way ANOVA (Analysis of Variance) was performed, and Bonferroni's post hoc test. Statistically significant differences were considered when p-value < 0.05. GraphPad Prism® 5.01 (GraphPad Software, San Diego, CA; www.graphpad. com) was used to perform the statistical analyses. All data were obtained of at least three independent experiments, carried out in triplicate and at different times.
Results
Chemical analysis and antioxidant potential
The extraction yield of CSf was approximately 20% per gram of dry alga, with a free-sulfate content of about 28%. No protein contaminants were detected. The molar mass of CSf was estimated at 425 kDa. A single broad peak observed in the GPC analysis indicated a highly polydisperse molar mass. The antioxidant activity analysis of CSf revealed low antioxidant properties in the ORAC assay (28.2 ± 2 µmol Eq. Trolox per gram of CSf ) and no activity in the DPPH and RP methods (Figure 1A and 1B). Although CSf has exhibited low antioxidant potential (less than 50%) compared to ascorbic acid, it showed a tendency toward H2O2 scavenging activity at a concentration of 1 mg/mL compared to the negative control. This effect became significant (P < 0.05) at a concentration of 2 mg/mL (Figure 1C).
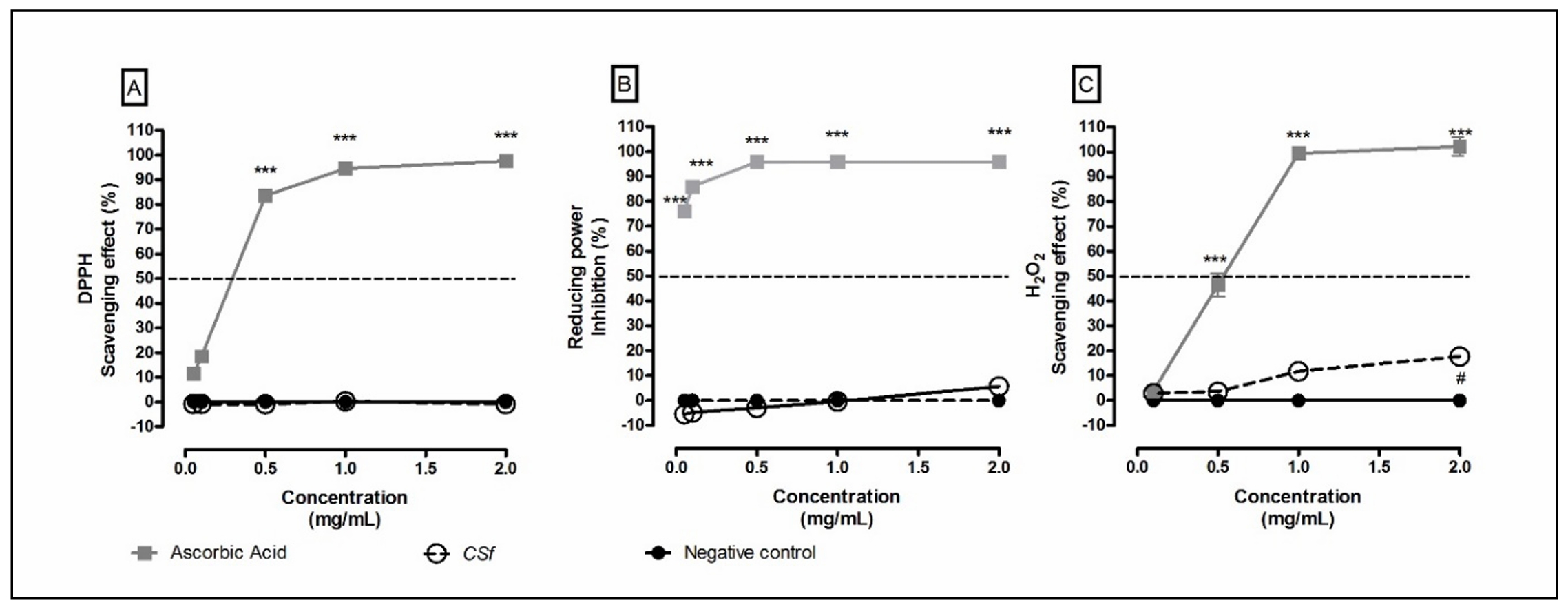
Figure 1. Antioxidant potential of CSf. (A) DPPH, (B) reducing power (RP), and (C) H2O2 assays, respectively. Figure 1A and 1C illustrate the scavenging activity of CSf at varying concentrations, while Figure 1B the inhibition of reducing power by CSf compared to ascorbic acid. Notably, in these assays, CSf demonstrates less than 50% of the antioxidant potential observed for ascorbic acid. The values correspond to mean ± SEM at least three independent experiments carried out in triplicate. Two-way ANOVA, Bonferroni test. ***P < 0.001, in comparison with the positive control (Ascorbic acid), and #P < 0.05, in comparison with the negative control.
Cytotoxicity and mitochondrial assessment
CSf (1 mg/mL) showed no cytotoxicity in the tested cell lines compared to their respective control groups (Figure 2). Furthermore, CSf (1 mg/mL) exhibited a capacity to increase the mitochondrial activity of 3T3 cells in relation to the control group (Figure 3A). Regarding the evaluation of neuroprotective activity on SH-SY5Y cells, it was possible to observe that the presence of CSf (1 and 0.6 mg/mL) protected the cells against mitochondrial activity changes (P < 0.001, 96.2 ± 0.05 and 62.4 ± 0.05, respectively), compared to 6-OHDA- treated group (42.7 ± 0.02). Moreover, this effect was also observed in the MMP depolarization assay (Figure 3B), where CSf (1 and 6 mg/mL) reduced the MMP depolarization (P < 0.001, 34.1 ± 0.02 and 54.6 ± 0.03, respectively) induced by 6-OHDA (99.8 ± 0.02). However, CSf was not able to return the MMP to basal levels (1.7 ± 0.001).
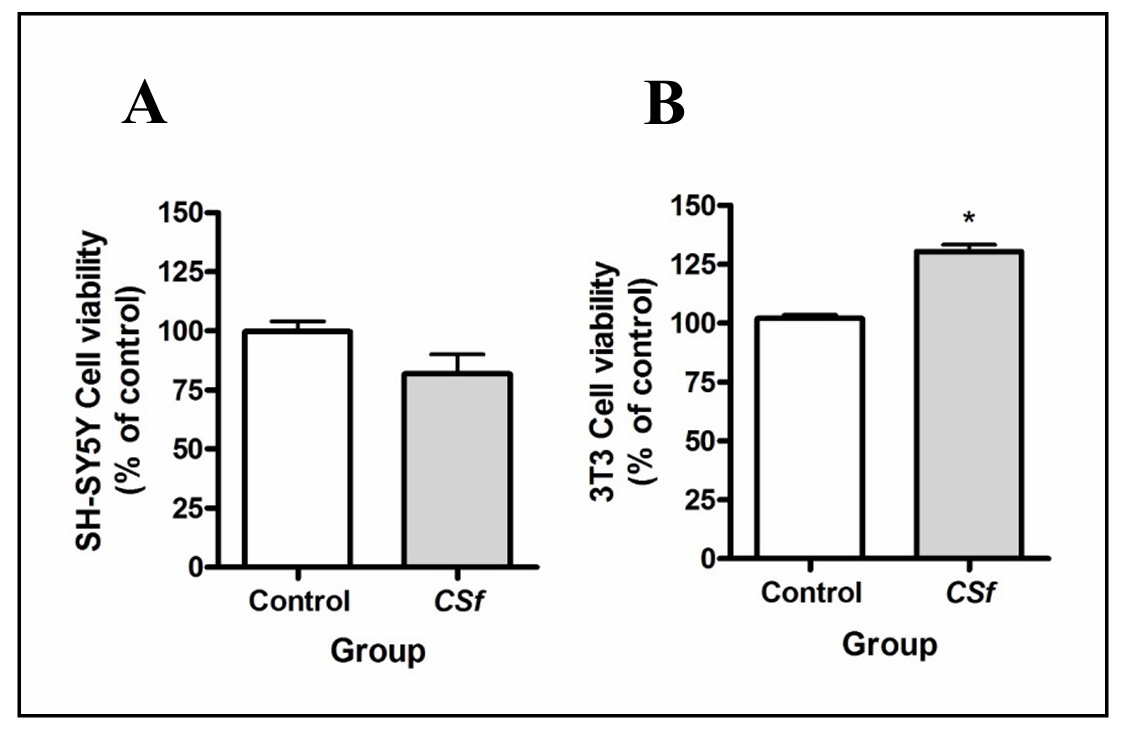
Figure 2. Cytotoxicity of CSf (1 mg/mL) on SH-SY5Y (A), and 3T3 cells (B), respectively. In Figure 2A, cellular viability does not show significant difference when compared to the control group, whereas in Figure 2B, an opposite trend is observed, indicating enhanced viability. Paired Student's t-test, #P < 0.05, when compared with control group.
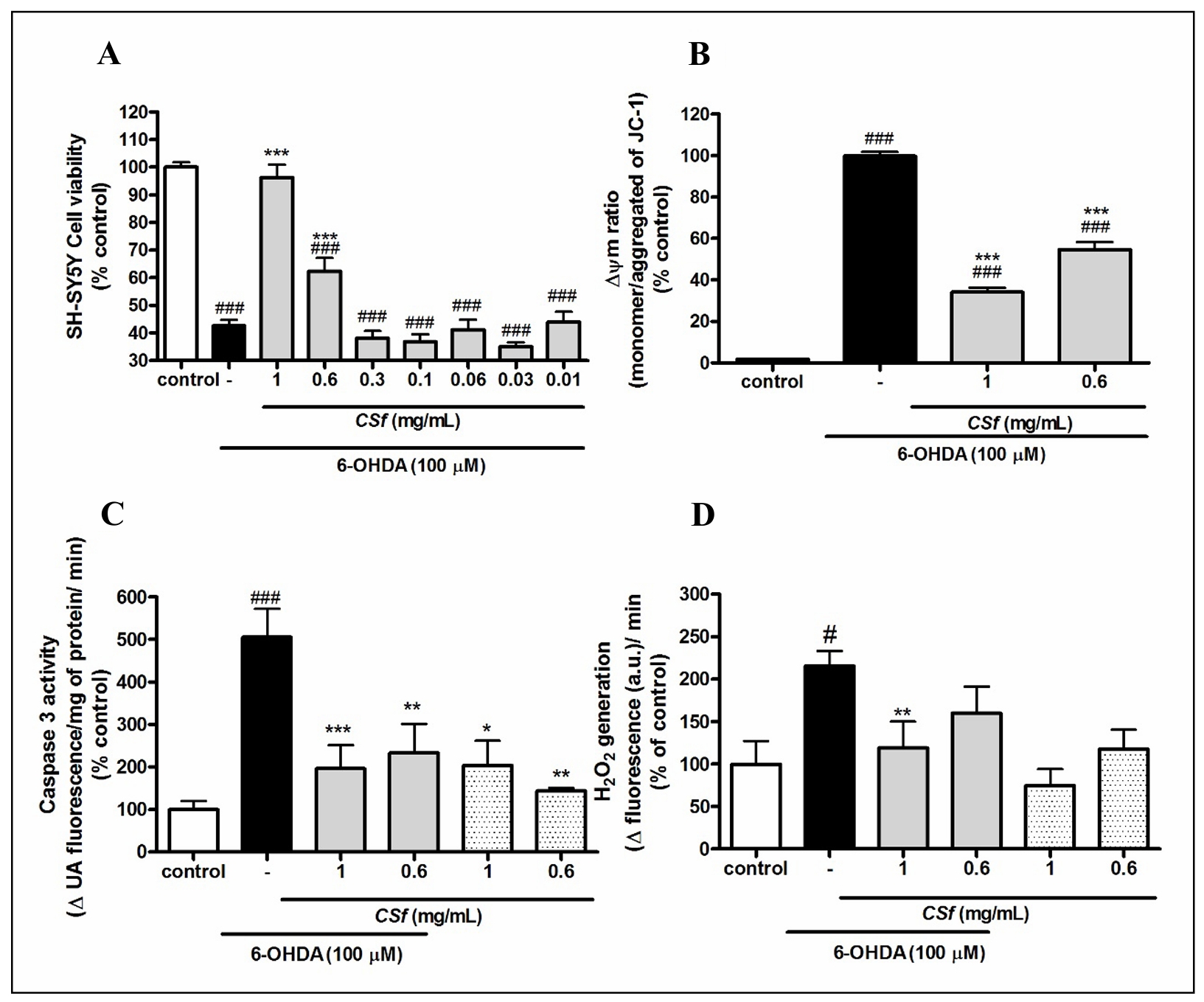
Figure 3. Neuroprotective effects of CSf in 6-OHDA-induced neurotoxicity model. (A) MTT assay, (B) MMP depolarization assay, (C) caspase-3 assay, and (D) H2O2 generation, respectively. In Figure 3A, seven concentrations of CSf were evaluated in the presence of 100 µM of the neurotoxin 6-OHDA. The most effective concentrations identified were subsequently analyzed further and are presented in Figure 3B, Figure C, and D. Figure 3C and D also include results obtained in the absence of the neurotoxin. The values correspond to mean ± SEM. One-way ANOVA, Bonferroni test. #P < 0.05 and ###P < 0.001, respectively, in relation with control group. *P < 0.05, **P < 0.01, and ***P < 0.001, respectively, in relation with 6-OHDA group.
Analysis of caspase-3 activity and H2O2 generation
Concerning the caspase-3 assay, the SH-SY5Y cells cultivated in presence of 6-OHDA exhibited a reduction of this enzyme activity when treated with CSf (1 mg/mL: P < 0.001, 196.3 ± 0.55; and 0.6 mg/mL: P < 0.01, 233.1 ± 0.68, respectively), in relation to the non-treated group (505 ± 0.67) (Figure 3C). Furthermore, CSf (1 and 0.6 mg/ mL) did not promote significant changes in caspase-3 activity in non-treated cells with the neurotoxin. Concomitantly, the analysis of H2O2 generation revealed high levels of H2O2 in cells exposed to 6-OHDA (P < 0.05, 215.6 ± 0.18), in relation to the control group (100.1 ± 0.27) (Figure 3D). On the other hand, SH-SY5Y cells treated with the highest concentration of CSf (1 mg/mL), maintained H2O2 generation at basal levels (119.1 ± 0.31). Additionally, CSf (1 and 0.6 mg/mL) did not stimulate significant changes in the H2O2 generation in non-treated cells with the neurotoxin, when compared to the control group.
Discussion
Because of structural heterogeneity and composition of
polymers from marine algae, a broad range of bioactivities
has been found. Furthermore, these polymers have shown
potential for various pharmacological and biotechnological applications. Among them, a chemical and structural
diversity of SPs have been investigated and classified
[29, 31, 32]. Despite being relatively new to the scientific
literature, neuroprotective activities from marine algae
polymers are increasingly reported. These studies have
been investigated and collaborated to a possible development of new therapeutic strategies and pharmacological
applications [29, 33-35]. In the present study, the chemical
characteristics, antioxidant potential, and cytotoxic and
neuroprotective effects of the SP isolated from S. filiformis
are reported. Indeed, the chemical features of the CSf have
been previously well-reported in the literature [20-23].
Evidently, the analysis performed in this study revealed
yield and sulphate content similar to the ɩ-carrageenan described by Araújo et al. [19]. Furthermore, a higher value
of molecular mass with a polydispersive characteristic
was identified. These findings are commonly exhibited by
SPs from marine algae, due to the grouping of polysaccharide chains [11].
Among the bioactivities, the antioxidant action can serve
as an indicator of a potential neuroprotective activity [36].
Antioxidants are compounds capable of either delay or
inhibiting oxidation processes and belong to the defense
mechanism of an organism against the development of
pathologies associated with the attack of free radicals [37].
The antioxidant potential of the SP isolated from S. filiformis has been previously reported [22, 25]. Nevertheless,
the results shown here revealed that CSf possesses weak
antioxidant properties in general. The differences observed
in the antioxidant capacity of a SP can be influenced by
various factors, such as concentration, the antioxidant assay as well as by the extraction method chosen to analysis.
Indeed, Peñuela et al. [25] reported a positive antioxidant
action of a SP from S. filiformis (5 mg/mL) when tested in
ABTS (2,2′-azino-bis-3-ethylbenzo thiazoline-6-sulfonic
acid) and FRAP (ferric reducing power) assays. However,
its activity was absent in the DPPH assay. According to
the authors, due to the limitations of each antioxidant
assay, the extraction method chosen might lead to underestimation or undetectability of the radical scavenging
activity of SPs. Considering these limitations, it is worth
mentioning that four different assays were performed in
the present study. Moreover, and interestingly, the CSf (1
mg/mL) showed potential for H2O2 scavenging capacity.
Cellular models have been proportioned to investigate molecular and physiologic findings
related to several pathogeneses, including those in PD studies. In previous studies,
SPs showed no toxic effects when analyzed in both in vitro and in vivo models [38-44].
Mehrban et al. [45] reported non-cytotoxic effects induced by ɩ-carrageenan on 3T3 cells.
Therefore, it was also decided to include the 3T3 cell line as a toxicological control.
In the present study, our data suggest that CSf does not induce a cytotoxic effect,
corroborating with previous in vitro cytotoxic studies performed on 3T3 and other cell
lines, such as colon epithelial cells derived from HT-29 (colorectal adenocarcinoma),
HCT-8 (human ileocecal colorectal adenocarcinoma), Caco-2 (human colorectal adenocarcinoma),
and HepG2 (human hepatoma) [45, 46]. Moreover, the CSf seems to stimulate the viability of
3T3 cells in the present study. According to Sun et al. [47], SPs (such as heparin, chondroitin
sulphate, λ‐carrageenan, and dextran sulphate) act on fibroblast growth factors and protect them
from denaturation. In addition, the CSf did not promote toxic effects on SH-SY5Y cells. Thereby,
the findings shown here align with previous studies, which suggest that the use of CSf is pharmacologically
safe on the cells analyzed, and its presence in the cell culture can
promote an increase in the viability of fibroblast cells.
Recently, neuroprotective activities of SPs isolated from seaweeds have been
reported in the literature [44, 48-50]. However, few studies have focused on the
application of carrageenans in neurodegeneration models. Equally to the SH-SY5Y
cell-line, the neurotoxin 6-OHDA has been useful to carrying out a widely recognized
model for experimental PD scientific studies [3, 4, 7, 51]. Mitochondrial dysfunction
has been shown to be related to the development of PD pathogenesis [52]. Actually, the
mitochondria are the main target of 6-OHDA, which leads to the membrane permeabilization
of this organelle and consequently to an apoptotic cascade in neuronal cells [53]. Our
findings agree with previously reported studies in the literature showing the mitochondrial
protective action stimulated by SPs [16, 29, 54]. Furthermore, our data suggest that ɩ-carrageenan
used in this study has a superior effect on mitochondrial protection, when compared with κ-carrageenan
isolated from red marine alga Hypnea musciformis in study by Souza et al. [29]. According to Ma et al. [55],
the sulphate content in SPs is directly related to the improvement of mitochondrial protection. Consequently,
the higher sulphate content in ɩ-carrageenan, in relation to the κ-carrageenan, was possibly responsible
for the superior effect observed. Hence, our data suggest that ɩ-carrageenan not only shown a protective
activity on mitochondria but also has a superior effect compared to κ-carrageenan.
The endoprotease caspase-3 has an apoptotic function that contributes to cell
death by degrading proteins and it has been associated to neurodegenerative diseases,
such as Alzheimer’s disease and PD [49, 56]. Sato et al. [12] reported that SPs possess
a capacity to modulate caspase-3 activity. Therefore, the neuroprotective effect of the
CSf on caspase-3 activity was investigated. In the last year, SPs from seaweeds have been
reported to regulate caspase-3 activity in in vitro models [16, 29, 49, 57]. For instance,
Wei et al. [49] showed that k-carrageenan found in marine red algae can modulate the caspase-3
pathway and decrease cellular apoptosis induced by fragment of beta-amyloid peptide. Similarly,
a k-carrageenan (H. Musciformis) has shown an antiapoptotic activity in SH-SY5Y cells treated
with 6-OHDA [29]. Corroborating these previous studies, the CSf
mediates antiapoptotic activity against 6-OHDA-induced cell death through caspase-3 modulation.
The neurotoxic effect of 6-OHDA results from oxidative stress induced by the production
of reactive oxygen species (ROS) through its auto-oxidation after being taken up by
the neuron via the dopamine transporter [58]. According to Kick et al. [59], a surplus
of endogenous ROS such as H2O2 is associated with mitochondrial disturbances, leading
to apoptotic factors release, caspase cascade activation, and finally, cellular death.
Therefore, the balance between the generation of H2O2 and its neutralization by endogenous
cellular defense mechanisms is one important factor in cellular homeostasis [60].
In the present study, the CSf attenuated H2O2 generation induced by 6-OHDA exposure
without inducing cytotoxicity on normal cells. Corroborating with the present data
from the H2O2 radical scavenging assay, these findings suggest that the ɩ-carrageenan
obtained from S. filiformis exhibits a homeostatic capacity, downmodulating the
endogenous H2O2 generation induced by neurotoxin to basal levels in SH-SY5Y cells.
Conclusions
The current investigation has provided insight into the pharmacological potential of CSf demonstrating neuroprotective effects in a neurotoxic model through the modulation of H2O2 generation and caspase-3 activity to basal levels, as well as protection of the mitochondria. Additionally, CSf exhibited no cytotoxicity in the tested cells, suggesting its potential pharmacological safety for use in the development of novel treatments for neurodegenerative disorders. Although, the presented results are promising, additional more complex model studies are necessary to provide deeper insights into the mechanisms of action and potential translational applicability of CSf.
Declarations
Financial support and sponsorship
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, and supported by Laboratório de Carboidratos e Lectinas (CARBOLEC, Brasil) and by Portuguese Foundation for Science and Technology (FCT) through the strategic project UID/MAR/04292/2020 to MARE—Marine and Environmental Sciences Centre, through POINT4PAC project (Oncologia de Precisão: Terapias e Tecnologias Inovadoras, SAICTPAC/0019/ 2015-LISBOA- 01-0145-FEDER-016405), through CROSS-ATLANTIC project (PTDC/BIA-OUT/29250/2017), co-financed by COMPETE (POCI-01-0145-FEDER-029250). This work was also funded by the Integrated Programme of SR&TD Smart Valorization of Endogenous Marine Biological Resources Under a Changing Climate (reference Centro-01-0145-FEDER-000018), co-funded by Centro 2020 Programme, Portugal 2020, European Union, through the European Regional Development Fund.
Conflict of interest
The authors have declared that no competing interests exist.
Ethical approval and informed consent
Not applicable.
References
1. Kulcsarova K, Skorvanek M, Postuma RB, & Berg D. Defining Parkinson's disease: past and future. J Parkinsons Dis, 2024, 14(s2): S257-s271. [Crossref]
2. Murakami H, Shiraishi T, Umehara T, Omoto S, & Iguchi Y. Recent advances in drug therapy for Parkinson's disease. Intern Med, 2023, 62(1): 33-42. [Crossref]
3. Li Y, Luo F, Wei L, Liu Z, & Xu P. Knockdown of glycogen synthase kinase 3 beta attenuates 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Neurosci Lett, 2011, 487(1): 41-46. [Crossref]
4. Gu H, Wang J, Du N, Tan J, Johnstone B, & Du Y. Adipose stromal cells-conditioned medium blocks 6-hydroxydopamine-induced neurotoxicity and reactive oxygen species. Neurosci Lett, 2013, 544: 15-19. [Crossref]
5. Glinka Y, Gassen M, & Youdim MB. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl, 1997, 50: 55-66. [Crossref]
6. Cui J, Zhao D, Xu M, Li Z, Qian J, Song N, et al. Characterization of graded 6-hydroxydopamine unilateral lesion in medial forebrain bundle of mice. Sci Rep, 2024, 14(1): 3721-3731. [Crossref]
7. Xicoy H, Wieringa B, & Martens GJ. The SH-SY5Y cell line in Parkinson's disease research: a systematic review. Mol Neurodegener, 2017, 12(1): 10-21. [Crossref]
8. Barros AS, Crispim RYG, Cavalcanti JU, Souza RB, Lemos JC, Cristino Filho G, et al. Impact of the chronic omega-3 fatty acids supplementation in hemiparkinsonism model induced by 6-hydroxydopamine in rats. Basic Clin Pharmacol Toxicol, 2017, 120(6): 523-531. [Crossref]
9. Hannan MA, Dash R, Haque MN, Mohibbullah M, Sohag AAM, Rahman MA, et al. Neuroprotective potentials of marine algae and their bioactive metabolites: pharmacological insights and therapeutic advances. Mar Drugs, 2020, 18(7): 347-357. [Crossref]
10. Catanesi M, Caioni G, Castelli V, Benedetti E, d'Angelo M, & Cimini A. Benefits under the sea: the role of marine compounds in neurodegenerative disorders. Mar Drugs, 2021, 19(1): 24-34. [Crossref]
11. Pomin VH. Structural and functional insights into sulfated galactans: a systematic review. Glycoconj J, 2010, 27(1): 1-12. [Crossref]
12. Sato Y, Nakanishi K, Tokita Y, Kakizawa H, Ida M, Maeda H, et al. A highly sulfated chondroitin sulfate preparation, CS-E, prevents excitatory amino acid-induced neuronal cell death. J Neurochem, 2008, 104(6): 1565-1576. [Crossref]
13. Dudas B, & Semeniken K. Glycosaminoglycans and neuroprotection. Handb Exp Pharmacol, 2012, (207): 325-343. [Crossref]
14. Nakanishi K, Ito M, Sato Y, & Oohira A. A highly-sulfated chondroitin sulfate, CS-E, adsorbs specifically to neurons with nuclear condensation. Neurosci Res, 2012, 74(3-4): 223-229. [Crossref]
15. Das IJ, & Bal T. Exploring carrageenan: from seaweed to biomedicine-a comprehensive review. Int J Biol Macromol, 2024, 268(Pt 2): 131822. [Crossref]
16. Bichiri D, Rente AR, & Jesus Â. Safety and efficacy of iota-carrageenan nasal spray in treatment and prevention of the common cold. Med Pharm Rep, 2021, 94(1): 28-34. [Crossref]
17. Aziz E, Batool R, Khan MU, Rauf A, Akhtar W, Heydari M, et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J Complement Integr Med, 2020. [Crossref]
18. Campo VL, Kawano DF, Silva DBd, & Carvalho I. Carrageenans: biological properties, chemical modifications and structural analysis – a review. Carbohydrate Polymers, 2009, 77(2): 167-180. [Crossref]
19. Araújo IWFd, Rodrigues JAG, Vanderlei EdSO, Paula GAd, Lima TdB, & Benevides NMB. Iota-carrageenans from Solieria filiformis (Rhodophyta) and their effects in the inflammation and coagulation. Acta Scientiarum-technology, 2012, 34: 127-135. [Crossref]
20. Murano E, Toffanin R, Cecere E, Rizzo R, & Knutsen SH. Investigation of the carrageenans extracted from Solieria filiformis and Agardhiella subulata from Mar Piccolo, Taranto. Marine Chemistry, 1997, 58(3): 319-325. Crossref]
21. de Araújo IWF, Vanderlei EdSO, Rodrigues JAG, Coura CO, Quinderé ALG, Fontes BP, et al. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydrate Polymers, 2011, 86(3): 1207-1215. [Crossref]
22. Sousa WM, Silva RO, Bezerra FF, Bingana RD, Barros FCN, Costa LEC, et al. Sulfated polysaccharide fraction from marine algae Solieria filiformis : structural characterization, gastroprotective and antioxidant effects. Carbohydr Polym, 2016, 152: 140-148. [Crossref]
23. Caamal-Fuentes E, Robledo D, & Freile-Pelegrín Y. Physicochemical characterization and biological activities of sulfated polysaccharides from cultivated Solieria filiformis Rhodophyta. Natural Product Communications, 2017, 12: 601-611. [Crossref]
24. Araújo IW, Chaves HV, Pachêco JM, Val DR, Vieira LV, Santos R, et al. Role of central opioid on the antinociceptive effect of sulfated polysaccharide from the red seaweed Solieria filiformis in induced temporomandibular joint pain. Int Immunopharmacol, 2017, 44: 160-167. [Crossref]
25. Ana P, Nathalie B, Gilles B, Daniel R, Tomás MS, & Yolanda FP. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int J Biol Macromol, 2021, 168: 322-330. [Crossref]
26. Coura CO, de Araújo IW, Vanderlei ES, Rodrigues JA, Quinderé AL, Fontes BP, et al. Antinociceptive and anti-inflammatory activities of sulphated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin Pharmacol Toxicol, 2012, 110(4): 335-341. [Crossref]
27. DuBois M, Gilles KA, Hamilton JK, Rebers PA, & Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 1956, 28(3): 350-356. [Crossref]
28. Dodgson KS, & Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J, 1962, 84(1): 106-110. [Crossref]
29. Souza RB, Frota AF, Silva J, Alves C, Neugebauer AZ, Pinteus S, et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis : Antimicrobial, anticancer and neuroprotective potential. Int J Biol Macromol, 2018, 112: 1248-1256. [Crossref]
30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72: 248-254. [Crossref]
31. Gomez-Zavaglia A, Prieto Lage MA, Jimenez-Lopez C, Mejuto JC, & Simal-Gandara J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants (Basel), 2019, 8(9): 406-416. [Crossref]
32. Silva J, Martins A, Alves C, Pinteus S, Gaspar H, Alfonso A, et al. Natural approaches for neurological disorders-the neuroprotective potential of codium tomentosum. Molecules, 2020, 25(22): 5478-5488. [Crossref]
33. Silva J, Alves C, Pinteus S, Mendes S, & Pedrosa R. Neuroprotective effects of seaweeds against 6-hydroxidopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complement Altern Med, 2018, 18(1): 58-68. [Crossref]
34. Silva J, Alves C, Freitas R, Martins A, Pinteus S, Ribeiro J, et al. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson's disease model. Mar Drugs, 2019, 17(2): 85-95. [Crossref]
35. Fallarero A, Loikkanen JJ, Männistö PT, Castañeda O, & Vidal A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G.Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine, 2003, 10(1): 39-47. [Crossref]
36. Pangestuti R, & Kim SK. Neuroprotective effects of marine algae. Mar Drugs, 2011, 9(5): 803-818. [Crossref]
37. Bhandari UR, Danish SM, Ahmad S, Ikram M, Nadaf A, Hasan N, et al. New opportunities for antioxidants in amelioration of neurodegenerative diseases. Mech Ageing Dev, 2024, 221: 111961. [Crossref]
38. das Chagas Faustino Alves MG, Dore CMPG, Castro AJG, do Nascimento MS, Cruz AKM, Soriano EM, et al. Antioxidant, cytotoxic and hemolytic effects of sulfated galactans from edible red alga Hypnea musciformis . Journal of Applied Phycology, 2012, 24(5): 1217-1227. [Crossref]
39. Navya P, & Khora SS. In vitro cytotoxicity analysis of sulfated polysaccharides from green seaweed Codium tomentosum stackhouse, 1797. Journal of Applied Pharmaceutical Science, 2017, 7: 33-36. [Crossref]
40. Assreuy AM, Gomes DM, da Silva MS, Torres VM, Siqueira RC, Pires Ade F, et al. Biological effects of a sulfated-polysaccharide isolated from the marine red algae Champia feldmannii . Biol Pharm Bull, 2008, 31(4): 691-695. [Crossref]
41. de Sousa Oliveira Vanderlei E, de Araújo IW, Quinderé AL, Fontes BP, Eloy YR, Rodrigues JA, et al. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed Gracilaria birdiae . Inflamm Res, 2011, 60(12): 1121-1130. [Crossref]
42. Rodrigues JAG, Vanderlei EdSO, Araújo IWFd, Quinderé ALG, Coura CO, & Benevides NMB. In vivo toxicological evaluation of crude sulfated polysaccharide from the green seaweed Caulerpa cupressoides var. lycopodium in Swiss mice. Acta Scientiarum-technology, 2013, 35: 603-610. [Crossref]
43. Carneiro JG, Rodrigues JA, de Sousa Oliveira Vanderlei E, Souza RB, Quinderé AL, Coura CO, et al. Peripheral antinociception and anti-inflammatory effects of sulphated polysaccharides from the alga Caulerpa mexicana . Basic Clin Pharmacol Toxicol, 2014, 115(4): 335-342. [Crossref]
44. Souza RB, Frota AF, Sousa RS, Cezario NA, Santos TB, Souza LM, et al. Neuroprotective effects of sulphated agaran from marine alga Gracilaria cornea in rat 6-hydroxydopamine Parkinson's disease model: behavioural, neurochemical and transcriptional alterations. Basic Clin Pharmacol Toxicol, 2017, 120(2): 159-170. [Crossref]
45. Mehrban N, Smith AM, & Grover LM (2009). Evaluation of iota-carrageenan as a potential tissue engineering scaffold.
46. McKim JM, Jr., Baas H, Rice GP, Willoughby JA, Sr., Weiner ML, & Blakemore W. Effects of carrageenan on cell permeability, cytotoxicity, and cytokine gene expression in human intestinal and hepatic cell lines. Food Chem Toxicol, 2016, 96: 1-10. [Crossref]
47. Sun C, Liu M, Sun P, Yang M, Yates EA, Guo Z, et al. Sulfated polysaccharides interact with fibroblast growth factors and protect from denaturation. FEBS Open Bio, 2019, 9(8): 1477-1487. [Crossref]
48. Wang T, Zhu M, & He ZZ. Low-molecular-weight fucoidan attenuates mitochondrial dysfunction and improves neurological outcome after traumatic brain injury in aged mice: involvement of Sirt3. Cell Mol Neurobiol, 2016, 36(8): 1257-1268. [Crossref]
49. Wei H, Gao Z, Zheng L, Zhang C, Liu Z, Yang Y, et al. Protective effects of fucoidan on Aβ25-35 and d-gal-induced neurotoxicity in PC12 cells and d-gal-induced cognitive dysfunction in Mice. Mar Drugs, 2017, 15(3): 77-87. [Crossref]
50. Yang WN, Chen PW, & Huang CY. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar Drugs, 2017, 15(6): 183-193. [Crossref]
51. Takahashi T, Deng Y, Maruyama W, Dostert P, Kawai M, & Naoi M. Uptake of a neurotoxin-candidate, (R)-1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline into human dopaminergic neuroblastoma SH-SY5Y cells by dopamine transport system. J Neural Transm Gen Sect, 1994, 98(2): 107-118. [Crossref]
52. Kupsch A, Schmidt W, Gizatullina Z, Debska-Vielhaber G, Voges J, Striggow F, et al. 6-Hydroxydopamine impairs mitochondrial function in the rat model of Parkinson's disease: respirometric, histological, and behavioral analyses. J Neural Transm (Vienna), 2014, 121(10): 1245-1257. [Crossref]
53. Gomez-Lazaro M, Galindo MF, Concannon CG, Segura MF, Fernandez-Gomez FJ, Llecha N, et al. 6-Hydroxydopamine activates the mitochondrial apoptosis pathway through p38 MAPK-mediated, p53-independent activation of Bax and PUMA. J Neurochem, 2008, 104(6): 1599-1612. [Crossref]
54. Josephine A, Amudha G, Veena CK, Preetha SP, Rajeswari A, & Varalakshmi P. Beneficial effects of sulfated polysaccharides from Sargassum wightii against mitochondrial alterations induced by Cyclosporine A in rat kidney. Mol Nutr Food Res, 2007, 51(11): 1413-1422. [Crossref]
55. Ma XT, Sun XY, Yu K, Gui BS, Gui Q, & Ouyang JM. Effect of content of sulfate groups in seaweed polysaccharides on antioxidant activity and repair effect of subcellular organelles in injured HK-2 cells. Oxid Med Cell Longev, 2017, 2017: 2542950. [Crossref]
56. McIlwain DR, Berger T, & Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol, 2015, 7(4): a026716. [Crossref]
57. Liu Y, Jiang L, & Li X. κ‑carrageenan‑derived pentasaccharide attenuates Aβ25‑35‑induced apoptosis in SH‑SY5Y cells via suppression of the JNK signaling pathway. Mol Med Rep, 2017, 15(1): 285-290. [Crossref]
58. Storch A, Kaftan A, Burkhardt K, & Schwarz J. 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: independent of mitochondrial energy metabolism. J Neural Transm (Vienna), 2000, 107(3): 281-293. [Crossref]
59. Kich DM, Bitencourt S, Alves C, Silva J, Pinteus S, Pedrosa R, et al. Neuromodulatory effects of Calyptranthes grandifolia extracts against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Biomed Pharmacother, 2016, 84: 382-386. [Crossref]
60. Georgieva E, Ivanova D, Zhelev Z, Bakalova R, Gulubova M, & Aoki I. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of "free radical diseases". Anticancer Res, 2017, 37(10): 5373-5381. [Crossref]