Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Gut microbiome and neurodegenerative disorders: therapeutic implications
# These authors contributed equally to this work.
* Corresponding author: Sugato Banerjee
Mailing address: Department of Pharmacology and Toxicology,
National
Institute of Pharmaceutical Education and Research,
Kolkata, West Bengal, India.
Email:banerjeesugato1@gmail.com
This article belongs to the Special Issue: The gut microbiome for improving neurological health
Revised: 27 September 2024 / Revised: 18 October 2024 / Accepted:11 November 2024 / Published:28 December 2024
DOI: 10.31491/APT.2025.03.164
Abstract
The gut microbiome, home to 100 trillion microorganisms, and its influence on brain health and disease via the gut-brain axis has encouraged interdisciplinary studies. The gut-brain axis is the two-way communication network connecting the gut microbiome to the central nervous system. Gut microbes produce neurotransmitter's such as serotonin and gamma-aminobutyric acid, which can impact mood and cognitive function. The microbial imbalance can lead to systemic inflammation, which may contribute to neuroinflammation and further cause neurodegeneration. Additionaly, it modulates immune responses and results in autoimmune disorders. Every day, new and progressive findings are surfacing related to microbiota-mediated neurodegenerative disorders, their mechanistic approach, and different therapeutic approaches to ameliorate these conditions. In this review, we aim to unfold the intricate relationship of the microbiota-gut-brain axis to overlay a better understanding of the microbiota-mediated pathogenesis of neurodegenerative disorders, popularly known as Alzheimer's and Parkinson's disease, including amyotrophic lateral sclerosis, multiple sclerosis, and Huntington's disease. Aging accelerates neurodegeneration by modulating microbial composition, altering metabolic processes and immune functions. Moreover, possible therapeutic strategies such as the use of probiotics, prebiotics, synbiotics and various dietary modulations to ameliorate neurodegenerative conditions have been outined in both preclinical and clinical studies. As the gut microbiome is highly individualistic, designing personalized prebiotic or probiotic formulations according to each person's microbiome profile is a future challenge.More research is needed to fully understand how the gut microbiota influences neurodegenerative processes at a mechanistic level, The long-term effects of microbiome-based interventions on neurodegenerative diseases need to be thoroughly investigated to establish their safety and efficacy.
Keywords
Gut microbiota, gut-brain axis, neurodegenerative disorders, probiotics, prebiotics, aging
Introduction
The gut microbiome is an environment composed of both beneficial and pathogenic microbes that plays an
important role in various functions related to health anddisease [1]. Cytophaga-favobacterium bacteroides
(CFB) and Firmicutes are the beneficial bacteria present in the gut microbiota of an individual.The CFB group consists ofBacteroides
with the majority of Prevotella, Porphyromonas , and Bifidobacterium, whereas Firmicutes are classified into Bacilli, Clostridia, Erysipelotrichia, Lactobacillus, Limnochordia, Clostridia, Negativicutes, Thermolithobacteria, and
Tissierellia [2]. These beneficia bacteria play a very important role in unlocking the nutrients we need from our food and also create beneficial byproducts like short-chain fatty acids(SCFAs),
consisting of butyrate, propionate, and acetate, which contribute to gut health and even influence mood, cognition, and brainfunction [3].
They are also involved in various other functions such as absorption of minerals, production of vitamins,
regulation of gut motility, conversion of bile acids and steroids, metabolism of xenobiotics, and destruction of mutagens,
toxins, and genotoxins [4]. Apart from these, some harmful bacteria, consisting of Clostridium perfringens, Staphylococcus and
Escherichia coli (E.coli), also reside in our intestines, which inhibit health by promoting aging and triggering various diseases [5]. When the natural equilibrium
between these beneficial and pathogenic bacteria is altered, it leads to gut dysbiosis that contributes to variousdiseases such as obesity, diabetes, cancer,neurodegenerative,
psychiatric, cardiovascular, and inflammatory bowel diseases [6]. Recent research has revealed the existenceof a bidirectional communication channel between the
gut microbiome and the brain through the microbiotagutbrain axis (MGBA) [7]. Despite the anatomical separation of the brain and gut,
several pathways have been suggested for the gut bacteria to interact with the central nervous system (CNS). These include the generation of neuroactive compounds, metabolites, and hormones that
modulate the neuroendocrine system, vagus nerve, entericneurological system (ENS), immune system,and cardiovascular system [8].
Research has demonstrated that the gut microbiota is capable of producing neurotransmitters such as γ-aminobutyric acid (GABA),
dopamine, and serotonin [9]. When these neurotransmitter levels are affected, it leads to various neurodegenerative diseases such as depression and anxiety, Alzheimer's disease(AD),
Parkinson's disease (PD), multiple sclerosis (MS), amyo-trophic lateral sclerosis (ALS), Huntington’s disease (HD) and so on [10].
The association of the abundance of different microorganisms in the respective neurodegenerative disorders has been depicted in Figure 1 [11].
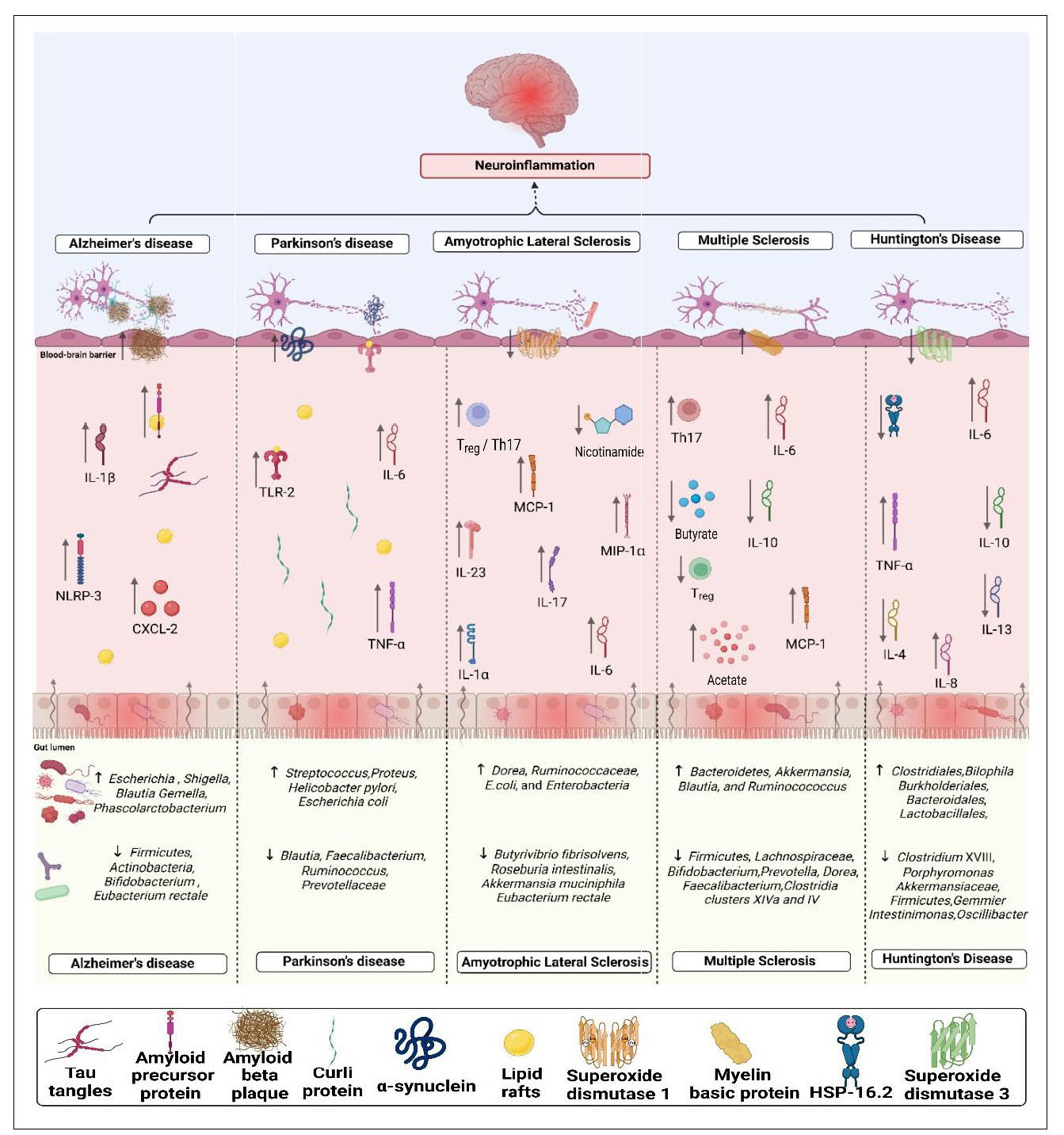
Pigure 1. Pictorial presentation of neuropathogenesis of gut microbiota-mediated neurodegenerative diseases. Dvsbiosis of the gut microbiota leads to disruption of the intestinal barrier, resulting in increased intestinal permeability. With a compromised intestinal barrier, pathogens enter the intestine and trigger the release of multiple pro-inflammatory factors such as C-X-C motif chemokine ligand 2 (CXCL-2), NOD-like receptor protein-3 (NLRp-3), Toll-like receptor-2 (TLR-2), tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-l alpha (MIP-1α), and disruption of the immune balance between'T helper 17 (Th17) cells and regulatory 'T cells (Tregs). loss of beneficial bacteria in a given disease resulted in altered expression of certain proteins, such as amyloid precursor protein (APP), tau protein, curli protein, superoxide dismutase, mvelin basic protein. heat shock protein-16.2 ((HSP-16.2). and reduced levels of nicotinamide, Levels of SCFAs were also affected by disruptions in the gut microbiota, such as increased acetate and decreased butyrate in a given disease. Neuroinfammation is facilitated by gut dysbiosis, which significantly decreases levels of anti-inflammatory interleukins (IL-4, IL-10, IL-13) and increases levels of pro-inflammatory interleukins (IL-1β, IL-6, IL-17, IL-23, IL-8).
To replenish the beneficial bacteria, prebiotics, probiotics, or synbiotics are given to enhance the body's defense system, improve nutrient absorption, and reduce digestive discomfort [12]. Prebiotics are defined as “a nondigestible dietary component that selectively stimulates the growth and/or activity of a limited number of bacteria in the colon, hence improving host health and providing a beneficial impact on the host". The common prebiotics include inulin, resistant starch, fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), and trans-galacto-oligosaccharides (TOS) [13]. In a recent study, mice fed a high-fat diet (HFD) that disrupted the gut microbiome and then treated with FOS and GOS showed reduced signs of depression and anxiety. This behavioral improvement contributed to a reduction in dysbiosis, an increase in bacteria that produced acetate (Bacillus acidifaciens and Bacillus dorei), and a decrease in intestinal permeability that eventually resulted in a reduction in both peripheral and central inflammation [14]. Probiotics are defined as microorganisms that, when administered in suficient quantities, provide health benefits to the host [15]. A recent study showed the effect of a probiotic diet (Lactobacillus plantarum KY1032 and Lactobacillus curvatus HY7601) on the transgenic 3xTg-AD mouse that inhibited the progression of mild cognitive impairment and neurodegeneration in AD pathology [16]. A recent preclinical study was conducted on a cuprizone-induced demyelination model in rats by administering probiotic Bifidobacterium breve which alleviated demyelination and oxidative stress levels in the corpus callosum and may be used as a supplementary strategy for the treatment of MS [17]. Synbiotics are a combination of probiotics and prebiotics employed to replenish the gut with beneficial microbes in situations where the microbiome is disrupted due to severe disease or clinical care treatments [18]. A recent preclinical study suggested that a 5xFAD transgenic AD mouse model, when treated with a synbiotic formulation (Clostridium sporogenes and xylan) for 30 days, promoted gut-derived indole-3-propionic acid and markedly enhanced cognitive performance, spatial memory as well as reduced amyloidbeta (Aβ) accumulation in the hippocampus and cortex of the brain [16]. Despite significant advances in drug development,many medications used to treat neurodegenerative diseases that lead to a range of side effects. This often complicates treatment decisions and can significantly impact a patient's quality of life [19]. The connection between diet and neurodegenerative diseases is an area of growing interest. While research is ongoing, there is compelling evidence that dietary choices can significantly impact brain health and potentially reduce the risk or slow the progression of neurodegenerative conditions such as AD, PD, MS, depression and anxiety [20]. This review summarizes the possible mechanisms,current advances, and development of potential novel probiotic, prebiotic and synbiotic interventions that may manage and treat AD, PD, ALS, MS and HD in Figure 2 .
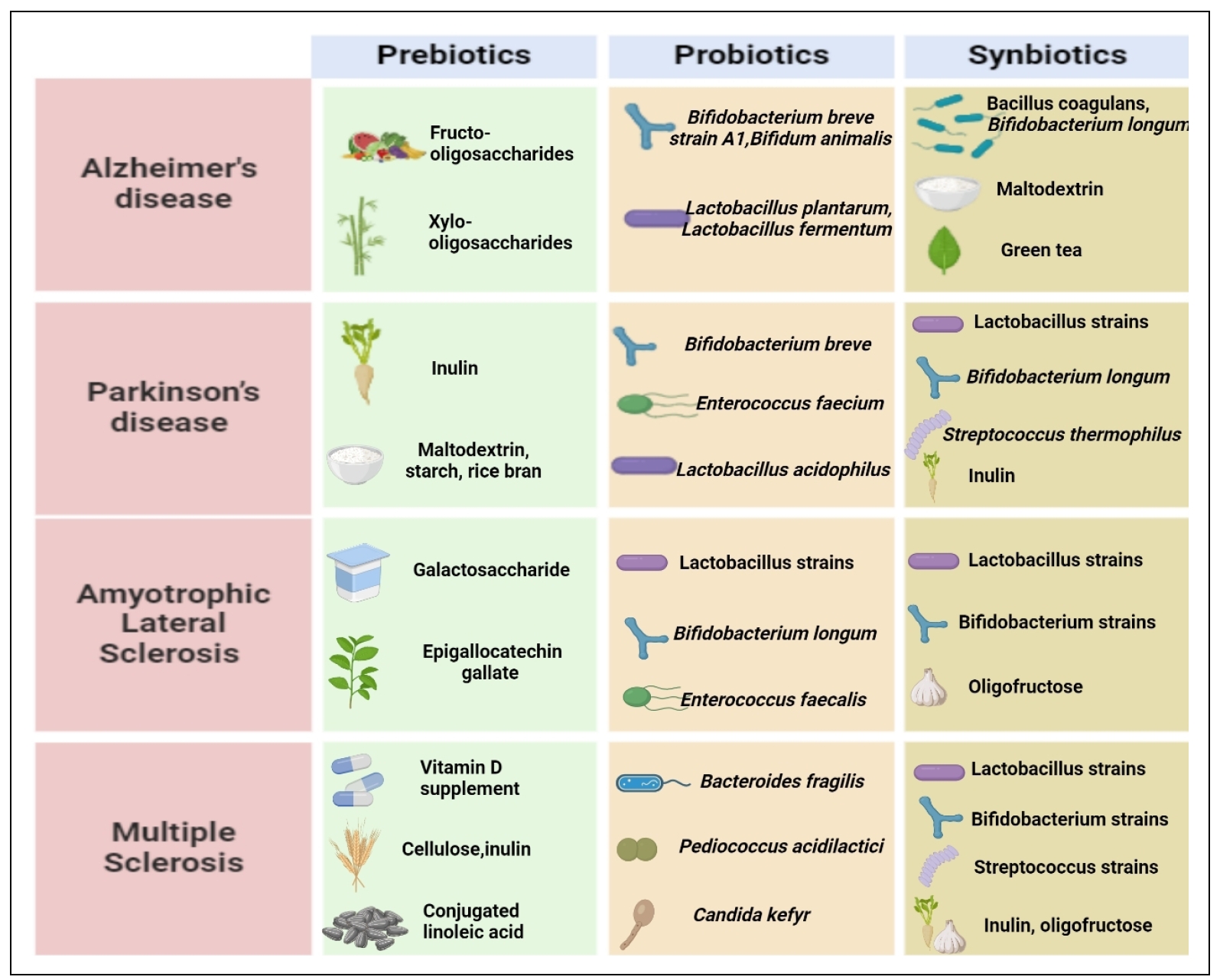
Figure 2. Consumption of diferent prebiotics, probiotics and synbiotics in ameliorating neurodegenerative diseases conducted in precinica and clinical studies. Prebiotics are indigestible fibers that promote the development of beneficial gut flora. The consumption of probiotics. which are live bacteria, can have beneficial efiects on our health. Synbiotics improve the survival and colonization of good bacteria in the gut by combining both probiotics and prebiotics. All ofthese elements are essential for maintaining the balance of the gut microbiota and overall health.
Gut microbiome and neurodegenerative disorders
Alzheimer’s disease (AD)
AD is a neurodegenerative disease that worsens over time.
causing damage to the neurons associated with memory,
language, and thinking, resulting in early symptoms such as memory loss, impaired thinking, and confusion [21].
Worldwide, 35.6 million people have dementia, and the number is expected to double by 2030 (65.7 million) and
triple by 2050 (11.4 million). Currently, 6.9 million Americans age 65 and older are reported to have Alzheimer's dementia.
Without medical advances to prevent or treat AD, this number could rise to 13.8 million by 2060 [22].
Alzheimer's dementia affects 5.0% of people aged 65 to 74, 13.1% of people aged 75 to 84, and 33.3% of people aged 85 and older. Data show that AD progresses with
age, which is one of the most important risk factors for AD [23]. AD treatments consist primarily of cholinesterase inhibitors, including donepezil (Aricept), rivastigmine Exelon),
and galantamine (Razadyne). These medications increase acetylcholine levels to temporarily reduce cognitive dysfunction [24].
However, they have significant limitations: they do not slow disease progression, they can cause side effects such as nausea and insomnia that
can affect patient adherence, and their effectiveness often decreases over time [25]. Ultimately, while they providesome symptomatic relief, they do not improve AD
pathology. Due to these undesirable side effects, research is more focused on alternative strategies that may have a more comprehensive safety profile and efficacy.
AD is mainly driven by a complex interaction of lifestyle genetic, and environmental variables that lead to neuronal
degeneration in the brain. Aβ peptides accumulated in the brain to form insoluble plaques, which play a vital role in
the pathophysiology of AD by impairing neuronal communication and triggering inflammatory responses [26].
Microglia are exposed to Aβ through amyloid compaction, which also plays a critical role in microglial activation
through NOD-like receptor and toll-like-receptor (TLR) signaling, resulting in the production of proinflammatory
cytokines [27]. Aβ accumulation is also linked to compromised synaptic signaling and plasticity, both of which
are crucial for learning and memory [28]. Aβ accumulation, impaired brain glucose metabolism, and impaired phosphorylation, dephosphorylating pathway enhances
tau hyperphosphorylation, which results in the development of neurofibrillary tangles [29]. In healthy neurons, tau protein plays a crucial role in stabilizing microtubules,
key components of the cytoskeleton that help transport cellular materials within axons and dendrites [30]. When tau protein becomes hyperphosphorylated,it undergoes structural changes that interfere with its ability to effectively bind to microtubules [31].
This destabilization leads to microtubule disruption, which disrupts the transport of vital proteins, organelles, and other molecules necessary for proper neuronal function and survival [32].
The cholinergic hypothesis of AD suggests that a significant decrease in the neurotransmitter acetylcholine (ACh) contributes to the cognitive decline observed in the disease [33].
A more recent theory, known as the lipid invasion model, postulates that disruptions in the blood-brain barrier allow external lipids to enter the brain, triggering neuroinflammation and oxidative stress [34]. This model
indicates that lipid influx may play a role in the formation of amyloid plaques and neurodegeneration, offering a different perspective on the mechanisms underlying AD [35].
Genetic mutations in APP [36], presenilin (PSEN1 and PSEN2) [37], and apolipoprotein E (APOE ε4 allele) [38], greatly elevate the likelihood of developing AD.
Although there are several theories explaining AD, the most well-known of which is the Aβ hypothesis, researchers are now focusing more on gut dysbiosis,
which is assumed to be involved in the etiology of the disease [39]. The term “dysbiosis” describes the disruptions in the microbial population of the intestine, its local deposition patterns,
its metabolic features, and the gut epithelial barrier when compared with the healthy individual’s microbiota [40]. In both clinical and murine research, a marked shift toward pro-inflammatory microorganisms and a reduction
in the variety of bacteria have been linked to AD [41]. According to a recently published study, the abundance
of Firmicutes families such as Turicibacteraceae, Peptostreptococcaceae, Clostridiaceae, Ruminococcaceae, and Mogibacteriaceae
decreases, while the abundance of the genera Phascolarctobacterium, Blautia, and Gemella increases in AD [42]. The primary indicator of gut dysbiosis
has been associated with an increase in the Firmicutes/Bacteroidetes ratio since the early stages of AD, which is subsequently associated with APP accumulation in the gut
[43]. Elevations of Aβ in the central nervous system and deficits in memory and spatial learning have been associated with alterations in the gut microbiome of APP/PS1
mice [44]. Increased levels of bile acids (BAs) produced
by bacteria in the bloodstream may cause tight junction
rupture, increasing blood-brain barrier (BBB) permeability and allowing peripheral cholesterol or BAs to enter the
central nervous system. As cellular cholesterol builds up
in the brain, it binds directly to APP, making it easier for APP to enter into the phospholipid monolayers that constitute the lipid rafts where the formation of Aβ occurs,
eventually promoting the production and accumulation of Aβ [45]. An essential component of the Actinobacteria
phylum, Bifidobacterium controls the GBA, and its degeneration contributes to the pathophysiology of tau, leading to the build-up of tangles that cause cell damage and
inflammation in AD [46]. Elevated levels of CXCL2, IL1β, and NOD-like receptor protein-3 (NLRP3) have been
related to increased levels of proinflammatory bacteria
such as Escherichia, Shigella along with decreased levels
of anti-inflammatory bacteria such as Eubacterium rectale were found in the plasma of patients suffering from brain
amyloidosis and cognitive impairment [47]. Different
pathways suggesting a link between gut microbiota and
AD have been investigated in different studies and are
summarized in Figure 3.
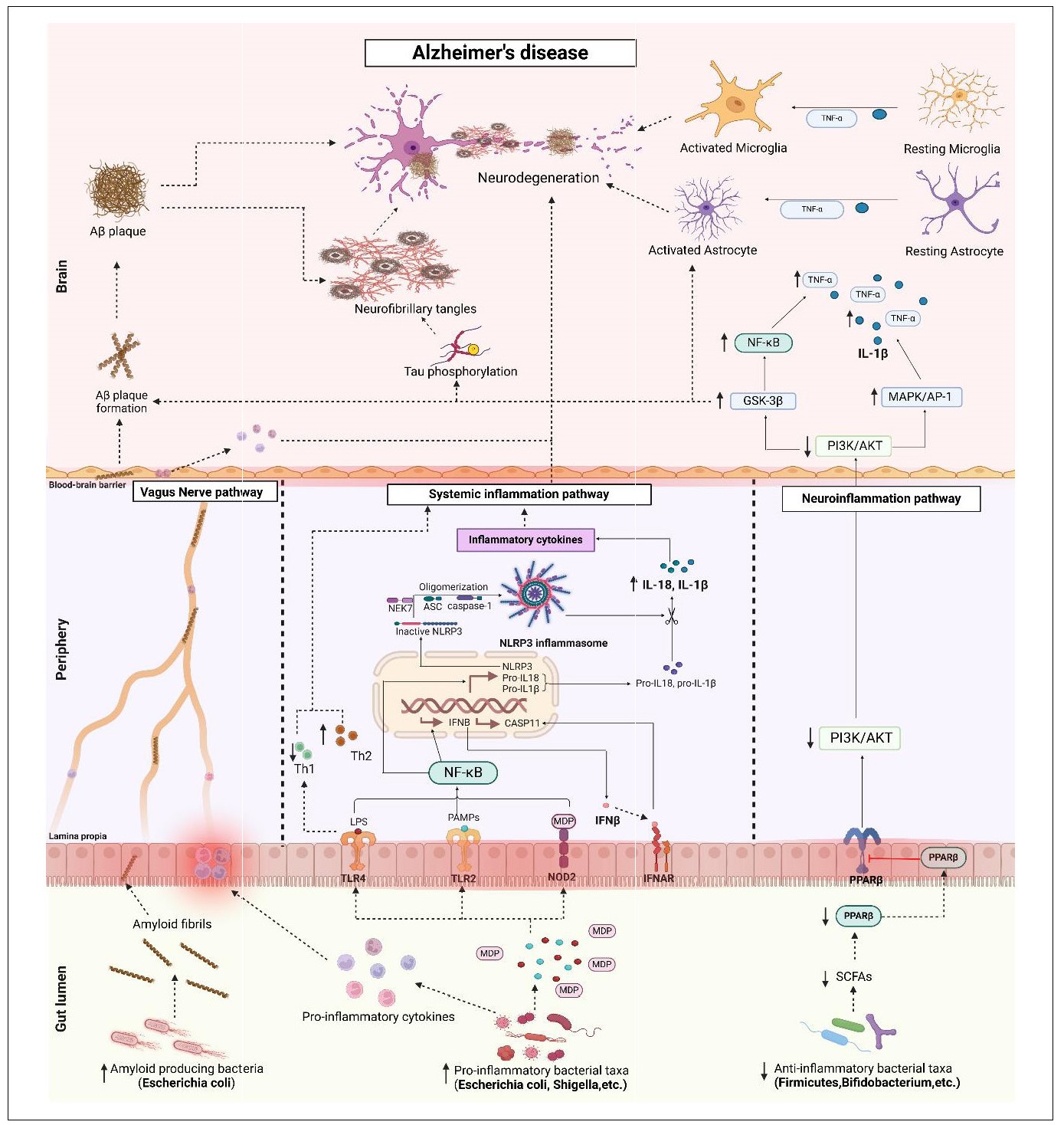
Figure 3. Potential signaling pathways linking gut dysbiosis with AD . The abundance of pro-inflammatory bacterial taxa over anti-inflammatory bacterial taxa resulting in gut dysbiosis may mediate AD. 1) Vagus nerve pathway: Pro-inflammatory bacteria secrete pro-inflammatory cytokines that cause increased intestinal permeability and inflammation, referred to as leaky gut. These pro-inflammatory cytokines travel through the vagus nerve and into the brain, where they cause neuroinflammation and neurodegeneration. Increased pro-inflammatory bacteria, particularly E. coli, also known as amyloid-producing bacteria, secrete amyloid into the intestinal lumen, which travels through the leaky gut and enters the vagus nerve. Amyloid travels through the vagus nerve and crosses the BBB, resulting in the formation of Aβ plaques. 2) Systemic inflammatory pathway: gut dysbiosis leads to increased production of lipopolysaccharide (LPS), pathogen-associated molecular patterns (PAMPs), and muramyl dipeptide (MDP). Upon recognition of these stimuli by TLR4, TLR2 and NOD2 receptors, NF-κB is activated, leading to the production of interferon-beta (IFN-β) and the transcription of NLRP3 and other pro-inflammatory genes. Activation of IFN-β by IFNAR leads to activation of the CASP11 and NLRP3 inflammasomes. Activation of the NLRP3 inflammasome involves oligomerization of the inactive NLRP3 protein, an adaptor protein called ASC, caspase-1, and NEK7. The NLRP3 inflammasome converts pro-IL-18 and pro-IL-1β to their active forms, IL-18 and IL-1β. These pro-inflammatory cytokines lead to systemic inflammation, followed by neuroinflammation and neurodegeneration. Stimulation of TLR4 by LPS leads to an imbalance between Th1 and Th2 cells, contributing to systemic inflammation. 3) Neuroinflammatory pathway: reduced levels of SCFAs lead to decreased expression of PPARβ, which inhibits the PI3K/AKT pathway and activates the GSK-3β and MAPK/AP-1 pathways, resulting in the production of IL18 and TNF-α. The GSK-3β pathway stimulates tau phosphorylation and Aβ plaque formation, leading to neurofibrillary tangles and AD progression. All of these pathways lead to AD pathogenesis. TLR, Toll-like-receptors; NOD2, nucleotide-binding oligomerization domain containing 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; CASP11, caspase 11; NLRP3, NOD-like receptor protein 3; ASC, apoptosis-associated speck-like protein; NEK7, NIMA-related kinase 7; IFNAR, interferon beta receptor; IL, interleukin; Th, T helper cells; PPAR-β, peroxisome proliferator-activated receptor beta; PI3K/AKT, phosphatidylinositol 3-kinase/protein kinase B; GSK-3β, glycogen synthase kinase 3 beta; MAPK/ AP-1, mitogen-activated protein kinase/activator protein 1; TNF-α, tumor necrosis factor alpha.
Prebiotics, probiotics, and synbiotics may prove useful as novel biological prophylactics in the treatment of AD because of their ability to improve cognition and metabolic activity, their antioxidant and anti-inflammatory properties, and their ability to produce metabolites essential for the gut and brain [48]. The most extensively studied prebiotic is FOS, which is naturally present in many fruits and vegetables and serves as a substrate for the growth of Bifidobacterium and Lactobacillus, thus promoting their production in AD patients [49]. When FOS was administered to transgenic AD mice, there was an increase in glucagon-like peptide-1 (GLP-1), a protein that readily crosses the BBB, stimulates pancreatic insulin production, and promotes delayed gastric emptying. As a result of the delayed glucose metabolism associated with AD patients, this increase in cerebral GLP-1 prevents CNS insulin resistance and slows neuronal death [50]. Enhancing the diversity of the gut microbiota through the introduction of xylooligosaccharides (XOS), which are naturally obtained from honey, fruits, bamboo sprouts, vegetables, and more recently from sugar cane biomass, showed reduced intestinal inflammation by lowering levels of immunosuppressive cytokine-like IL-10 as well as pro-inflammatory cytokines such as IL-1β and IL-6, which were elevated in APP/PS1 mice [51]. Probiotic species such as Bifidum animalis, Lactobacillus plantarum and Lactobacillus fermentum have shown the ability to produce antioxidants in large quantities [52]. According to Kobayashi et al., the probiotic strains Bifidobacterium infantis and Bifidobacterium breve strain A1 enhanced the proportion of superoxide dismutase (SOD) and decreased the deposition of IL-1, Aβ, and TNF-α in the hippocampal region of the brain in Aβ-induced AD rats [53]. Administration of Bifidobacterium increases hippocampal plasma acetate levels, which improves cognitive function and inhibits the production of immune-reactive genes. From this, it can be deduced that Bifidobacterium can prevent neuroinflammation and control the immune response that arises from Aβ toxic exposure in brain tissue [54]. Bifidobacterium breve MCC1274 treatment in wild-type (WT) mice decreased the AD-related pathologies by lowering the levels of phosphorylated tau, presenilin 1 protein, and soluble hippocampal Aβ1-42. Also, it enhanced synaptic proteins and decreased neuroinflammation [55]. In astrocytes, Lactobacillus reuterican reduce neuroinflammation by promoting the synthesis of indole-3-propionic acid and indole3-aldehyde by subsequently passing through the BBB [56]. For neural defense, nuclear factor erythroid-related factor 2 (NRF-2) is essential because it can stimulate the expression of genes that are cytoprotective, anti-inflammatory, and antioxidant, which are reduced in the pathology of AD [57]. Curcumin, found in saffron, has neuroprotective qualities. According to Patel et al., curcumin and Lactobacillus rhamnosus may work together as an adjuvant to enhance memory and learning and raise antioxidant enzyme levels in mice with scopolamine-induced dementia [58]. Synbiotic powder (containing vitalon probiotics (VP) powder -Bifidobacterium longum, Bacillus coagulans, Bifidobacterium breve, protease, and maltodextrin) and prebiotics (composed of 650 mL of healthy green tea that contains 2.5% inulin freeze-dried into powder) were mixed in a 1:7 ratio and dissolved in water at 0.5 g/ mL and administered to 3-month-old APP and wild-type (WT) mice [48]. The results showed a significant decrease in Aβ42 levels between the treatment and control groups. Thus, the consumption of synbiotics was able to effectively reduce the accumulation of Aβ42, the most pathogenic species among the different Aβ lengths. Doublecortin (DCX), a marker of neurogenesis, was also found to be significantly increased in APP mice treated with the symbiotic mixture, indicating that the symbiotic treatment can promote neurogenesis. Levels of pro-inflammatory cytokines such as TNF-α were also reduced between groups [59]. An interesting study was conducted on a transgenic humanized Drosophila melanogaster model that exhibited an AD phenotype caused by BACE1-APP. The insects were given the synbiotic containing the polyphenol-rich plant prebiotic Triphala and three metabolically active probiotics (Lactobacillus fermentum, Lactobacillus plantarum, and Bifidobacterium longum spp. infantis), and the results showed suppression of Aβ aggregation as well as a reduction in neuroinflammation by generating a secondary source of antioxidants [60].
Parkinson’s disease (PD)
PD is a complex neurodegenerative disease that progresses with age and results in uncontrollable or unintended
movements, including stiffness, tremor, and difficulty with
balance and coordination [61]. Worldwide, approximately
10 million people are affected by PD, with approximately
one million people in the United States, which is expected to increase to 1.2 million by 2030. According to
a survey conducted by the 2022 Parkinson’s Foundation,
approximately 90,000 Americans are diagnosed with PD
each year, a sharp increase of 50% from the previously
estimated rate of 60,000 diagnoses per year [62]. PD affects 0.3% of individuals between the ages of 55 and 64,
1.0% of individuals between the ages of 65 and 74, 3.1%
of individuals between the ages of 75 and 84, and 4.3%
of individuals over the age of 85 [63]. The facts clearly
indicate that PD accelerates with age, making it one of
the most significant risk factors for the disease. First-line
treatment for PD consists of levodopa, often combined
with carbidopa (Sinemet), and dopamine agonists such as
pramipexole (Mirapex) and ropinirole (Requip) [64]. Levodopa is highly effective for motor symptoms by increasing dopamine levels, while dopamine agonists are useful
in the early stages or as adjunctive therapy. However,
long-term use of levodopa can lead to reduced efficacy
and motor fluctuations, and dopamine agonists can cause
side effects such as nausea and sleep disturbances [65].
Importantly, neither treatment addresses the non-motor
symptoms that greatly impact a patient’s quality of life.
As a result of these undesirable side effects, prebiotics,
probiotics, or synbiotic treatments are receiving more attention in research as they may have a better safety profile and greater efficacy.
A mechanistic link between aging and PD has been established through various pathways, including decreased
dopamine levels, abnormal accumulation of alpha-synuclein, loss of protein homeostasis, neuroinflammation,
genomic instability, oxidative damage, and impaired stress
responses [66]. Dopamine is a neurotransmitter responsible for coordinating millions of nerve and muscle cells
involved in movement and is broken down by an enzyme
called monoamine oxidase B (MAO-B), which leads to
a reduction in dopamine levels. Dopamine levels decline
by about 13% each decade after age 45 in brain regions
associated with motor and cognitive function. This decline coincides with an increase in brain levels of MAOB [67]. The dopamine transporter (DAT) is the primary
defense mechanism of dopaminergic neurons against this
oxidative stress. DAT transports the damaging dopamine
to the nerve terminal where it can be repackaged into synaptic vesicles by the vesicular monoamine transporter 2
(VMAT2). There have been reports of a decline in DAT
expression in the dorsal layer of the substantia nigra (SN)
with age, which may explain some of the neuronal vulnerability to loss in PD [68]. The abnormal accumulation of
alpha-synuclein protein as Lewy bodies is a key feature of
PD. These aggregates disrupt cellular function and induce
neuronal toxicity, leading to the degeneration of dopaminergic neurons in the substantia nigra [69]. This neuronal
loss leads to the motor symptoms of PD, including tremor,
rigidity, and bradykinesia. Lewy bodies not only characterize the disease, but also highlight the pathogenic processes that drive its progression [70]. Approximately 15%
of individuals with PD have a family history of the disease, and these can be caused by genetic abnormalities in
a variety of genes, such as α-synuclein (α-Syn), LRRK2,
PARK2, PARK7, PINK1, or the SNCA genes [71].
While there are many theories to explain PD, a growing
variety of research suggests a connection between gut dysbiosis and the development and progression of PD. The
potential pathobionts are from the genera Escherichia,
including Shigella, Streptococcus, Proteus, Enterococcus,
Enterobacteriaceae, and Helicobacter pylori were significantly increased, whereas Blautia, Faecalibacterium,
Ruminococcus, Prevotellaceae, and Enterobacteriaceae were significantly decreased in PD subjects compared to
healthy controls [72]. Curli is one of the amyloid proteins
produced by E. coli and other strains of Gram-negative
bacteria that form biofilms that promote bacterial adhesion
and colonization. Repeated oral administration of Curliproducing bacteria to aged wild-type (WT) rats resulted in
the formation of intestinal α-Syn deposits, and brain samples from these animals showed increased levels of TLR2, IL-6, TNF, along with enhanced microgliosis, astrogliosis, and neuronal α-Syn deposition in both the intestinal
and brain tissues of α-Syn-overexpressing rats [73]. α-Syn
aggregates cause dysfunction in presynaptic and postsynaptic transmission and disrupt dopaminergic transmission.
In this way, CURLI subunits may facilitate the development of Parkinson’s disease [74]. It has been observed
that variations in gut microbiota populations are influenced by dopamine production through changes in levels
of the gut hormone ghrelin. Ghrelin is a gastrointestinal
hormone that controls appetite and obesity and is found
in the endogenous ghrelin receptor (GHSR) located in the
hypothalamus [75]. The intestinal mucus barrier becomes
more permeable due to the growth of the Akkermansia
genus, which breaks down the mucus layer and uses the
mucus as energy to perform its function [76]. Variations
in the intestinal microbiota population affect the integrity
and permeability of the BBB by altering tight junctions
such as occludin, claudin, and zonula occludens-1 (ZO1), which maintain normal BBB permeability. The altered
subcellular distribution of ZO-1 and occludin, as well as
the decreased expression level of colonic occludin, have
shown that intestinal permeability is altered in PD patients
[77]. The potential pathways linking gut dysbiosis to PD
are summarized in Figure 4.
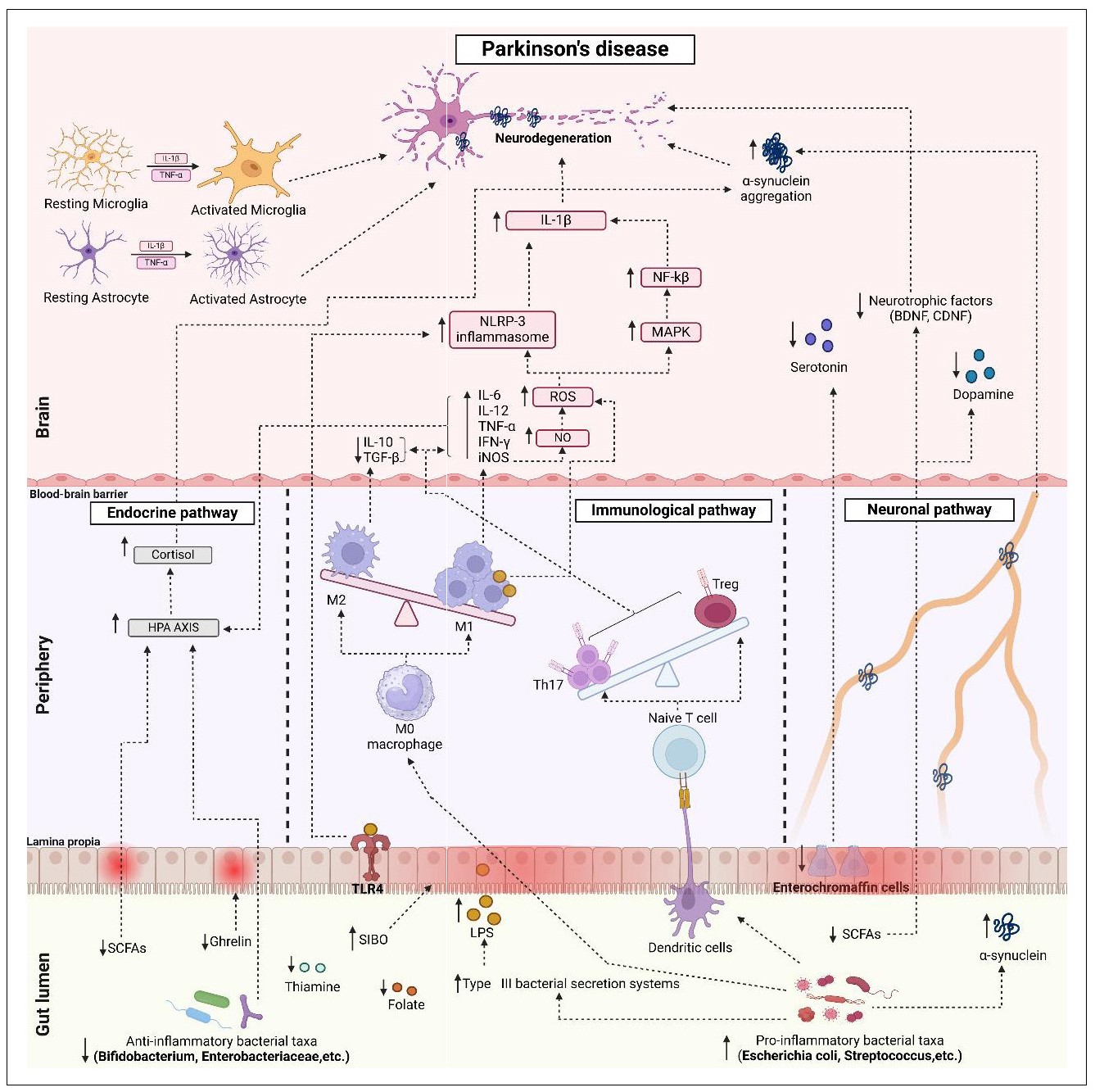
Figure 4. Potential signaling pathways of pathogenesis of gut microbiota-mediated Parkinson’s disease. The dominance of pro-inflammatory bacterial taxa over anti-inflammatory bacterial taxa (gut dysbiosis) initiates different pathways for the pathogenesis of PD. 1) Endocrine pathway: Gut dysbiosis results in decreased production of SCFAs, thiamine, folate, and ghrelin, and increased levels of LPS (via an enhanced type III bacterial secretion system). Reduced SCFAs and increased LPS lead to activation of the HPA axis and release of stress hormones. Increased plasma levels of cortisol promote α-syn aggregation and neurodegeneration. Reduced levels of SCFAs and ghrelin cause intestinal inflammation and leaky gut by reducing mucin secretion. In addition, ghrelin is responsible for maintaining and protecting the normal function of nigrostriatal dopaminergic neurons. 2) Immunological pathway: Gut dysbiosis increases SIBO, which increases intestinal permeability and creates an imbalance between Th17 and Treg and M1 and M2 macrophages leading to the formation of ROS, Inos. It induces the production of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-6, and IL-12) and decreases the anti-inflammatory cytokines IL-10 and TGF-β. iNOS produces NO and potentiates ROS production. Similarly, depletion of Treg cells and increase in Th17 produce pro-inflammatory cytokines and ROS. Increased ROS can activate the NLRP-3 inflammasome and the MAPK/NF-κβ pathway. In addition, the presence of inflammatory cytokines such as TNF-α and IL-1β activates astrocytes and microglia leading to neurodegeneration. 3) Neuronal pathway: Decrease in SCFAs and enterochromaffin epithelial cells affects the formation of serotonin, dopamine and brain-derived neurotrophic factor (BDNF) in the brain via the vagus nerve and accelerates the accumulation of α-syn. All of these factors contribute to neurodegeneration in PD. SIBO, small intestinal bacterial overgrowth; SCFA, short chain fatty acid; LPS, lipopolysaccharides; α-syn, alpha synuclein; HPA, hypothalamus-pituitary adrenal; ROS, reactive oxygen species; IL, interleukin; IFN-γ, interferon γ; TNFα, tumor necrosis factor α; iNOS, inducible nitric oxide synthase; NO, nitric oxide.
Probiotics, prebiotics, or synbiotics may prevent and alleviate Parkinson’s disease (PD) symptoms by regulating intestinal microecology, reducing oxidative stress damage and inflammatory response, and enhancing neurotrophic factor and dopamine production through the gut-brain axis (Figure 2). A recent open-label, non-randomized study was conducted in a small cohort of PD participants who received a prebiotic fiber intervention consisting of rice bran, inulin, resistant maltodextrin, and resistant starch incubated with human stool obtained from healthy controls. The bacterial population enriched by the administration of the prebiotic intervention was from the genera Ruminococaceae, Prevotella, and Lachnospiraceae promoted by resistant starch; the genera Ruminoccocus, Bacteroides, and Doreaa promoted by rice bran; the genera Bifidobacterium, Blautia, and Anaerostipes promoted by inulin; and the genus Parabacteroides promoted by resistant maltodextrin. In addition, changes in the gut microbiota, increased SCFA, decreased calprotectin (responsible for intestinal inflammation) and zonulin (a potential indicator of intestinal barrier inflammation) were observed, along with a small statistically significant decrease in NfL (a neurodegenerative marker) [78]. A four-strain probiotic comprised of Lacticaseibacillus rhamnosus, Enterococcus faecium, Lactiplantibacillus plantarum, and Lactobacillus acidophilus was administered to PD patients in a multi-center randomized controlled trial that resulted in significant decrease in plasma levels of IL-6 and TNF-α as well as improvements in both motor and non-motor symptoms [79]. A recent preclinical study was conducted in female Sprague-Dawley (SD) rats to evaluate the effect of the probiotic Bifidobacterium breve (Bif11) and it was observed that the probiotic ameliorated motor symptoms in the methylphenyl tetrahydropyridine hydrochloride (MPTP) induction rat model assessed by the rotarod test. The results indicated the downregulation of tyrosine hydroxylase (TH) in the midbrain by MPTP, which was reversed by a higher dosage of Bif11, suggesting a potential compensatory mechanism in midbrain biosynthesis and signaling. TH is recognized as a potential indicator in the onset and progression of PD. It is also the rate-limiting enzyme in the biosynthesis of dopamine, and it has been reported that TH levels tend to decline in both animal models of PD patients with the disease. The neuroprotection provided by Bif11 may be a result of reduced levels of reactive nitrogen species (RNS) and reactive oxygen species (ROS), as well as inflammatory cytokines such as IL1β and IL-6, and the corresponding inflammatory protein expression [80]. A double-blind, placebo and randomized controlled trial was carried out on PD patients to evaluate the effect of synbiotic sachet comprised of five strains of beneficial probiotics, including Lactobacillus acidophilus (LA-5), Lactobacillus plantarum (LAP-10), Lactobacillus rhamnosus (LAR-7), Bifidobacterium longum (BIA-8), Streptococcus thermophilus along with 4 g of inulin that served as a prebiotic which showed a significant reduction in serum oxidative stress index (OSI), malondialdehyde (MDA), and increase in serum glutathione (GSH) level and total antioxidant capacity (TAC) and led to significant improvement in depression, cognitive dysfunction and improvement in the activities of daily living which was hindered in PD patients was measured by the scale known as Parkinson’s disease quality of life (PDQ-39) [81].
Amyotrophic lateral sclerosis (ALS)
ALS is defined as a degenerative motor neuron disease,
often associated with pathogenic neuronal hyperexcitability, characterized by impairment of motor neurons in the
spinal cord, primary motor cortex, and brain stem [82].
According to the National ALS Registry, the age-adjusted
prevalence estimate is 6.6 per 100,000. According to a
2023 study, the prevalence of ALS in the United States is
approximately 9.1 cases per 100,000 people [83]. The age
range of 18 to 39 years old had the lowest incidence rates
(0.6 age-adjusted rates per 100,000), whereas the age categories of 60–69 (4.2–4.4 age-adjusted rates per 100,000)
and 70–79 (19.5 age-adjusted rates per 100,000) age
groups had the highest incidence rates across all years,
suggesting that aging is a significant risk factor for disease
progression [84]. The only ALS drug approved by the U.S.
Food and Drug Administration (FDA) is riluzole, which
works by blocking glutamatergic transmission and lowering glutamate levels to protect motor neurons from degeneration caused by excitotoxicity [85]. The most common
side effects associated with riluzole are dizziness, general
malaise, and elevated liver enzyme levels, which may
indicate possible liver damage [86]. Prebiotics, probiotics and synbiotic therapies are gaining importance due
to these unfavorable side effects because of their better
safety profile and higher efficacy in the treatment of ALS
[87].
Remarkably, the pathological features of ALS common
to both hereditary and sporadic variants coincide with
markers of aging, including telomere attrition, disrupted
intercellular communication, inflammation, loss of proteostasis, mitochondrial failure, cellular senescence, and
genomic instability/DNA damage. [88]. In the SOD1G93A rodent model, telomerase knockout promotes telomere
shortening and an accelerated ALS phenotype [89]. Connexin-based gap junctions, such as Cx43, facilitate
communication between astrocytes. Astrocytic-mediated
neurotoxicity is associated with abnormally elevated Cx43
expression in mSOD1 mice and in cortical and spinal cord
(SC) astrocytes of ALS patients [90]. The SOD1G93A transgenic rat has elevated expression of pro-inflammatory
markers like IL-1β, NF-κB, and IL-18 along with NLRP3
inflammasome and caspase-1. Active caspase 1, IL-18,
and apoptosis-associated speck-like proteins containing
a caspase-1 recruitment domain (ASC) are also found
in higher concentrations of the spinal cord astrocytes of
SOD1G93A mice and sporadic ALS patients [91]. Furthermore, with aging, oxidative damage from reactive oxygen species (ROS) or sugars can modify proteins posttranslationally, which leads to the creation of advanced
glycation end products (AGEs) [92]. This AGE accumulation in neurofilament protein prolongs neuronal damage
in ALS patients by producing superoxide, blocking nitric
oxide-mediated responses, and causing covalent crosslinking [93]. When compared to non-transgenic or asymptomatic transgenic rats, senescence markers such as loss
of nuclear lamin B1 expression and significantly elevated
levels of matrix metalloproteinase-1 (MMP-1), p53 and
p16INK4a were observed [94]. Senescence in naturally aging neurons may therefore impair their viability and make
them more susceptible to various diseases. However, more
research is needed to elucidate the relationship between
senescence and the pathogenesis of ALS [95].
Although there are several theories to explain ALS, a
growing collection of research indicates a link between
intestinal dysbiosis and the development and progression
of ALS. When compared to controls, the genus Dorea was
overexpressed, while the genera Anaerostipes, Lachnospira, andOscillibacter were comparatively underexpressed
in ALS patients [96]. Apart from this, specific species like Butyrivibrio fibrisolvens, Roseburia intestinalis, Akkermansia muciniphila, and Eubacterium rectale were also
decreased along with a decrease in Firmicutes/Bacteroidetes (F/B) ratio along with lower abundance of Clostridium and some yeasts [97, 98]. In the ALS group, a higher proportion of uncultured Ruminococcaceae, E. coli, and Enterobacteria were also found [99]. Certain taxa, including Sphingomonas, Gaiella, Lachnospiraceae, and Klebsiella have been identified to be significant ALS predictors [100].
Increased risk of ALS was shown to be related to Enterobacteriaceae and unclassified Acidaminococcaceae. It is
probable that γ-glutamyl amino acids may have a negative
correlation with the possibility of developing the disease
where γ-glutamyl phenylalanine is a specific risk factor for
the condition. It was discovered that both of its metabolites, 3-methyl-2-oxobutyrate and 1-arachidonoyl-GPI, increase the possibility of developing ALS, whereas higher
4-acetylaminobutyric acid levels may lower the incidence
of ALS [101]. It was reported that butyrate-producing
bacteria (e.g., Butyrivibrio Fibrisolvens) are substantially
reduced in ALS patients than in healthy controls. These
changes affect not only the generation of SCFAs but also
exhibit the potential to aggravate gut inflammation locally
and initiate a neuroinflammatory or systemic response
[102]. According to Niccolai et al., stool samples from
patients with ALS showed considerably greater levels of
inflammatory biomarkers, including IL-1α, IL-6, IL-18,
and IL-27, monocyte chemoattractant protein-1 (MCP1) as well as macrophage inflammatory protein-1 alpha
(MIP-1α). Additionally, it has been shown that the serum
and CSF fluid of ALS patients contained higher amounts
of circulating inflammatory cytokines, such as IL-23 and
IL-17, which indicated a Treg/Th17 imbalance [103]. Sagi
et al. reported that alterations in the gut microbiota and F/B ratio were linked with significant metabolic abnormalities in mice lacking the antioxidant enzyme superoxide
dismutase 1 (SOD1), a well-known animal model of ALS
[104]. SOD1 deficiency increased oxidative stress, which
prevented hepatic gluconeogenesis and facilitated lipid accumulation. Furthermore, a crucial enzyme in glycolysis,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH),
was linked to increased nitrosylation and subsequent deactivation in relation to redox imbalance. Chronic variations in the metabolism of carbohydrates might negatively
affect the energy balance and progression of the ALS disease [105]. The presence of Akkermansia muciniphila (A.
muciniphila) has been linked to better clinical outcomes,
a possible increase in motor neuron survival, and a rise
in nicotinamide, which is also linked to improvements in
motor and functional abilities in ALS patients [106]. The
immediate link of bacteria, A. muciniphilais highlighted
due to its capacity to elevate GABA/glutamate ratios in
the hippocampus in the pathogenesis of the disease [107].
Prebiotics, probiotics and synbiotics offer a promising avenue for exploring novel therapeutic strategies by modulating the gut microbiome and gut-brain axis function,
reducing inflammation and conferring neuroprotection
for debilitating disease [108]. A common prebiotic is galactosaccharide (GOS), which, when administered along
with prebiotic curd rich in GOS to SOD1G93A mice, their
life duration significantly increased, motor neuron loss
was also decreased, and the production of inflammatory
markers TNF-α and iNOS was also blocked [109]. According to a study conducted on SOD1G93A mice, the polyphenol Epigallocatechin gallate (EGCG) present in green
tea is the main ingredient promoting the increase in A.
muciniphila levels. Additionally, EGCG can boost SCFA
levels substantially and particularly promote the proliferation of Bifidobacterium and Lactobacillus, thereby alleviating the symptoms of the disease [110]. The ALS mice
expressing human mutant of transactive response DNA
binding protein of 43 kDa (TDP43) were treated with probiotic formulation VSL#43 (comprised of Streptococcus
thermophilus, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus helveticus, Bifidobacterium breve,
Lactobacillus paracasei, Bifidobacterium longum and Bifidobacterium infantis) by oral gavage for three weeks
daily. The probiotic therapy enhanced Butyrivibrio fibrisolvens, Butyryl-coenzyme A CoA transferase as well as
smooth muscle actin (α-SMA), ZO-1, and Claudin-5 in the
colon, spinal cord, and brain. It also decreased the expression of inflammatory cytokines (IL-6, IL-17, and IFN-γ),
GFAP, and TDP43 [111]. A longitudinal study was carried
out to assess the changes in the morphology of the colon
and ileum in mutant SOD1G93A transgenic mice models of
ALS by using immunofluorescence and Western blotting.
A multistrain probiotic combination (LBE) comprised of
live bacteria of Enterococcus faecalis, Bifidobacterium
longum, and Lactobacillus acidophilus was given to the
mice beginning from 60 days of age and continued until
the disease reached its fatal stage. Oral administration of
LBE- not only protected the neuronal cells in the gut but
also in the spinal cord of SOD1G93Amice, decreased abnormal SOD1 aggregation, and improved the pro-inflammatory response. Additionally, intestinal microbiota, SCFA
levels, and autophagy function were all enhanced through
LBE therapy [112]. Another interesting finding from prospective longitudinal research, including 50 ALS patients
and 50 controls, demonstrated an imbalance between
microbial groups that may be beneficial, such as Bacteroidetes, and those that may be neurotoxic or have proinflammatory functions, like Cyanobacteria. Moreover, Cyanobacteria produce additional neurotoxic compounds
such as nodularin, which damages the cytoskeleton [113];
saxitoxin, which paralyzes voluntary muscle contraction
[114]; and also microcystins, which are undesirable for
the brain [115]. The microbial groups that altered the most
over time were Bacteroidetes, and related families serve
as a defense mechanism against neurotoxicity because
of their many roles in the generation of butyrate, the activation of T cell-mediated responses, the metabolism of
toxic and/or mutagenic compounds, and the synthesis of
bile acid [116]. These findings may serve as a basis for
further research on compounds associated with cytotoxicrelated Cyanobacteria in the blood of ALS patients in
order to validate the theory that these bacteria play a role
in the pathogenesis of the disease [116]. The most widely
used synbiotics are mostly made up of oligofructose and Bifidobacterium or Lactobacillus [117]. The impact of
synbiotics on ALS patients or animal models has not been
extensively studied in research [97].
Multiple sclerosis (MS)
MS is an autoimmune neurodegenerative disease characterized by progressive destruction of the myelin sheath
surrounding nerve fibers by reactive T cells. Demyelination of nerve fibers causes axonal inflammation and
results in a lack of coordination in walking and standing, including tremors, muscle spasms, irregular bowel movements, and cognitive impairment [118]. It is thought to
be of non-traumatic origin and predominates in young
women. MS has been subdivided into benign MS (BMS),
progressive relapsing MS (PRMS), primary progressive
MS (PPMS), relapsing and remitting MS (RRMS) and
secondary progressive MS (SPMS). In RRMS patients,
relapses are mostly reversible with occasional exacerbations, whereas in SPMS there is continuous disease progression with or without relapses. PPMS is characterized
by the recurrence of neurological symptoms with continuous disease progression and no response to treatment
[119].
Although the origin of MS is still unknown, current research suggests that gut microbiota dysbiosis promotes the
development and progression of MS [120]. The microbial
diversity is affected in all subcategories of MS patients
[121]. The significant drop in relative abundance of Bifidobacterium, Firmicutes, Lachnospiraceae, Prevotella,
Roseburia, Coprococcus, Dorea, Lachnospira, Faecalibacterium, and Butyricicoccushas been reported in gut
microbiome of MS patients including a marked increment
in Bacteroidetes, Akkermansia, Blautia, and Ruminocococcus population compared to healthy people [122].
The depletion of Prevotella concentration is responsible
for relapsing episodes of RRMS patients and the expansion of Th17 cells [123]. Another study reported reduced
levels of Clostridia clusters XIV and IV, resulting in less
amount of SCFAs, Treg cells, and anti-inflammatory cytokines like 1L-10 production in RRMS patients [124, 125]. Clostridium is known for the production of Treg cells and
anti-inflammatory cytokines like IL-10. Firmicutes, Bifidobacterium, and Prevotellaare mainly responsible for
the production of explicit microbial metabolite, SCFAs,
and immune regulation. Therefore, SCFA levels in the serum of MS patients were observed low [126, 127], mainly
butyrate [128, 129]. Similarly, Coprococcus, Butyricicoccus, Lachnospira and Roseburiaare butyrate-producing
bacteria. Faecalibacterium can convert other SCFAs,
such as acetate and lactate, into butyrate. Therefore, the
low concentration of the above-mentioned significant
butyrate-producing bacteria in MS patients results in
low SCFAs and butyrate molecules. SCFAs and butyrate
have anti-inflammatory properties and have important
immunomodulatory functions by enhancing Treg cells
[130]. Moreover, SCFAs can cross BBB and can reduce
the neuro-inflammatory cytokines [131], which trigger
an inflammatory state favouring neuroinflammation in
MS patients. Akkermansiais a mucin degrading bacteria
and mucin degradation may cause intestinal inflammation
[132, 133]. Blautia can release acetate, which stimulates
insulin release and encourages hyperglyceridemia, fatty
liver disease, and insulin resistance [134]. The reduced
alpha diversity of gut microbiota was detected in RRMS
patients [126]; however, an increase in alpha diversity was
shown in PPMS [127]. The population of Adlercreutzia
was lessened in MS patients, resulting in enhancement in
oxidative stress, including inflammatory cytokines (IL-6)
and chemo-attracting proteins-1 [135]. The consequences
of the gut microbial community in MS are generally studied on an experimental autoimmune encephalomyelitis
(EAE) animal model [136] (Figure 1).
The introduction of various therapeutic agents such as
probiotics and prebiotics to improve the condition of MS
patients by modulating gut dysbiosis, reducing oxidative stress and improving mental health has been studied
[137]. Probiotic supplementation has slowed the onset
and progression of the disease, including improved motor coordination by regulating immune and inflammatory
factors [138]. The two probiotics named Lactobacillus
plantarum and Lactobacillus paracasei restrained neurological symptoms in the EAE animal model [139]. The
administration of probiotics Lactobacillus casei shirota,
Bacteroides fragilis, Bifidobacterium bifidum, and Bifidobacterium animalis in EAE mice, suppressed TNF-α,
IL-17 which further reduced Th1 and Th17 immune cells
and triggers Treg and IL-10 secretion and improves the
diseased condition [140]. Many more probiotics like Candida kefir [141], Lactobacillus lactis [142], Lactobacillus and Bifidobacterium [143], Bifidobacterium animalis Pediococcus acidilactici [144], a combination of L. plantarum and B. animalis has proven the positive effect
in EAE animal model. Similar results have been observed
in clinical studies as well. Various probiotics individually
[145, 146], as well as a combination of probiotics (L. casei, L. fermentum, L. acidophilus, and B. bifidum) [147],
have made MS disease progression sluggish. The intake
of different prebiotics in the form of non-fermentable dietary fiber (cellulose-rich diet) has healed gut dysbiosis
by enriching Ruminococcaceae, Helicobacteraceae , and Enterococcaceae and lowering the Sutterellaceae and Coriobacteriaceae and prevents EAE in animals. Further,
it reduces Th2 immune responses [148]. Recently, in randomized and cross-over trials, prebiotics (Prebiotin, containing oligofructose enriched inulin) and probiotics (Visbiome, containing Lactobacillus, Bifidobacterium, and Streptococcus species) were administered to MS patients
for six weeks. Both the supplements were well tolerated,
but prebiotics were preferable to probiotics among MS patients; however, probiotics significantly improved bowel
control than prebiotics [149] (Figure 2).
Moreover, few techniques like fecal microbial transplantation (FMT) have been reported the successful amelioration
of MS symptoms [150, 151]. Diet modification studies in
MS patients have shown promising results in alleviating
the chronic symptoms of MS. Intervention with vitamin
D supplements in a low-calorie diet and intake of dietary
polyphenols like resveratrol, quercetin has rebalanced the
gut microbiota, consequently leading to improved mitochondrial function [152], reduced oxidative biomarkers,
better motor function and balance. Additionally, these diet
modifications hampered the production of inflammatory
cytokines (TNF-α) and autoimmune T cells and demyelination in MS and contributed to a better quality of life
in MS patients [141-143]. A ketogenic diet consumed for
6 months by MS patients reversed the composition of the
gut microbiome to normal, especially the abundance of
Akkermansia strains [144]. One more method that has recently drawn attention in combating MS symptoms is intermittent fasting (IF), which enriched the gut population of Bacteroidaceae, Lactobacillaceae , and Prevotellaceae in EAE animals. Additionally, it showed an immunomodulatory effect by reducing Th17 and stimulating Treg
cells [153, 154]. Many other variations in diet such as oral
supplementation of SCFAs and introduction of polyunsaturated fatty acids (PUFAs) like omega-3 have indicated
a therapeutic potential for MS. SCFAs such as propionate
or butyrate treatment has promoted neuroprotection and
remyelination, axonal density was also recovered with
better immune responses which slowed relapse rate and
severity of disease progression [152, 155]. Conjugated
linoleic acid supplementation lowered CNS inflammation
and demyelination, which corresponded with proliferation
in Porphyromonadaceae, Lachnospiraceae, Bacteroides,
Lactobacillus, and Akkermansia in EAE animals [156].
Thus, the imbalance between beneficial and pathogenic
bacteria in the gut microbiome is an important player
in the onset and progression of MS, and its modulation
through various therapeutic interventions has been shown
to benefit MS patients.
Huntington’s disease (HD)
HD is an inherited autosomal dominant neurodegenerative
disorder characterized by progressive cognitive decline
and behavioral disturbances along with movement disorders such as dystonia and chorea [157]. Recent advances
in the study of the gut microbiome have established a link
between gut dysbiosis and the development of HD. Both
wild-type (WT) male and female mice with HD exhibit
a characteristic gut bacterial dominance. WT male HD
mice had dominance of Clostridiales, Bacteroidales, and Lactobacillales compared to control WT male mice which
showed bacterial gut dominance of Clostridiales, Bacteroidales, Deferribacterales, Erysipelotrichales, and Lactobacillales. Similarly in WT female HD mice, the abundance of Clostridiales, Bacteroidales, and Lactobacillales were reported in compared with control WT female mice
where abundance of Coriobacteriales, Clostridiales,
Erysipelotrichales, Bacteroidales, and Burkholderiales were observed [143]. In transgenic R6/1 HD mice, the increased level of Bacteroidetes and decreased level of Firmicutes was noted however in R6/2 mice Firmicutes were
found in lower quantities whereas Proteobacteria and Bacteroidetes were found in greater quantities [158, 159].
Similar results, as depleted population of Firmicutes,
Lachnospiraceae and Akkermansiaceae were observed in
HD gene expansion carriers (HDGECs) male mice [160].
HD patients have a significant drop in a metabolite named
4-hydroxyphenyl acetic acid level which is derived from
the diet [161]. In HD patients, lower species abundance
of both α-diversity and β-diversity of gut microbiota
were observed. Significant differences in phylum level of Euryarchaeota, Firmicutes, and Verrucomicrobia were
noticed in HD male patients compared to healthy control.
Additionally, in family level of Enterobacteriaceae, Bacteroidaceae, Peptostreptococcaceae, Bifidobacteriaceae,
Erysipelotrichaceae, Christensenellaceae, Peptococcaceae, Coriobacteriaceae, Flavobacteriaceae, Akkermansiaceae, Eggerthellaceae, Lachnospiraceae, Methanobacteriaceae, Rikenellaceae, Acidaminococcaceae , and Clostridiaceae differences were highlighted however no
major differences were observed in women. These observations were correlated with the cognitive inability of HD
patients [162]. Another study results depicted, the lower
abundance of genus Intestinimonas and higher abundance
of genus Bilophila indicated the modulation of immune
responses of HD patients as plasma level of anti-inflammatory cytokines (IL-4) was decreased and proinflammatory IL-6 level was increased. The gut population of Porphyromonas was positively correlated with the plasma
concentration of IL-4, IL-10, and IL-13, whereas Oscillibacter and Gemmier were negatively correlated with IL-6
and Clostridium_XVIII were also negatively correlated
with TNF-α and IL-8 [163] (Figure 1).
With the advancement of research, various therapies
have been proposed to prevent the pathogenesis and progression of HD. The use of various polyphenols such as
rutin, resveratrol, and grape seed polyphenol extract has
counteracted neuroinflammation and neurodegeneration
progression in HD. Rutin induces nuclear localization of
DAF-16, which normalizes SOD-3 and HSP-16.2 gene
expression, which disrupts sensory terminals and provides neuroprotection in C. elegans models of HD [164].
Resveratrol stimulates Lactobacillus and Aβ clearance of
R6/2 transgenic HD mice which prevents apoptosis and
Bax gene and stimulates Bcl-2 genes. This cascade of
process delays neurodegeneration [165]. The grape seed
polyphenol extract delays motor incoordination and enhances longevity in R6/2 transgenic HD mice [166]. Other
polyphenols such as fisetin, hesperidin, and hesperetin
significantly enrich Lachnospiraceae and activate ERK/
MAPK signalling, which ameliorates the pathogenesis of
HD [167-169].
The most widely used probiotic strain, Lactobacillus and Bifidobacterium, provides neuroprotection in other neurodegenerative disorders, including HD [170]. The other
probiotics including Lactobacillus rhamnosus, Lactobacillus reutri, Lactobacillus casei or Lactobacillus acidophilus, Saccharomyces modulate gut dysbiosis and related
immune response [171]. These probiotics benefit in recovering intestinal permeability and promoting anti-inflammatory responses. Additionally, the production of SCFAs
can be stimulated and can significantly ameliorate the
neuropathogenesis of HD by lowering neuroinflammation
and neurodegeneration [172]. Well-planned studies, both
pre-clinical as well as clinical, needed to be carried out to
analyse the ideal probiotic for HD and to understand its
microbiota-based molecular mechanism. Other microbiota-based therapeutic approaches for HD are modifications
in diet, for example, the introduction of a high-fiber diet
[173], ketogenic diet [174], or Mediterranean diet to HD
patients [175]. These diets promote an abundance of beneficial bacteria, push gut dysbiosis to eubiotics, promote
SCFA production, and cease oxidative stress and neuronal
dysfunction, which may positively influence the neurogenesis of HD. However, advanced research is needed
to choose the prime dietary regimen for HD patients and
to interpret the mechanisms to control HD pathogenesis
and progression [173]. The other major technique that is
usually beneficial in other neurodegenerative diseases is FMT. FMT can restore the gut microbiota and its function
and modulate the brain axis; therefore, well-planned clinical trials are needed to confirm the safety and beneficial
effects of FMT in HD patients and to identify the precise
gut microbiota and metabolites that could act as therapeutic targets [174].
Clinical trials and evidence
Many clinical trials are currently underway to understand the clinical significance of various prebiotics, probiotics, or synbiotics and their mechanistic approach to ameliorating neurodegenerative disorders, which are listed in Table 1[176].
Table 1.
Application of different prebiotics, probiotics, or synbiotics in neurodegenerative disorders in ongoing clinical trials.
| Sl. No. | Neurodegenerative diseases | Therapeutic intervention | Trial outcome | Title of the study | Phase | NCT Number |
|---|---|---|---|---|---|---|
| 1 | AD | Probiotic blend capsule [20 million CFU(Bifidobacterium breve, Lactobacillus helveticus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus rhamnosus)]. |
• Pro-inflammatory cytokines (IL-6, IL-1β, CXCL2, NLRP3)
and anti-inflammatory cytokines (IL-10) are peripheral
inflammation indicators linked to the pathophysiology of
mild AD separated from plasma in blood. • 16S rDNA gene sequencing for bacterial identification, taxonomic profiling. |
Effect of probiotics on cognitive functioning of patients with mild Alzheimer's disease | Early Phase 1 | NCT06181513 |
| 2 | PD |
Ecologic® Barrier 849 [Maize starch, maltodextrin, vegetable protein, potassium chloride, +/- probiotic bacteria (L. casei W56, B. lactis W51, B. lactis W52, L. acidophilus W37, L. brevis W63, L. salivarius W24, Lc. lactis W19, Lc. lactis W58, B. bifidum W23; ≥ 2.5 × 109 CFU/g), magnesium sulphate, manganese sulphate sachet, two times daily dosing for a total of 2 g (viable cell count of 2.5 × 109 CFU/g) per day]. |
• The 12-item Parkinson anxiety scale (PAS) is a selfreported measure with three subscales: avoidance behavior,
episodic anxiety, and persistent anxiety; a likert scale (0–4) is
employed to rate it. • The beck depression inventory (BDI) is a multiple-choice, 21-question self-report measure with a 0-to-3 scale. |
Treating anxiety in Parkinson's disease with a multi-strain probiotic—a randomized, placebo-controlled trial | Phase 2 | NCT03968133 |
| Prebiotic fibers and a probiotic [Lactobacillus acidophilus-10 billion CFU] |
• The Movement Disorder Society Unified Parkinson's
Disease Rating Scale (MDS-UPDRS) will be used to assess
patients clinically both at baseline and 3 months later. • Blood samples will be collected at baseline and after 3 months |
Clinical study evaluating the efficacy and safety of synbiotic as an adjuvant therapy in the treatment of Parkinson's disease | Phase 3 | NCT05576818 | ||
| 3 | ALS | Probiotic formulation containing 15 billion CFU. |
• The study aimed to assess the changes in lipidomic profiles
within and between placebo and probiotic groups after 24
weeks of intervention. • The study aimed to evaluate changes in polar metabolite profiles within and between probiotic and placebo groups during a 24-week period. Key microbiota metabolites that will be targeted, includes SCFAs, trimethylamine N-oxide (TMAO), and tryptophan-derived metabolites. |
Effects of probiotics on lipidomic profile and disease evolution in ALS-FTDSD patients: a randomized multicenter, double-blind, phase II, placebocontrolled, parallel trial. | Phase 2 | NCT06051123 |
| 4 | MS |
Vivomixx® [1.800 bio bacteria/day] Conjugated linoleic acid (CLA/Tonalin® FFA 80) |
• T2 lesions were selected as the main outcome because they
have been utilized in a number of recent MS studies as a
surrogate indicator of treatment effectiveness. • The number of new or growing T2-weighted hyperintense lesions after 48 weeks of intervention is determined by comparing the brain MRI images to baseline. |
Effects of incorporating conjugated linoleic acid (CLA/Tonalin® FFA 80) with probiotics (Vivomixx®/VSL#3) as addon to a first-line immunotherapy in the treatment of RRMS. | Not applicable | NCT05920018 |
| Bouchard Belgian dark chocolate probiotic Napolitains, containing Bifidobacterium longum |
• Percent change in Treg cells from baseline to week 6. • Percent change in SCFA levels from baseline to week 6. |
The effects of probiotics on inflammatory biomarkers in MS patients and their family members with MS. | Not applicable | NCT06475183 |
Future directions
Research on the gut-brain axis is advancing rapidly, providing exciting new opportunities to explore the potential benefits of probiotics, prebiotics and synbiotics in the treatment of age-related and neurodegenerative diseases. Elucidate the mechanisms by which the gut microbiota influences neurodegenerative disease, such as the production of neuroactive metabolites, immune modulation, and gut-brain axis signaling, and characterize the distinct gut microbiome composition of patients to identify specific strains or microbial imbalances that could be targeted. Determining the specific indicators that distinguish neurodegenerative disease patients from healthy individuals and characterizing the gut flora in neurodegenerative disease are critical challenges. To address such challenges, high-quality metabolomic data from multiple long-term cohort studies and strain-level resolution metagenomic data would be extremely beneficial. Furthermore, precise mechanistic knowledge of the pathways by which gut microbes and their by-products affect the brain is still lacking. Designing personalized prebiotic or probiotic formulations according to each person’s microbiome profile may help identify specific bacteria that are important for disease development or progression and prevention. Investigating the possibilities of integrating probiotics, prebiotics, and synbiotics with additional therapeutic treatments, including drugs or dietary changes, may help alleviate disease.
Conclusions
The continued rise in the prevalence of neurodegenerative diseases worldwide, coupled with the ineffectiveness of FDA-approved drugs, highlights the need for a different approach to identifying effective therapeutic targets. Modern technologies are helping us to understand the complex interactions between gut bacteria and the brain in neurodegenerative diseases. Interventions that modulate the microbiome, such as probiotics, synbiotics, and prebiotics, could reduce the severity of symptoms by decreasing proinflammatory bacteria and increasing SCFA production, which may reduce the inflammatory tone associated with neurodegenerative diseases. This review aims to explore the various possible pathways and mechanisms related to gut dysbiosis and aging in various neurodegenerative diseases such as AD, PD, ALS, MS, and HD. Various studies have shown that prebiotics, probiotics and synbiotics can improve brain function and overall health and can be used as an alternative treatment strategy, thereby improving the overall quality of life of an individual diagnosed with a neurodegenerative disease.
Declarations
Acknowledgements
We would like to acknowledge the National Institute of Pharmaceutical Education and Research Kolkata for infrastructural support.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Not applicable.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther, 2022, 7(1): 135-145. [Crossref]
2. Chen Y, Zhou J, & Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol, 2021, 11: 625913. [Crossref]
3. Erny D, Dokalis N, Mezö C, Castoldi A, Mossad O, Staszewski O, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab, 2021, 33(11): 2260-2276.e2267. [Crossref]
4. Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, & Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci, 2015, 16(4): 7493-7519. [Crossref]
5. Ren J, Li H, Zeng G, Pang B, Wang Q & Wei J. Gut microbiome-mediated mechanisms in aging-related diseases:are probiotics ready for prime time? Front Pharmacol, 2023, 14: 1178596. [Crossref]
6. Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease:unveiling the relationship. Front Microbiol, 2022, 13: 999001. [Crossref]
7. Ahmed H, Leyrolle Q, Koistinen V, Kärkkäinen O, Layé S, Delzenne N, et al. Microbiota-derived metabolites as drivers of gut-brain communication.Gut Microbes, 2022, 14(1): 2102878. [Crossref]
8. Liu L, Huh JR, & Shah K. Microbiota and the gut-brainaxis: implications for new therapeutic design in the CNS. EBioMedicine, 2022, 77: 103908. [Crossref]
9. Strandwitz P, Kim KH, Terekhova D, Liu jK, Sharma A, Levering J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol, 2019, 4(3): 396-403. [Crossref]
10. Teleanu Rl, Niculescu AG, Roza E, Vladácenco O, Grumezescu AM, & Teleanu DM. Neurotransmitters-keyfactors in neurological and neurodegenerative disorders ofthe central nervous system. Int J Mol Sci, 2022, 23(11): 5954-5964.[Crossref]
11. Koutsokostas C, Merkouris E, Goulas A, Aidinopoulou K, Sini N, Dimaras T, et al. Gut microbes associated with neurodegenerative disorders: a comprehensive review of the literature. Microorganisms, 2024, 12(8): 17351745. [Crossref]
12. Pandey KR, Naik SR, & Vakil BV. Probiotics, prebiotics and synbiotics-a review. J Food Sci Technol, 2015, 52(12): 7577-7587. [Crossref]
13. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition,types, sources, mechanisms, and clinical applications. Foods, 2019, 8(3): 92-102. [Crossref]
14. Paiva IHR, Maciel LM, Silva RSD, Mendonça IP, Souza JRB, & Peixoto CA. Prebiotics modulate the microbiota-gut-brain axis and ameliorate anxiety and depressionlike behavior in HFD-fed mice. Foods Res Int, 2024, 182: 114153. [Crossref]
15. Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, & Müller DJ. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int J Mol Sci, 2022, 23(9): 4494-4506. [Crossref]
16. Medeiros D, McMurry K, Pfeiffer M, Newsome K, Testerman T, Graf J, et al. Slowing Alzheimer’s disease progression through probiotic supplementation. Front Neurosci, 2024, 18: 1309075. [Crossref]
17. Hasaniani N, Ghasemi-Kasman M, Halaji M, & Rostami Mansoor S. Bifidobacterium breve probiotic compared to Lactobacillus casei causes a better reduction in demyelination and oxidative stress in cuprizone-induced demyelination model of rat. Mol Neurobiol, 2024, 61(1): 498-509. [Crossref]
18. Rello J, van Engelen TSR, Alp E, Calandra T, Cattoir V, Kern WV, et al. Towards precision medicine in sepsis: a position paper from the European society of clinical microbiology and infectious diseases. Clin Microbiol Infect, 2018, 24(12): 1264-1272. [Crossref]
19. Wareham LK, Liddelow SA, Temple S, Benowitz LI, Di Polo A, Wellington C, et al. Solving neurodegeneration: common mechanisms and strategies for new treatments.Mol Neurodegener, 2022, 17(1): 23-35. [Crossref]
20. Puri S, Shaheen M, & Grover B. Nutrition and cognitive health: a life course approach. Front Public Health, 2023, 11: 1023907. [Crossref]
21. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement, 2024, 20(5): 3708-3821. [Crossref]
22. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, & Ferri CP. The global prevalen of dementia: a systematic review and meta analysis. Alzheimers Dement, 2013, 9(1): 63-75.e62 [Crossref]
23. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement, 2023, 19(4): 1598-1695. [Crossref]
24. Singh R, & Sadiq NM: Cholinesterase inhibitors. In: StatPearls. 2024, edn. Stat Pearls Publishing LLC.
25. Ruangritchankul S, Chantharit P, Srisuma S, & Gray LC. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: a comprehensive literature review. Ther Clin Risk Manag, 2021, 17: 927-949. [Crossref]
26. Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin , 2017, 38(9): 1205-1235. [Crossref]
27. García-Revilla J, Alonso-Bellido IM, Burguillos MA, Herrera AJ, Espinosa-Oliva AM, Ruiz R, et al. Reformulating pro-oxidant microglia in neurodegeneration. J Clin Med, 2019, 8(10): 1719-1729. [Crossref]
28. Parihar MS, & Brewer GJ. Amyloid-β as a modulator of synaptic plasticity. J Alzheimers Dis, 2010, 22(3): 741- 763. [Crossref]
29. Gong CX, & Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem, 2008, 15(23): 2321-2328. [Crossref]
30. Dolan PJ, & Johnson GV. The role of tau kinases in Alzheimer’s disease. Curr Opin Drug Discov Devel, 2010, 13(5): 595-603.
31. Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, et al. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci, 2018, 12: 338-348. [Crossref]
32. Mazanetz MP, & Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov, 2007, 6(6): 464-479.[Crossref]
33. Chen ZR, Huang JB, Yang SL, & Hong FF. Role of cholinergic signaling in Alzheimer’s disease. Molecules, 2022, 27(6): 1816-1823. [Crossref]
34. Rudge JD. A new hpothesis for Alzheimer’s dsease: te lpid ivasion mdel. J Alzheimers Dis Rep, 2022, 6(1): 129- 161. [Crossref]
35. Rudge JD. The lipid invasion model: growing evidence for this new explanation of Alzheimer’s disease. J Alzheimers Dis, 2023, 94(2): 457-470. [Crossref]
36. Schilling S, Pradhan A, Heesch A, Helbig A, Blennow K, Koch C,et al. Differential effects of familial Alzheimer’s disease-causing mutations on amyloid precursor protein (APP) trafficking, proteolytic conversion, and synaptogenic activity. Acta Neuropathol Commun, 2023, 11(1): 87-97. [Crossref]
37. Sun Y, Islam S, Michikawa M, & Zou K. Presenilin: a multifunctional molecule in the pathogenesis of Alzheimer’s disease and other neurodegenerative diseases. Int J Mol Sci, 2024, 25(3): 1757-1768. [Crossref]
38. Raulin AC, Doss SV, Trottier ZA, Ikezu TC, Bu G, & Liu CC. ApoE in Alzheimer’s disease: pathophysiology and therapeutic strategies. Mol Neurodegener, 2022, 17(1): 72-88. [Crossref]
39. Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, & Chen L. Recent advances in Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther, 2024, 9(1): 211-223. [Crossref]
40. Mahdavi-Roshan M, Salari A, Kheirkhah J, & Ghorbani Z. The effects of probiotics on inflammation, endothelial dysfunction, and atherosclerosis progression: a mechanistic overview. Heart Lung Circ, 2022, 31(5): e45-e71. [Crossref]
41. Wang SS, Li XH, Liu P, Li J, & Liu L. The relationship between Alzheimer’s disease and intestinal microflora structure and inflammatory factors. Front Aging Neurosci, 2022, 14: 972982. [Crossref]
42. Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep, 2017, 7(1): 13537. [Crossref]
43. Brandscheid C, Schuck F, Reinhardt S, Schäfer KH, Pietrzik CU, Grimm M, et al. Altered gut microbiome composition and Tryptic activity of the 5xFAD Alzheimer’s mouse model. J Alzheimers Dis, 2017, 56(2): 775-788. [Crossref]
44. Shen L, Liu L, & Ji HF. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis, 2017, 56(1): 385-390. [Crossref]
45. Liu S, Gao J, Zhu M, Liu K, & Zhang HL. Gut microbiota and dysbiosis in Alzheimer’s disease: implications for pathogenesis and treatment. Mol Neurobiol, 2020, 57(12): 5026-5043. [Crossref]
46. Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, & Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis, 2018, 50(5): 421-428. [Crossref]
47. Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging, 2017, 49: 60-68. [Crossref]
48. Arora K, Green M, & Prakash S. The microbiome and Alzheimer’s disease: potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front Bioeng Biotechnol, 2020, 8: 537847. [Crossref]
49. Chandra S, Sisodia SS, & Vassar RJ. The gut microbiome in Alzheimer’s disease: what we know and what remains to be explored. Mol Neurodegener, 2023, 18(1): 9-21. [Crossref]
50. Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun, 2018, 9(1): 3555-3576. [Crossref]
51. Ávila PF, Franco Cairo JPL, Damasio A, Forte MBS, & Goldbeck R. Xylooligosaccharides production from a sugarcane biomass mixture: effects of commercial enzyme combinations on bagasse/straw hydrolysis pretreated using different strategies. Food Res Int, 2020, 128: 108702. [Crossref]
52. Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, & Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci, 2017, 74(20): 3769-3787. [Crossref]
53. Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep, 2017, 7(1): 13510. [Crossref]
54. Desbonnet L, Garrett L, Clarke G, Bienenstock J, & Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res, 2008, 43(2): 164-174. [Crossref]
55. Abdelhamid M, Zhou C, Jung CG, & Michikawa M. Probiotic Bifidobacterium breve MCC1274 mitigates Alzheimer’s disease-related pathologies in wild-type mice. Nutrients, 2022, 14(12): 2543-25. [Crossref]
56. Montgomery TL, Eckstrom K, Lile KH, Caldwell S, Heney ER, Lahue KG,et al. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome, 2022, 10(1): 198-207. [Crossref]
57. Li Q, Zheng T, Ding H, Chen J, Li B, Zhang Q, et al. Exploring the benefits of probiotics in gut inflammation and diarrhea-from an antioxidant perspective. Antioxidants, 2023, 12(7): 1342-1355. [Crossref]
58. Patel C, Pande S, & Acharya S. Potentiation of antiAlzheimer activity of curcumin by probiotic Lactobacillus rhamnosus UBLR-58 against scopolamine-induced memory impairment in mice. Naunyn Schmiedebergs Arch Pharmacol, 2020, 393(10): 1955-1962. [Crossref]
59. Deng SM, Chen CJ, Lin HL, & Cheng IH. The beneficial effect of synbiotics consumption on Alzheimer’s disease mouse model via reducing local and systemic inflammation. IUBMB Life, 2022, 74(8): 748-753. [Crossref]
60. Nimgampalle M, & Kuna Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J Clin Diagn Res, 2017, 11(8): Kc01-kc05. [Crossref]
61. Tolosa E, Garrido A, Scholz SW, & Poewe W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol, 2021, 20(5): 385-397. [Crossref]
62. Fasano A, Canning CG, Hausdorff JM, Lord S, & Rochester L. Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord, 2017, 32(11): 1524-1536. [Crossref]
63. de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meché FG, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology, 1995, 45(12): 2143-2146. [Crossref]
64. Aradi SD, & Hauser RA. Medical management and prevention of motor complications in Parkinson’s disease. Neurotherapeutics, 2020, 17(4): 1339-1365. [Crossref]
65. Zahoor I, Shafi A, & Haq E: Pharmacological treatment of Parkinson’s disease. In: Parkinson’s Disease: Pathogenesis and Clinical Aspects. 2018, edn. Codon Publications.
66. Coleman C, & Martin I. Unraveling Parkinson’s disease neurodegeneration: does aging hold the clues? J Parkinsons Dis, 2022, 12(8): 2321-2338. [Crossref]
67. Tong J, Rathitharan G, Meyer JH, Furukawa Y, Ang LC, Boileau I, et al. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain, 2017, 140(9): 2460-2474. [Crossref]
68. Gopinath A, Mackie P, Hashimi B, Buchanan AM, Smith AR, Bouchard R, et al. DAT and TH expression marks human Parkinson’s disease in peripheral immune cells. NPJ Parkinsons Dis, 2022, 8(1): 72-87. [Crossref]
69. Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, & Moratalla R. Modeling Parkinson’s disease with the alpha-synuclein protein. Front Pharmacol, 2020, 11: 356-367. [Crossref]
70. McGregor MM, & Nelson AB. Circuit mechanisms of Parkinson’s disease. Neuron, 2019, 101(6): 1042-1056. [Crossref]
71. Klein C, & Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med, 2012, 2(1): a008888. [Crossref]
72. Huang Y, Liao J, Liu X, Zhong Y, Cai X, & Long L. Review: the role of intestinal dysbiosis in Parkinson’s disease. Front Cell Infect Microbiol, 2021, 11: 615075. [Crossref]
73. Miraglia F, & Colla E. Microbiome, Parkinson’s disease and molecular mimicry. Cells, 2019, 8(3): 222-235. [Crossref]
74. Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, & Ghiglieri V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis, 2023, 14(3): 176-188. [Crossref]
75. Torres-Fuentes C, Golubeva AV, Zhdanov AV, Wallace S, Arboleya S, Papkovsky DB, et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. Faseb J, 2019, 33(12): 13546-13559. [Crossref]
76. Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord, 2017, 32(5): 739-749. [Crossref]
77. van ISCD, & Derkinderen P. The intestinal barrier in Parkinson’s disease: current state of knowledge. J Parkinsons Dis, 2019, 9(s2): S323-s329. [Crossref]
78. Hall DA, Voigt RM, Cantu-Jungles TM, Hamaker B, Engen PA, Shaikh M, et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat Commun, 2023, 14(1): 926-933. [Crossref]
79. Leta V, Mandal G, Zinzalias P, Staunton J, Batzu L, Trivedi D, et al. Effects of a four-strain probiotic on inflammatory markers in Parkinson’s disease: a multicentre randomised controlled trial (the SymPD study) (N5.001). Neurology, 2024, 102(17 supplement 1): 3969-3985. [Crossref]
80. Valvaikar S, Vaidya B, Sharma S, Bishnoi M, Kondepudi KK, & Sharma SS. Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem Int, 2024, 174: 105691. [Crossref]
81. Mehrabani S, Khorvash F, Heidari Z, Tajabadi-Ebrahimi M, & Amani R. The effects of synbiotic supplementation on oxidative stress markers, mental status, and quality of life in patients with Parkinson’s disease: a double-blind, placebo-controlled, randomized controlled trial. Journal of Functional Foods, 2023, 100: 105397. [Crossref]
82. van den Bos MAJ, Geevasinga N, Higashihara M, Menon P, & Vucic S. Pathophysiology and diagnosis of ALS: insights from advances in neurophysiological techniques. Int J Mol Sci, 2019, 20(11): 2818-2832. [Crossref]
83. Mehta P, Raymond J, Zhang Y, Punjani R, Han M, Larson T, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener, 2023: 1-7. [Crossref]
84. Mehta P, Raymond J, Punjani R, Han M, Larson T, Kaye W, et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Frontotemporal Degener, 2023, 24(1-2): 108-116. [Crossref]
85. Lu H, Le WD, Xie YY, & Wang XP. Current therapy of drugs in amyotrophic lateral sclerosis. Curr Neuropharmacol, 2016, 14(4): 314-321. [Crossref]
86. In: LiverTox: clinical and research information on druginduced liver injury. 2012, edn. National Institute of Diabetes and Digestive and Kidney Diseases.
87. Noor Eddin A, Alfuwais M, Noor Eddin R, Alkattan K, & Yaqinuddin A. Gut-modulating agents and amyotrophic lateral sclerosis: current evidence and future perspectives. Nutrients, 2024, 16(5): 590-612. [Crossref]
88. Jagaraj CJ, Shadfar S, Kashani SA, Saravanabavan S, Farzana F, & Atkin JD. Molecular hallmarks of ageing in amyotrophic lateral sclerosis. Cell Mol Life Sci, 2024, 81(1): 111-123. [Crossref]
89. Linkus B, Wiesner D, Meßner M, Karabatsiakis A, Scheffold A, Rudolph KL, et al. Telomere shortening leads to earlier age of onset in ALS mice. Aging, 2016, 8(2): 382- 393. [Crossref]
90. Spitale FM, Vicario N, Rosa MD, Tibullo D, Vecchio M, Gulino R, et al. Increased expression of connexin 43 in a mouse model of spinal motoneuronal loss. Aging, 2020, 12(13): 12598-12608. [Crossref]
91. Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia, 2015, 63(12): 2260-2273. [Crossref]
92. Nowotny K, Jung T, Höhn A, Weber D, & Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules, 2015, 5(1): 194- 222. [Crossref]
93. Chou SM, Wang HS, Taniguchi A, & Bucala R. Advanced glycation endproducts in neurofilament conglomeration of motoneurons in familial and sporadic amyotrophic lateral sclerosis. Mol Med, 1998, 4(5): 324-332.
94. Trias E, Beilby PR, Kovacs M, Ibarburu S, Varela V, Barreto-Núñez R, et al. Emergence of microglia bearing senescence markers during paralysis progression in a rat model of inherited ALS. Front Aging Neurosci, 2019, 11: 42-55. [Crossref]
95. Pandya VA, & Patani R. Region-specific vulnerability in neurodegeneration: lessons from normal ageing. Ageing Res Rev, 2021, 67: 101311. [Crossref]
96. Martin S, Battistini C, & Sun J. A gut feeling in amyotrophic lateral sclerosis: microbiome of mice and men. Front Cell Infect Microbiol, 2022, 12: 839526. [Crossref]
97. Chen S, Cai X, Lao L, Wang Y, Su H, & Sun H. Brain-gutmicrobiota axis in amyotrophic lateral sclerosis: a historical overview and future directions. Aging Dis, 2024, 15(1): 74-95. [Crossref]
98. Mazzini L, Mogna L, De Marchi F, Amoruso A, Pane M, Aloisio I, et al. Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J Clin Gastroenterol,2018, 52 Suppl 1, proceedings from the 9th probiotics, prebiotics and new foods, nutraceuticals and botanicals for nutrition & human and microbiota health meeting, held in Rome, Italy from September 10 to 12, 2017: S68-s70. [Crossref]
99. Fournier CN, Houser M, Tansey MG, Glass JD, & Hertzberg VS. The gut microbiome and neuroinflammation in amyotrophic lateral sclerosis? Emerging clinical evidence. Neurobiol Dis, 2020, 135: 104300. [Crossref]
100. Liu K, Guo Q, Ding Y, Luo L, Huang J, & Zhang Q. Altera tions in nasal microbiota of patients with amyotrophic lateral sclerosis.Chin Med J (Engl), 2024, 137(2): 162- 171. [Crossref]
101. Hong D, Zhang C, Wu W, Lu X, & Zhang L. Modulation of the gut-brain axis via the gut microbiota: a new era in treatment of amyotrophic lateral sclerosis. Front Neurol, 2023, 14: 1133546. [Crossref]
102. Sun J, Huang T, Debelius JW, & Fang F. Gut microbiome and amyotrophic lateral sclerosis: a systematic review of current evidence. J Intern Med, 2021, 290(4): 758-788. [Crossref]
103. Niccolai E, Di Pilato V, Nannini G, Baldi S, Russo E, Zuc chi E, et al. The gut microbiota-immunity axis in ALS: a role in deciphering disease heterogeneity? Biomedicines, 2021, 9(7): 753-762. [Crossref]
104. Sagi H, Shibuya S, Kato T, Nakanishi Y, Tsuboi A, Moriya S, et al. SOD1 deficiency alters gastrointestinal microbiota and metabolites in mice. Exp Gerontol, 2020, 130: 110795. [Crossref]
105. Lerskiatiphanich T, Marallag J, & Lee JD. Glucose metabolism in amyotrophic lateral sclerosis: it is bitter-sweet. Neural Regen Res, 2022, 17(9): 1975-1977. [Crossref]
106. Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice.Nature, 2019, 572(7770): 474-480. [Crossref]
107. Dooling SW, & Costa-Mattioli M. Gut bacteria seize control of the brain to prevent epilepsy. Cell Host Microbe, 2018, 24(1): 3-5. [Crossref]
108. Meher AK, Acharya B, & Sahu PK. Probiotics: bridging the interplay of a healthy gut and psychoneurological well-being. Food Bioengineering, 2024, 3(1): 126-147. [Crossref]
109. Song L, Gao Y, Zhang X, & Le W. Galactooligosaccharide improves the animal survival and alleviates motor neuron death in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuroscience, 2013, 246: 281-290. [Crossref]
110. Moțățăianu A, Șerban G, & Andone S. The role of shortchain fatty acids in microbiota-gut-brain cross-talk with a focus on amyotrophic lateral sclerosis: a systematic review. Int J Mol Sci, 2023, 24(20): 15094. [Crossref]
111. Zhang Y, Xia Y, & Sun J. Probiotics and microbial metabolites maintain barrier and neuromuscular functions and clean protein aggregation to delay disease progression in TDP43 mutation mice. Gut Microbes, 2024, 16(1): 2363880. [Crossref]
112. Xin Z, Xin C, Huo J, Liu Q, Dong H, Li R, et al. Neuroprotective effect of a multistrain probiotic mixture in SOD1(G93A) mice by reducing SOD1 aggregation and targeting the microbiota-gut-brain axis. Mol Neurobiol, 2024. [Crossref]
113. Yoshizawa S, Matsushima R, Watanabe MF, Harada K, Ichihara A, Carmichael WW, et al. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J Cancer Res Clin Oncol, 1990, 116(6): 609-614. [Crossref]
114. Llewellyn LE. Saxitoxin, a toxic marine natural product that targets a multitude of receptors.Nat Prod Rep, 2006, 23(2): 200-222. [Crossref]
115. Feurstein D, Stemmer K, Kleinteich J, Speicher T, & Dietrich DR. Microcystin congener- and concentrationdependent induction of murine neuron apoptosis and neurite degeneration. Toxicol Sci, 2011, 124(2): 424-431. [Crossref]
116. Thomas F, Hehemann JH, Rebuffet E, Czjzek M, & Michel G. Environmental and gut bacteroidetes: the food connection. Front Microbiol, 2011, 2: 93-104. [Crossref]
117. Yadav MK, Kumari I, Singh B, Sharma KK, & Tiwari SK. Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol, 2022, 106(2): 505-521. [Crossref]
118. Dobson R, & Giovannoni G. Multiple sclerosis - a review. Eur J Neurol, 2019, 26(1): 27-40. [Crossref]
119. Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol, 2018, 9: 3116-3127. [Crossref]
120. Roy Sarkar S, & Banerjee S. Gut microbiota in neurodegenerative disorders. J Neuroimmunol, 2019, 328: 98- 104. [Crossref]
121. Reynders T, Devolder L, Valles-Colomer M, Van Remoortel A, Joossens M, De Keyser J, et al. Gut microbiome variation is associated to multiple sclerosis phenotypic subtypes. Ann Clin Transl Neurol, 2020, 7(4): 406-419. [Crossref]
122. Ordoñez-Rodriguez A, Roman P, Rueda-Ruzafa L, Campos-Rios A, & Cardona D. Changes in gut microbiota and multiple sclerosis: a systematic review. Int J Environ Res Public Health, 2023, 20(5): 4624-4635. [Crossref]
123. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep, 2016, 6: 28484. [Crossref]
124. Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PLoS One, 2015, 10(9): e0137429. [Crossref]
125. Preiningerova JL, Jiraskova Zakostelska Z, Srinivasan A, Ticha V, Kovarova I, Kleinova P, et al. Multiple sclerosis and microbiome. Biomolecules, 2022, 12(3): 433-445. [Crossref]
126. Choileáin SN, Kleinewietfeld M, Raddassi K, Hafler DA, Ruff WE, & Longbrake EE. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J Transl Autoimmun, 2020, 3: 100032. [Crossref]
127. Kozhieva M, Naumova N, Alikina T, Boyko A, Vlassov V, & Kabilov MR. Primary progressive multiple sclerosis in a Russian cohort: relationship with gut bacterial diversity. BMC Microbiol, 2019, 19(1): 309-320. [Crossref]
128. Saresella M, Marventano I, Barone M, La Rosa F, Piancone F, Mendozzi L, et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front Immunol, 2020, 11: 1390-1402. [Crossref]
129. Levi I, Gurevich M, Perlman G, Magalashvili D, Menascu S, Bar N, et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiomemetabolome analysis. Cell Rep Med, 2021, 2(4): 100246. [Crossref]
130. Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity ,2016, 44(4): 951-953. [Crossref]
131. Silva YP, Bernardi A, & Frozza RL. The role of short-chain fatty acids from gut microbiota in Gut-brain communication. Front Endocrinol, 2020, 11: 25-34. [Crossref]
132. Melbye P, Olsson A, Hansen TH, Søndergaard HB, & Bang Oturai A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol Scand, 2019, 139(3): 208- 219. [Crossref]
133. Esposito S, Bonavita S, Sparaco M, Gallo A, & Tedeschi G. The role of diet in multiple sclerosis: a review. Nutr Neurosci, 2018, 21(6): 377-390. [Crossref]
134. Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature, 2016, 534(7606): 213-217. [Crossref]
135.Schepici G, Silvestro S, Bramanti P, & Mazzon E. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant, 2019, 28(12): 1507-1527. [Crossref]
136. Noto D, & Miyake S. Gut dysbiosis and multiple sclerosis. Clin Immunol, 2022, 235: 108380. [Crossref]
137. Rahimlou M, Hosseini SA, Majdinasab N, Haghighizadeh MH, & Husain D. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Nutr Neurosci, 2022, 25(2):411-422. [Crossref]
138. Li Y, Zhang B, Zhou Y, Wang D, Liu X, Li L, et al. Gut microbiota changes and their relationship with inflammation in patients with acute and chronic insomnia. Nat Sci Sleep, 2020, 12: 895-905. [Crossref]
139. Morshedi M, Hashemi R, Moazzen S, Sahebkar A, & Hosseinifard ES. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. J Neuroinflammation, 2019, 16(1): 231-244. [Crossref]
140. Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA, 2020, 117(44): 27509-27515. [Crossref]
141. Ghaiad HR, Nooh MM, El-Sawalhi MM, & Shaheen AA. Resveratrol promotes remyelination in cuprizone model of multiple sclerosis: biochemical and histological study. Mol Neurobiol, 2017, 54(5): 3219-3229. [Crossref]
142. Siahpoosh A, Majdinasab N, Derakhshannezhad N, Khalili HR, & Malayeri A. Effect of grape seed on quality of life in multiple sclerosis patients. 2018.
143. Chatterjee A, Kumar S, Roy Sarkar S, Halder R, Kumari R, Banerjee S, et al. Dietary polyphenols represent a phytotherapeutic alternative for gut dysbiosis associated neurodegeneration: a systematic review. J Nutr Biochem, 2024, 129: 109622. [Crossref]
144. Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with Ketogenic diet. Front Microbiol, 2017, 8: 1141-1154. [Crossref]
145. Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM, et al. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother, 2017, 95: 1535- 1548. [Crossref]
146. Hashemi B, Abdollahi M, Abbaspour-Aghdam S, Hazrati A, Malekpour K, Meshgi S, et al. The effect of probiotics on immune responses and their therapeutic application: a new treatment option for multiple sclerosis. Biomed Pharmacother, 2023, 159: 114195. [Crossref]
147. Kouchaki E, Tamtaji OR, Salami M, Bahmani F, Daneshvar Kakhaki R, Akbari E, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind,placebocontrolled trial. Clin Nutr, 2017, 36(5): 1245-1249. [Crossref]
148. Berer K, Martínez I, Walker A, Kunkel B, Schmitt-Kopplin P, Walter J, et al. Dietary non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status.Sci Rep, 2018, 8(1): 10431. [Crossref]
149. Straus Farber R, Walker EL, Diallo F, Onomichi K, Riley C, Zhang L, et al. A randomized cross-over trial of prebiotics and probiotics in multiple sclerosis: trial feasibility, supplement tolerability and symptom abatement. Mult Scler Relat Disord, 2024, 89: 105762. [Crossref]
150. Borody TJ, Leis S, Campbell J, Torres M, & Nowak A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS): 942. Am J Gastroent, 2011, 106: S352.
151. Makkawi S, Camara-Lemarroy C, & Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm, 2018, 5(4): e459. [Crossref]
152. Park J, Wang Q, Wu Q, Mao-Draayer Y, & Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation.Sci Rep, 2019, 9(1): 8837-8848. [Crossref]
153. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab, 2018, 27(6): 1222-1235.e1226. [Crossref]
154. Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, et al. An imbalance in interleukin-17-producing T and Foxp3⁺ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod, 2011, 26(11): 2964-2971. [Crossref]
155. Calvo-Barreiro L, Eixarch H, Cornejo T, Costa C, Castillo M, Mestre L, et al. Selected clostridia strains from the human microbiota and their metabolite, butyrate, improve experimental autoimmune encephalomyelitis. Neurotherapeutics, 2021, 18(2): 920-937. [Crossref]
156. Fleck AK, Hucke S, Teipel F, Eschborn M, Janoschka C, Liebmann M, et al. Dietary conjugated linoleic acid links reduced intestinal inflammation to amelioration of CNS autoimmunity. Brain, 2021, 144(4): 1152-1166. [Crossref]
157. Stoker TB, Mason SL, Greenland JC, Holden ST, Santini H, & Barker RA. Huntington’s disease: diagnosis and management. Pract Neurol, 2022, 22(1): 32-41. [Crossref]
158. Kong G, Cao KL, Judd LM, Li S, Renoir T, & Hannan AJ. Microbiome profiling reveals gut dysbiosis in a transgenic mouse model of Huntington’s disease. Neurobiol Dis, 2020, 135: 104268. [Crossref]
159. Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med, 2008, 205(8): 1869-1877. [Crossref]
160. Farshim PP, & Bates GP. Mouse models of Huntington’s disease.Methods Mol Biol,2018, 1780: 97-120. [Crossref]
161. Konjevod M, Nikolac Perkovic M, Sáiz J, Svob Strac D, Barbas C, & Rojo D. Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J Pharm Biomed Anal, 2021, 194: 113681. [Crossref]
162. Wasser CI, Mercieca EC, Kong G, Hannan AJ, McKeown SJ, Glikmann-Johnston Y, et al. Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun, 2020, 2(2): fcaa110. [Crossref]
163. Du G, Dong W, Yang Q, Yu X, Ma J, Gu W, et al. Altered gut microbiota related to inflammatory responses in patients with Huntington’s disease. Front Immunol, 2020, 11: 603594. [Crossref]
164. Cordeiro LM, Soares MV, da Silva AF, Machado ML, Bicca Obetine Baptista F, da Silveira TL, et al. Neuroprotective effects of rutin on ASH neurons in Caenorhabditis elegans model of Huntington’s disease. Nutr Neurosci, 2022, 25(11): 2288-2301. [Crossref]
165. Yadav E, Yadav P, Khan MMU, Singh H, & Verma A. Resveratrol: a potential therapeutic natural polyphenol for neurodegenerative diseases associated with mitochondrial dysfunction.Front Pharmacol , 2022, 13: 922232. [Crossref]
166. Wang J, Pfleger CM, Friedman L, Vittorino R, Zhao W, Qian X, et al. Potential application of grape derived polyphenols in Huntington’s disease. Transl Neurosci, 2010, 1(2): 95-100. [Crossref]
167. Maher P. Preventing and treating neurological disorders with the flavonol fisetin. Brain Plast, 2021, 6(2): 155- 166. [Crossref]
168. Jewell DE, Jackson MI, Cochrane CY, & Badri DV. Feeding fiber-bound polyphenol ingredients at different levels modulates colonic postbiotics to improve gut health in dogs. Animals (Basel), 2022, 12(5): 627-638. [Crossref]
169. Wasser CI, Mercieca EC, Kong G, Hannan AJ, Allford B, McKeown SJ, et al. A randomized controlled trial of probiotics targeting gut dysbiosis in Huntington’s disease. J Huntingtons Dis, 2023, 12(1): 43-55. [Crossref]
170. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr, 2019, 38(6): 2569-2575. [Crossref]
171. Nova E, Wärnberg J, Gómez-Martínez S, Díaz LE, Romeo J, & Marcos A. Immunomodulatory effects of probiotics in different stages of life. Br J Nutr, 2007, 98 Suppl 1: S90- 95. [Crossref]
172. Gaudana SB, Dhanani AS, & Bagchi T. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br J Nutr, 2010, 103(11): 1620-1628. [Crossref]
173. Gubert C, Kong G, Costello C, Adams CD, Masson BA, Qin W, et al. Dietary fibre confers therapeutic effects in a preclinical model of Huntington’s disease. Brain Behav Immun, 2024, 116: 404-418. [Crossref]
174. Zikou E, Koliaki C, & Makrilakis K. The role of fecal microbiota transplantation (FMT) in the management of metabolic diseases in humans: a narrative review. Biomedicines, 2024, 12(8): 1871-1884. [Crossref]
175. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut, 2020, 69(7): 1218-1228. [Crossref]
176. Clinicaltrials.gov/study [https://clinicaltrials.gov/ study/NCT06181513?term=NCT06181513&rank=1 ]
Cite this article as: Ruwatia R, Sarkar SR, Panda S, & Banerjee S. Gut microbiome and neurodegenerative disorders: therapeutic implications. Aging Pathobiol Ther, 2025, 7(1): 05-24. doi:10.31491/APT.2025.03.164