Open Access | Mini Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
The potential of astaxanthin as a natural compound for decelerating skin aging: an update review
* Corresponding author: Teresa Liliana Wargasetia
Mailing address: Master Program in Skin Ageing and Aesthetics, Faculty of Medicine, Maranatha Christian University, Bandung 40164, Jawa Barat, Indonesia
Email: teresa.lw@med.maranatha.edu
Received: 18 September 2024 / Revised: 08 October 2024 / Accepted: 03 December 2024 / Published: 28 December 2024
DOI: 10.31491/APT.2024.12.159
Abstract
Astaxanthin, a potent antioxidant found in natural sources such as algae, shrimp and salmon, is emerging as a promising agent for slowing skin aging. This review thoroughly examines the molecular mechanisms by which astaxanthin exerts its anti-aging effects. It is interesting to note that astaxanthin activates critical cellular pathways such as Nrf2/ARE, NF-κB and TGF-β. By enhancing the Nrf2/ARE pathway, astaxanthin increases the expression of antioxidant enzymes that protect skin proteins such as collagen and elastin from oxidative damage. In addition, astaxanthin suppresses the NF-κB pathway, thereby reducing inflammation, and promotes collagen synthesis by activating the TGF-β pathway. These effects inhibit collagen-degrading enzymes such as MMP1, thereby preventing wrinkle formation. Astaxanthin also modulates the MAPK pathway, which is involved in several cellular processes, including the response to oxidative stress, inflammation, and cell proliferation. Numerous studies provide strong evidence for the benefits of astaxanthin in preventing and treating skin aging, inhibiting wrinkles, increasing skin elasticity, reducing IL-1α, TNF-α, IL-6, IL-8, MMP1, ROS activity, brightening skin, increasing collagen production, and maintaining overall skin health and beauty, as well as contributing to skin repair and regeneration. With its potent antioxidant, anti-inflammatory and collagen-stimulating properties, astaxanthin has significant potential as an ingredient in anti-aging and photoprotective skin care products. Understanding these mechanisms provides valuable insight into the role of astaxanthin in preventing aging and improving skin health.
Keywords
Anti-inflammation, antioxidant, collagen stimulation, skincare
Introduction
Skincare trends have gained significant traction recently, reflecting a growing focus on maintaining youthful, healthy skin. Advances in technology and scientific
research have spurred innovation in anti-aging skincare products, making them a crucial part of the beauty and wellness industry. In 2021, the global anti-aging market
was valued at approximately 62.6 billion USD, with an expected compound annual growth rate (CAGR) of around 7% between 2022 and 2027
[1]. Several factors, including increased skincare awareness [2], technological
advancements in anti-aging products, and the rising population of elderly individuals worldwide, drive this rapid growth [1]. By
2050, the global population aged 60 years and older is projected to reach 1.6 billion, underscoring the increasing demand for effective anti-aging solutions
[1]. Therefore, the anti-aging and aesthetics sector industry allows continuous innovation to develop new products and services
attractive to consumers, thereby expanding the overall market for preventing aging [3].
Skin aging is a multifactorial process influenced by intrinsic factors such as genetics, hormonal changes, metabolism, and extrinsic factors, including ultraviolet
(UV) exposure, pollution, and lifestyle [4, 5]. Intrinsic aging, driven by genetic and
biological processes, results in the gradual loss of skin elasticity, hydration, and collagen over time [6]. Hormonal changes,
particularly the decrease in estrogen in women and testosterone in men, contribute to thinning, dryness, and decreased skin elasticity
[7, 8]. Meanwhile, extrinsic factors like UV radiation accelerate premature aging by
damaging collagen and elastin fibers, leading to wrinkles, hyperpigmentation, and overall loss of skin integrity
[5, 9, 10].
One of the most effective strategies to counteract skin aging, particularly from UV damage, is using antioxidants. Antioxidants can neutralize reactive oxygen species
(ROS) generated by UV exposure, reducing oxidative stress and preventing cellular damage [11,
12]. Topical as well as oral antioxidants can support skin brightening, growth of extracellular matrix proteins, and anti-aging
[13-15].
Among natural antioxidants, astaxanthin (3,3'-dihydroxy-β, β'-carotene-4,4'-dione), a xanthophyll carotenoid, has gained attention for its powerful antioxidant,
anti-inflammatory, and photoprotective properties. Astaxanthin has demonstrated the ability to reduce oxidative stress, promote skin health, and protect against
UV-induced damage, making it a promising compound in the anti-aging and skin care industries
[16-19].
Astaxanthin not only protects against photoaging but also supports skin elasticity [20], hydration, and the reduction of
wrinkles and fine lines. Several studies have highlighted astaxanthin's potential in improving skin texture, reducing hyperpigmentation, and enhancing wound healing
[19, 21, 22]. Despite these benefits,
comprehensive research on the specific mechanisms through which astaxanthin slows skin aging remains limited. This review aims to assess the potential of astaxanthin as
a natural anti-aging agent, focusing on its role in preventing skin wrinkles and promoting overall skin health.
Skin aging and pathways involved in skin aging
Skin aging is a complex process characterized by a decline in skin quality due to the combined effects of chronological aging, hormonal deficiencies, premature
aging, and environmental factors [5]. The factors contributing to skin aging are classified into two categories: intrinsic and
extrinsic. Intrinsic factors, affecting cells throughout the body, include aging, genetics, metabolic processes, and hormonal changes. On the other hand, extrinsic
factors encompass environmental and lifestyle influences such as exposure to ultraviolet (UV) radiation, pollution, and harmful chemicals
[23].
Intrinsic aging is driven by several physiological changes, including hormone reduction, glycation, apoptosis, methylation, immune system decline, and the production of
free radicals. Additionally, intrinsic aging involves the degeneration of extracellular matrix components such as elastin, collagen, and fibrillin, along with
alterations in oligosaccharides. Conversely, extrinsic aging is primarily influenced by external factors like an unhealthy lifestyle, poor dietary habits, stress, and
environmental exposure. Environmental factors such as UV radiation, pollution, and smoking particularly damage the skin [4].
UV radiation, a major contributor to skin aging, induces oxidative stress. Oxidative stress is an imbalance between ROS formed and antioxidant defense mechanisms. ROS
are reactive oxygen compounds and secondary products of aerobic metabolism. ROS imbalance is caused by increased ROS production, reduced antioxidant production, or both.
Oxidative stress causes oxidative damage to various cell components, disrupts communication processes between cells, stimulates apoptosis, and is involved in various
diseases associated with aging. Oxidative stress damages the skin's natural renewal and repair processes and damages DNA directly, causing cell mutations and premature
aging. This oxidative stress is the leading cause of photoaging and premature skin aging. Both ultraviolet B (UVB) and ultraviolet A (UVA) can damage the skin. UVB is
the main skin stress trigger that can damage escDNA and UVA increases the production of DNA toxic ROS [4].
ROS are unstable free radicals because they have unpaired electrons in the outer orbit. These compounds attract electrons from surrounding molecules to complete the
electrons in the outer orbit and produce a very dangerous free radical chain reaction. Such free radicals are superoxide anion (O2-), hydroxyl radical (-OH),
and peroxynitrate (ONOO-). Hydrogen peroxide (H2O2) and nitric oxide (NO) are not free radicals, but are classified as ROS, because they
trigger oxidation-reduction reactions and form free radicals. ROS plays a role in causing skin aging through cellular oxidation processes, activation of nuclear
factor kappa B [23], activation of mitogen-activated pathway (MAP) kinase, and stimulation of pro-inflammatory cytokines.
Increased ROS will damage proteins, lipids, and cell DNA, resulting in skin aging [4].
Intrinsic aging is associated with a reduction in transforming growth factor-beta (TGF-β) levels and ROS accumulation. In contrast, extrinsic aging, primarily caused by
UV exposure, increases ROS in the dermal layer, initiating a cascade of molecular reactions. ROS activates the transcription factor AP-1, which, in turn, stimulates the
expression of matrix metalloproteinase (MMP) enzymes, particularly collagenase-1 (MMP-1). These enzymes degrade collagen, contributing to skin aging
[4].
Several molecular pathways are involved in the skin aging process, including the Nrf2/ARE, NF-κB, TGF-β, MAPK, Wnt/β-catenin, RAGE (Receptor for Advanced Glycation
End Products), and p53 pathways [22, 24,
25]. The Nrf2/ARE pathway plays a crucial role in antioxidant defense and detoxification processes, while NF-κB regulates
inflammatory and immune responses. Activation of the NF-κB pathway leads to the production of pro-inflammatory cytokines, promoting inflammation and tissue damage
[26].
The TGF-β pathway is often dysregulated during aging, resulting in reduced collagen production, increased activity of extracellular matrix-degrading enzymes, and
impaired cell regeneration. Similarly, the MAPK pathway mediates cellular responses to oxidative stress, inflammation, and proliferation. The Wnt/β-catenin pathway,
essential for cell proliferation, differentiation, and tissue regeneration, also becomes less active with age, leading to decreased skin regeneration and collagen
synthesis. Furthermore, the RAGE pathway, which is involved in the accumulation of advanced glycation end products (AGEs), exacerbates the formation of wrinkles and skin
elasticity loss when overactivated. Finally, the p53 protein, which regulates apoptosis and cell proliferation, becomes more active with age, further reducing the
skin's regenerative capacity [25].
The potential of astaxanthin in decelerating skin aging
Natural sources of astaxanthin
For decades, natural products have been researched for a wide range of uses in health due to their adaptability, safety, and cost-effectiveness
[27]. There is also a continuous increase in the research involving the use of natural bioactive in cosmetics due to the
increasing health and environmental concerns.
Green algae, especially Haematococcus pluvialis, are rich natural sources of astaxanthin with carotenoid pigments that have strong antioxidant properties. Due
to its ability to produce astaxanthin in large quantities, Haematococcus pluvialis has become an important research subject in the fields of biotechnology and
nutrition [28]. Astaxanthin extracted from Haematococcus pluvialis is used as a food supplement, an additional
ingredient in food and beverage products, as well as in various skincare and health products. Astaxanthin is the most potent natural antioxidant
[29] which comes from green algae [30-41],
red algae [15], shrimp [41-48],
crayfish [49], trout [50-56], krill
[57-59], salmon [54,
56, 60-64], and yeast
[65, 66] which has several essential biological functions including reducing
pigmentation and protecting skin wrinkles against the effects of UV light. Even though it is contained in many natural sources, green algae is the largest producer of
astaxanthin [67].
Mechanism of astaxanthin to prevent skin aging
Astaxanthin exerts its anti-aging effects primarily by modulating several key cellular pathways, including the Nrf2/ARE, NF-κB, and TGF-β pathways
[22, 24]. One of its primary actions is enhancing the activity of the Nrf2/ARE
pathway, which is critical for the cellular antioxidant defense system. When astaxanthin activates this pathway, it leads to the upregulation of antioxidant genes and
detoxification enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) [68]. These
enzymes play a crucial role in neutralizing reactive oxygen species (ROS) and combating oxidative stress, a major contributor to skin aging. By increasing the cell's
capacity to counter oxidative damage, astaxanthin helps protect essential structural proteins in the skin, such as collagen and elastin, from degradation caused by free
radicals generated during UV exposure. Moreover, astaxanthin has been shown to reduce the activity of MMP-1, an enzyme responsible for collagen breakdown, significantly
contributing to wrinkle formation [36, 69,
70].
In addition to its antioxidant properties, astaxanthin can suppress the activation of the NF-κB pathway, a signaling cascade involved in the inflammatory response
[23]. Chronic inflammation accelerates skin aging by degrading connective tissue and promoting the overproduction of
pro-inflammatory cytokines. By inhibiting NF-κB activation, astaxanthin helps to reduce skin inflammation, thus preventing inflammation-induced tissue damage and
preserving skin integrity [22].
The TGF-β pathway is another critical signaling mechanism affected by astaxanthin. This pathway regulates cell proliferation, differentiation, and the production of
extracellular matrix components, particularly collagen [24]. By activating the TGF-β pathway, astaxanthin helps to maintain the
structure and function of skin connective tissue. This activation leads to enhanced collagen production, which not only improves skin texture but also reduces wrinkles
and other signs of aging. Increased collagen synthesis inhibits the activity of collagen-degrading enzymes, such as MMP-1, further supporting the skin's regenerative
processes [71].
Additionally, astaxanthin has been shown to inhibit the expression of MMPs in various cell types, including macrophages and chondrocytes, and in human dermal
fibroblasts, where it suppresses both MMP-1 and MMP-3 expression, leading to increased collagen content
[72-74]. Furthermore, astaxanthin increases pathway activity MAPK
(Mitogen-Activated Protein Kinase). MAPKs are involved in various cellular processes, including responses to oxidative stress, inflammation, and cell proliferation
[25]. Astaxanthin is becoming an attractive ingredient in anti-aging and photoprotection skincare products.
Understanding the natural sources of astaxanthin, its biological benefits, and its mechanisms of action allows for better application in anti-aging strategies.
Astaxanthin's potential to protect, repair, and renew skin makes it a compelling ingredient in modern skincare formulations aimed at slowing the aging process
(Figure 1).
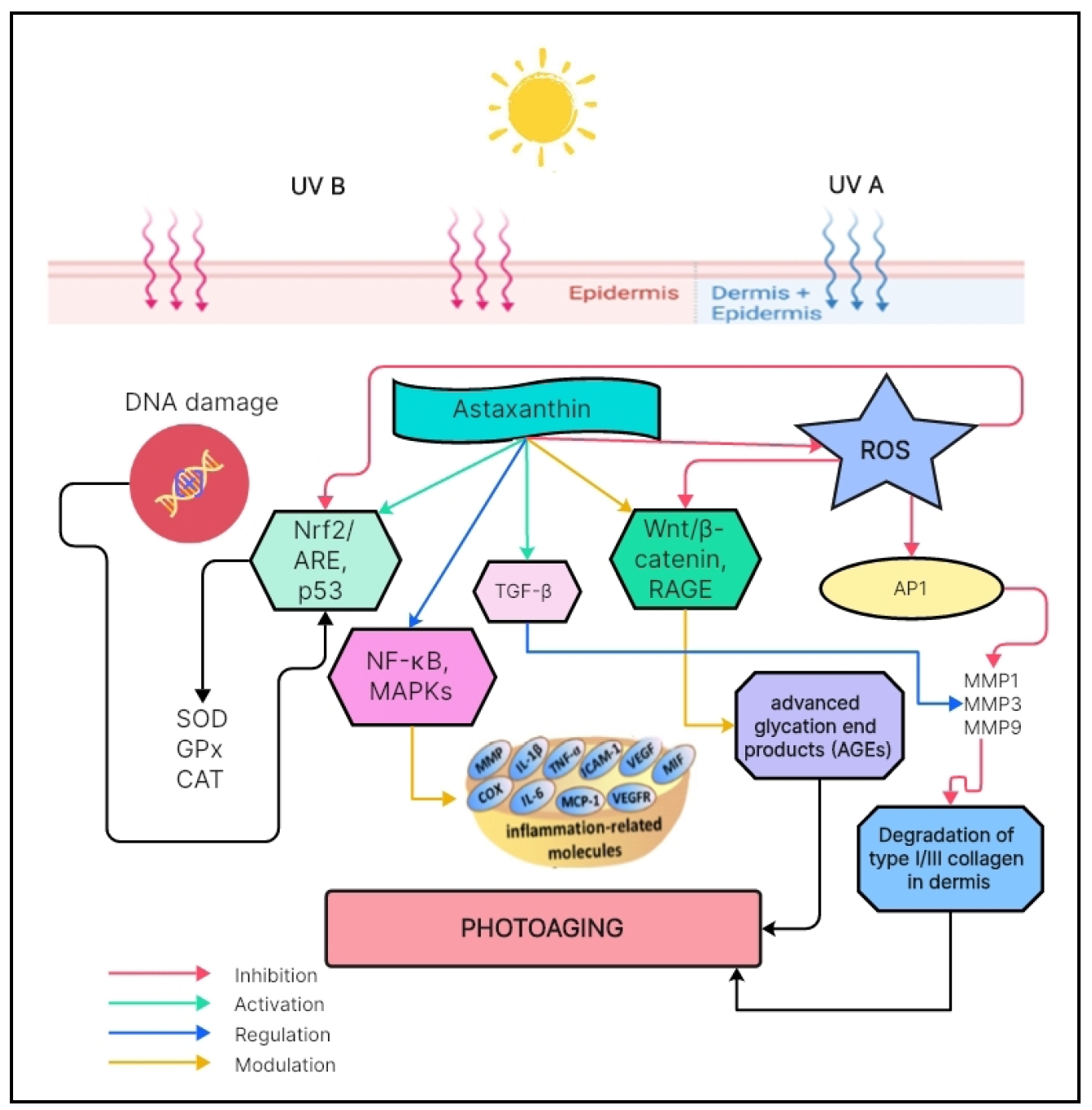
Figure 1. Effects of astaxanthin in many molecular pathways related with skin aging. Astaxanthin increases the activity of the Nrf2/ARE, TGF-β, and MAPKs pathway, reducing MMP activity, in addition to suppressing the activation of the NF-κB pathway, which can reduce inflammation and damage caused by excessive inflammatory responses in the skin.
In vitro, in vivo, in silico, and clinical studies have provided strong evidence of the benefits of astaxanthin in preventing and treating
skin aging, slowing wrinkles, increasing skin elasticity, reducing IL-1α, TNF-α, IL-6, IL-8, MMP-1, ROS activity, brightening skin, increasing collagen production, and
maintaining overall skin health and beauty (Table 1).
The main mechanism actions of astaxanthin include: 1) antioxidant activity, 2) scavenging free radicals, 3) protection against DNA damage, 4) anti-inflammation, 5)
stimulation of collagen production, 6) repair of skin damage, and 7) skin penetration. The mechanism of astaxanthin as an antioxidant able to fight damage caused by free
radicals produced by exposure to UV rays and pollution [78]. Free radicals can damage skin cells, cause skin aging, and
increase the risk of skin cancer. Astaxanthin neutralizes free radicals produced by UV rays by capturing them before they damage the skin structure
[6]. Astaxanthin works as an antioxidant by neutralizing ROS in the body. ROS are highly reactive oxygen molecules and can cause
cellular damage if produced too much or are out of balance with the body's antioxidant system. ROS include free radicals such as superoxide anion, hydrogen peroxide, and
hydroxyl radicals. Astaxanthin acts as a free radical scavenger, meaning it can bind ROS and prevent them from damaging other biological molecules, including DNA,
proteins, and lipids in the body's cells. In this way, astaxanthin helps protect the body's cells from oxidative damage that can lead to various disease conditions and
aging [71].
Table 1
Effect of astaxanthin on the skin.
| Intervention | Model | Duration | Results | Ref. |
|---|---|---|---|---|
| Astaxanthin supplementation | 65 healthy female participants | 16 weeks | IL-1α ↓ TNF-α ↓ IL-6 ↓ IL-8 ↓ MMP-1 ↓ Wrinkle ↓ Elasticity ↑ |
[75] |
| Astaxanthin supplementation | 23 healthy volunteers | 10 weeks | Rough skin ↓ Skin texture ↓ |
[76] |
| Astaxanthin oral treatments | Hairless mice | 8 weeks | Wrinkle ↓ Epidermal thickness ↓ Density of collagen fibers ↑ Density of capillary vessels ↑ ROS activity ↓ |
[6] |
| Astaxanthin topical and oral treatments | 60 women | 84 days | Wrinkles ↓ Roughness ↓ Spots (brown and visible) ↓ Elasticity ↑ Hydration ↑ Firmness ↑ Luminosity ↑ |
[72] |
| Astaxanthin topical and oral treatments | 60 women | 84 days | Wrinkles ↓ Roughness ↓ Spots (brown and visible) ↓ Elasticity ↑ Hydration ↑ Firmness ↑ Luminosity ↑ |
[72] |
| Astaxanthin administration | Protein data bank | Null | MMP-1 ↓ MMP-3 ↓ |
[71] |
| Astaxanthin administration | Normal human epidermal keratinocytes (NHEKs) | 2 hours | ROS production ↓ Apoptotic ↑ |
[18] |
| Astaxanthin nano emulsion administration | Human skin | 0-120 minutes | Wrinkle ↓ Skin-brightening ↑ |
[77] |
Exposure to UV rays cause damage to skin cell DNA. Astaxanthin has shown the ability to protect skin DNA from damage which may help prevent cell mutations and skin cancer [71, 79, 80]. Astaxanthin has anti-inflammatory properties that can help reduce skin inflammation due to exposure to UV rays [18, 81-83]. This can help prevent redness, swelling, and skin irritation. In addition, astaxanthin has been known to stimulate collagen production by skin cells, which can help maintain skin elasticity and suppleness [6]. Collagen is the main structural protein in the skin that provides strength and elasticity [72, 75]. Astaxanthin is also proven for its ability to repair existing skin damage, including wrinkles, fine lines, and age spots [72], by penetrating the layers of the skin, providing protection from the inside and outside. This makes it an effective supplement for skin health, whether used topically or through supplement consumption [84]. The use of astaxanthin in skin care can help reduce skin damage due to exposure to UV rays, increase skin elasticity, and reduce signs of skin aging [85].
Conclusions
Compounds derived from natural sources are gaining attention in the cosmetic industry and may be able to actively treat a variety of skin problems. This review attempts to shed light on the potential of the natural compound astaxanthin for slowing skin aging. Astaxanthin is an antioxidant that can prevent skin aging by increasing the activity of the Nrf2/ARE pathway and TGF-β, thereby reducing MMP-1 activity, and suppressing the activation of the NF-κB pathway, which can reduce inflammation and damage caused by excessive inflammatory responses in the skin. It also increases the activity of the MAPK pathway, which is involved in several cellular processes, including the response to oxidative stress, inflammation and cell proliferation. Many studies provide strong evidence of the benefits of astaxanthin in preventing and treating skin aging, slowing wrinkles, increasing skin elasticity, reducing IL-1α, TNF-α, IL-6, IL-8, MMP-1, ROS activity, brightening skin, increasing collagen production, and maintaining overall skin health and beauty. Finally, the use of astaxanthin in skincare products has become a potential product in the beauty industry. However, further studies are needed to fully understand the biological activity and precise mechanism of action of astaxanthin in slowing skin aging.
Declarations
Acknowledgments
We thank Maranatha Christian University for providing the necessary resources and support.
Author contributions
RS collected articles and wrote the first draft of manuscript, TLW initiated the study and revised the manuscript, and HR finalized the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Competing interests
All authors declare that they have no competing interests.
References
1. Nations U (2023). "Leaving no one behind in an ageing world: world social report 2020". from https://desapublications.un.org/publications/world-social-report-2023-leaving-no-one-behind-ageing-world (accessed 20.04.04).
2. Zouboulis CC, Blume-Peytavi U, Kosmadaki M, Roó E, Vexiau-Robert D, Kerob D, et al. Skin, hair and beyond: the impact of menopause. Climacteric, 2022, 25(5): 434-442. [Crossref]
3. D P. Anti-aging market size worldwide 2021-2027. 2024.
4. Yusharyahya SN. Mekanisme penuaan kulit sebagai dasar pencegahan dan pengobatan kulit menua. EJournal Kedokteran Indonesia, 2021, 9(2): 150-160. [Crossref]
5. Chaudhary M, Khan A, & Gupta M. Skin ageing: pathophysiology and current market treatment approaches. Curr Aging Sci, 2020, 13(1): 22-30. [Crossref]
6. Li X, Matsumoto T, Takuwa M, Saeed Ebrahim Shaiku Ali M, Hirabashi T, Kondo H, et al. Protective effects of astaxanthin supplementation against ultraviolet-induced photoaging in hairless mice. Biomedicines, 2020, 8(2): 18-28. [Crossref]
7. Keaney TC. Aging in the male face: intrinsic and extrinsic factors. Dermatol Surg, 2016, 42(7): 797-803. [Crossref]
8. Bravo B, Penedo L, Carvalho R, Dal Vesco C, Calomeni M, Gapanowicz D, et al. Dermatological changes during menopause and HRT: what to expect? Cosmetics, 2024, 11(1): 9-19. [Crossref]
9. Zouboulis CC, & Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol, 2011, 29(1): 3-14. [Crossref]
10. Lima SGM, Freire M, Oliveira VDS, Solisio C, Converti A, & de Lima Á AN. Astaxanthin delivery systems for skin application: a review. Mar Drugs, 2021, 19(9): 511-521. [Crossref]
11. Panich U, Sittithumcharee G, Rathviboon N, & Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int, 2016, 2016: 7370642. [Crossref]
12. Sari WP, Gaya ML, Dv S, Irianto MG, Sp F, Karima N, et al. Managemen topikal anti-aging pada kulit topical. Anti-aging management of the skin. Medula, 2019, 9(2): 228–234.
13. Bosch R, Philips N, Suárez-Pérez JA, Juarranz A, Devmurari A, Chalensouk-Khaosaat J, et al. Mechanisms of photoaging and cutaneous photocarcsinogenesis, and photoprotective strategies with phytochemicals. Antioxidants, 2015, 4(2): 248-268. [Crossref]
14. Kanlayavattanakul M, & Lourith N. Plants and natural products for the treatment of skin hyperpigmentation-a review. Planta Med, 2018, 84(14): 988-1006. [Crossref]
15. Davinelli S, Nielsen ME, & Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients, 2018, 10(4): 522-532. [Crossref]
16. Ambati RR, Phang SM, Ravi S, & Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications-a review. Mar Drugs, 2014, 12(1): 128-152. [Crossref]
17. Singh KN, Patil S, & Barkate H. Protective effects of astaxanthin on skin: recent scientific evidence, possible mechanisms, and potential indications. J Cosmet Dermatol, 2020, 19(1): 22-27. [Crossref]
18. Chung BY, Park SH, Yun SY, Yu DS, & Lee YB. Astaxanthin protects ultraviolet b-induced oxidative stress and apoptosis in human keratinocytes via intrinsic apoptotic pathway. Ann Dermatol, 2022, 34(2): 125-131. [Crossref]
19. Imokawa G. The xanthophyll carotenoid astaxanthin has distinct biological effects to prevent the photoaging of the skin even by its postirradiation treatment. Photochem Photobiol, 2019, 95(2): 490-500. [Crossref]
20. Lephart ED. Equol's efficacy is greater than astaxanthin for antioxidants, extracellular matrix integrity & breakdown, growth factors and inflammatory biomarkers via human skin gene expression analysis. Journal of Functional Foods, 2019, 59: 380-393. [Crossref]
21. Bouzroud S, El Maaiden E, Sobeh M, Merghoub N, Boukcim H, Kouisni L, et al. Biotechnological approaches to producing natural antioxidants: anti-ageing and skin longevity prospects. Int J Mol Sci, 2023, 24(2): 1397-1403. [Crossref]
22. Davinelli S, Saso L, D'Angeli F, Calabrese V, Intrieri M, & Scapagnini G. Astaxanthin as a modulator of Nrf2, NF-κB, and their crosstalk: molecular mechanisms and possible clinical applications. Molecules, 2022, 27(2): 502-512. [Crossref]
23. Cao C, Xiao Z, Wu Y, & Ge C. Diet and skin aging-from the perspective of food nutrition. Nutrients, 2020, 12(3): 870-880. [Crossref]
24. Shen M, Chen K, Lu J, Cheng P, Xu L, Dai W, et al. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-β1 expression and autophagy. Mediators Inflamm, 2014, 2014: 954502. [Crossref]
25. Kavitha K, Kowshik J, Kishore TK, Baba AB, & Nagini S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim Biophys Acta, 2013, 1830(10): 4433-4444. [Crossref]
26. Garg C. Molecular mechanisms of skin photoaging and plant inhibitors. Int J Green Pharmacy, 2017, 11(2): 1031-1042. [Crossref]
27. Fernandes A, Rodrigues PM, Pintado M, & Tavaria FK. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine, 2023, 115: 154824. [Crossref]
28. Firmansyah A, Winingsih W, Ababiel Z, Nurmeilasari N, & Setiasih A. Produksi astaxanthin dari mikroalga Haematococcus pluvialis menggunakan ekstraksi karbon dioksida superkritikal yang dimodifikasi. Production of astaxanthin from microalgae Haematococcus pluvialis using modified supercritical carbon dioxide extraction. Jurnal Sains dan Teknologi Farmasi Indonesia, 2019, 8(2): 113-123. [Crossref]
29. Snell TW, & Carberry J. Astaxanthin bioactivity is determined by stereoisomer composition and extraction method. Nutrients, 2022, 14(7): 1522-1532. [Crossref]
30. Wang HD, Chen CC, Huynh P, & Chang JS. Exploring the potential of using algae in cosmetics. Bioresour Technol, 2015, 184: 355-362. [Crossref]
31. García Prieto CV, Ramos FD, Estrada V, Villar MA, & Diaz MS. Optimization of an integrated algae-based biorefinery for the production of biodiesel, astaxanthin and PHB. Energy, 2017, 139: 1159-1172. [Crossref]
32. Dini I. The potential of algae in the nutricosmetic sector. Molecules, 2023, 28(10): 4032-4042. [Crossref]
33. Focsan AL, Polyakov NE, & Kispert LD. Photo protection of Haematococcus pluvialis algae by astaxanthin: unique properties of astaxanthin deduced by EPR, optical and electrochemical studies. Antioxidants, 2017, 6(4): 80-89. [Crossref]
34. Berthon JY, Nachat-Kappes R, Bey M, Cadoret JP, Renimel I, & Filaire E. Marine algae as attractive source to skin care. Free Radic Res, 2017, 51(6): 555-567. [Crossref]
35. Agatonovic-Kustrin S, & Morton DW. Cosmeceuticals derived from bioactive substances found in marine algae. Oceanography, 2013, 1: 1-11. [Crossref]
36. Pereira L. Seaweeds as source of bioactive substances and skin care therapy—cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics, 2018, 5(4): 68-78.
37. Jinu M, & Mohan Chandra K. Astaxanthin: An algaebased natural compound with a potential role in human health-promoting effect: an updated comprehensive review. Journal of Applied Biology & Biotechnology, 2021. [Crossref]
38. Dwivedi S, & Ahmad IZ (2020). Bioactive compounds from microalgal source as UV protective agents.
39. Rai AK, & Gurung SA (2022). Microalgal promise to the next generation: a dual potential perspective as cosmeceuticals and biofuels. Micro-algae: Next-generation Feedstock for Biorefineries: Cultivation and Refining Processes. P. Verma. Singapore, Springer Nature Singapore: 55-82.
40. Kanatt SR (2023). Antioxidants from the red algae Kappaphycus alvarezii. Marine Antioxidants. S.-K. Kim, K.-H. Shin and J. Venkatesan, Academic Press: 457-472.
41. Haque R, Sawant PB, Sardar P, Xavier KAM, Varghese T, Chadha NK, et al. Synergistic utilization of shrimp shell waste-derived natural astaxanthin with its commercial variant boosts physio metabolic responses and enhances colouration in discus (Symphysodon aequifasciatus). Environmental Nanotechnology, Monitoring & Management, 2021, 15: 100405. [Crossref]
42. Gomez-Estaca J, Comunian TA, Montero P, Ferro-Furtado R, & Favaro-Trindade CS. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocolloids, 2016, 61: 155-162. [Crossref]
43. Gómez-Estaca J, Calvo MM, Álvarez-Acero I, Montero P, & Gómez-Guillén MC. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem, 2017, 216: 37-44. [Crossref]
44. Roy VC, Getachew AT, Cho Y-J, Park J-S, & Chun B-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Penaeus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. The Journal of Supercritical Fluids, 2020, 159: 104773. [Crossref]
45. Messina CM, Manuguerra S, Arena R, Renda G, Ficano G, Randazzo M, et al. In vitro bioactivity of astaxanthin and peptides from hydrolisates of shrimp (Parapenaeus longirostris) by-products: from the extraction process to biological effect evaluation, as pilot actions for the strategy "from waste to profit". Mar Drugs, 2021, 19(4): 216-227. [Crossref]
46. Weeratunge WK, & Perera BG. Formulation of a fish feed for goldfish with natural astaxanthin extracted from shrimp waste. Chem Cent J, 2016, 10: 44-54. [Crossref]
47. Cheong JY, & Muskhazli M (2021). Turning leftover to treasure: an overview of astaxanthin from shrimp shell wastes. Global Perspectives on Astaxanthin. G. A. Ravishankar and A. Ranga Rao, Academic Press: 253-279.
48. El Boumlasy S, Mangraviti D, Arena K, Cacciola F, Asraoui F, & Debdoubi A. Determination of astaxanthin and astaxanthin esters in three samples of shrimp waste (Parapenaeus longirostris) by high performance liquid chromatography coupled photo-diode array and mass spectrometry detection. Nat Prod Res, 2024, 38(16): 2901-2908. [Crossref]
49. Chen S, Jiang S, & Jiang H. A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. Journal of Bioresources and Bioproducts, 2020, 5(4): 238-247. [Crossref]
50. Besharat M, Rajabi Islami H, Soltani M, & Abdolmajid Mousavi S. Effect of different levels of nanoliposome-coated astasxanthin on growth performance, body proximate composition, liver enzyme activity and pigmentation of rainbow trout ( Oncorhynchus mykiss). Aquaculture Research, 2021. [Crossref]
51. Wilkins LGE, Marques da Cunha L, Menin L, Ortiz D, Vocat-Mottier V, Hobil M, et al. Maternal allocation of carotenoids increases tolerance to bacterial infection in brown trout. Oecologia, 2017, 185(3): 351-363. [Crossref]
52. Zorriehzahra MJ. Efficacy of astaxanthin fed to rainbow trout (Oncorhynchus mykiss): effect on growth, pigmentation and blood indices. International Journal of Oceanography Aquaculture, 2020. [Crossref]
53. Wu K, Cleveland BM, Portman M, Sealey WM, & Lei XG. Supplemental microalgal DHA and astaxanthin affect astaxanthin metabolism and redox status of juvenile rainbow trout. Antioxidants, 2020, 10(1): 16-26. [Crossref]
54. Noori A, & Razi A. Effects of dietary astaxanthin on the growth and skin and muscle pigmentation of sexually immature rainbow trout Oncorhynchus mykiss (Walbaum, 1792) (Teleostei: Salmonidae). Iranian Journal of Ichthyology, 2018, 4(4): 361-374. [Crossref]
55. Kurnia AIN, Satoh S, Haga Y, Kudo H, Nakada M, Matsumura H, et al. Muscle coloration of rainbow trout with astaxanthin sources from marine bacteria and synthetic astaxanthin. Journal of Aquaculture Research and Development, 2015, 6: 1-4. [Crossref]
56. Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Fašmon Durjava M, et al. Safety and efficacy of a feed additive consisting of astaxanthin-rich Phaffia rhodozyma for salmon and trout (Igene Biotechnology, Inc.). Efsa j, 2022, 20(2): e07161. [Crossref]
57. Xie D, Gong M, Wei W, Jin J, Wang X, Wang X, et al. Antarctic krill (Euphausia superba) oil: a comprehensive review of chemical composition, extraction technologies, health benefits, and current applications. Compr Rev Food Sci Food Saf, 2019, 18(2): 514-534. [Crossref]
58. Barros MP, Poppe SC, & Bondan EF. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients, 2014, 6(3): 1293-1317. [Crossref]
59. Zhang S, Sun X, & Liu D. Preparation of (3R, 3′R)-astaxanthin monoester and (3R, 3′R)-astaxanthin from Antarctic krill (Euphausia superba Dana). Eur Food Res Technol, 2015, 240: 295−299.
60. Landry JD, Torley PJ, & Blanch EW. Quantitation of carotenoids and fatty acids from Atlantic salmon using a portable raman device. Analyst, 2022, 147(19): 4379-4388. [Crossref]
61. Weichert FG, Axén C, Förlin L, Inostroza PA, Kammann U, Welling A, et al. A multi-biomarker study on Atlantic salmon (Salmo salar L.) affected by the emerging red skin disease in the baltic sea. J Fish Dis, 2021, 44(4): 429-440. [Crossref]
62. Brown MR, Kube PD, Taylor RS, & Elliott NG. Rapid compositional analysis of Atlantic salmon (Salmo salar) using visible-near infrared reflectance spectroscopy. Aquaculture Research, 2014, 45(5): 798-811. [Crossref]
63. Grünenwald M, Carter CG, Nichols DS, Adams MB, & Adams LR. Heterogeneous astaxanthin distribution in the fillet of Atlantic salmon post-smolt at elevated temperature is not affected by dietary fatty acid composition, metabolic conversion of astaxanthin to idoxanthin, or oxidative stress. Aquaculture, 2020, 521: 735096. [Crossref]
64. Makri V, Feidantsis K, Papadopoulos D, Lattos A, Georgoulis I, Michaelidis B, et al. Natural-like pigmentation in cultured fish stocks, not only a matter of nutrition. A review of salmonidae and sparidae families, with a particular focus on the red porgy pagrus pagrus. Aquaculture Research, 2021, 52(7): 2942-2953. [Crossref]
65. Barredo JL, García-Estrada C, Kosalkova K, & Barreiro C. Biosynthesis of astaxanthin as a main carotenoid in the heterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J Fungi, 2017, 3(3): 44-54. [Crossref]
66. Tseng CC, Lin YJ, Liu W, Lin HY, Chou HY, Thia C, et al. Metabolic engineering probiotic yeast produces 3S, 3'Sastaxanthin to inhibit B16F10 metastasis. Food Chem Toxicol, 2020, 135: 110993. [Crossref]
67. Panis G, & Carreon JR. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: a microalgae process model and a techno-economic assessment all through production line. Algal Research, 2016, 18: 175-190. [Crossref]
68. Brotosudarmo THP, Limantara L, Setiyono E, & Heriyanto. Structures of astaxanthin and their consequences for therapeutic application. Int J Food Sci, 2020, 2020: 2156582. [Crossref]
69. An İ, & SG. B (2023). Sun protection diets.
70. Kaga K, Honda M, Adachi T, Honjo M, Wahyudiono, Kanda H, et al. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): effect of PVP and astaxanthin Z-isomer content. The Journal of Supercritical Fluids, 2018, 136: 44-51. [Crossref]
71. Sajjad W, Abbasi SW, & Ali L. Molecular docking study of astaxanthin derived from radio-resistant bacterium Deinococcus sp. strain WMA-LM9 to Matrix Metalloproteinase-1, 3 (MMP-1, MMP-3). Life Sciences, 2021, 2: 6-16. [Crossref]
72. Manzanares S, Arias EJG, Floriach N, M. S, & Roldán FA. Synergistic antiaging and dermal restorative effects of an oral bioactive procollagen and astaxanthin supplement with a topical retinyl palmitate, vitamin c, hyaluronic acid and alpha hidroxy acid based regimen. Journal of Dermatology Research, 2021.
73. Chen WP, Xiong Y, Shi YX, Hu PF, Bao JP, & Wu LD. Astaxanthin reduces matrix metalloproteinase expression in human chondrocytes. Int Immunopharmacol, 2014, 19(1): 174-177. [Crossref]
74. Chou HY, Lee C, Pan JL, Wen ZH, Huang SH, Lan CW, et al. Enriched astaxanthin extract from Haematococcus pluvialis augments growth factor secretions to increase cell proliferation and induces MMP1 degradation to enhance collagen production in human dermal fibroblasts. Int J Mol Sci, 2016, 17(6): 955-965. [Crossref]
75. Tominaga K, Hongo N, Fujishita M, Takahashi Y, & Adachi Y. Protective effects of astaxanthin on skin deterioration. J Clin Biochem Nutr, 2017, 61(1): 33-39. [Crossref]
76. Ito N, Seki S, & Ueda F. The protective role of astaxanthin for uv-induced skin deterioration in healthy people-a randomized, double-blind, placebo-controlled trial. Nutrients, 2018, 10(7): 817-827. [Crossref]
77. Nurdianti L, Setiawan F, Rusdiana T, Gozali D, & Cahyati KI. Physical and chemical evaluations of topical radiance serum containing nanoemulsion combination of astaxanthin and zeaxanthin: designed as anti-wrinkle and skin-brightening serum. International Journal of Applied Pharmaceutics, 2023. [Crossref]
78. Nakajima H, Terazawa S, Niwano T, Yamamoto Y, & Imokawa G. The inhibitory effects of anti-oxidants on ultraviolet-induced upregulation of the wrinkling-inducing enzyme neutral endopeptidase in human fibroblasts. PLoS One, 2016, 11(9): e0161580. [Crossref]
79. Chen Y-T, Kao C-J, Huang H-Y, Huang S-Y, Chen C-Y, Lin Y-S, et al. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of in vitro and in vivo models in melanoma. Journal of Functional Foods, 2017, 31: 20-31. [Crossref]
80. Jayawardhana H, Jayawardena TU, Sanjeewa KKA, Liyanage NM, Nagahawatta DP, Lee HG, et al. Marine algal polyphenols as skin protective agents: current status and future prospectives. Mar Drugs, 2023, 21(5): 285-295. [Crossref]
81. Donoso A, González-Durán J, Muñoz AA, González PA, & Agurto-Muñoz C. Therapeutic uses of natural astaxanthin: an evidence-based review focused on human clinical trials. Pharmacol Res, 2021, 166: 105479. [Crossref]
82. Park JW, & Song HS. Effect of astaxanthin on antiinflammatory and anti-oxidative effects of astaxanthin treatment for atopic dermatitis-induced mice. Journal of Acupuncture Research, 2021. [Crossref]
83. Lee YS, Jeon SH, Ham HJ, Lee HP, Song MJ, & Hong JT. Improved anti-inflammatory effects of liposomal astaxanthin on a phthalic anhydride-induced atopic dermatitis model. Front Immunol, 2020, 11: 565285. [Crossref]
84. Kohandel Z, Farkhondeh T, Aschner M, PourbagherShahri AM, & Samarghandian S. Anti-inflammatory action of astaxanthin and its use in the treatment of various diseases. Biomed Pharmacother, 2022, 145: 112179. [Crossref]
85. Miguel SP, Ribeiro MP, Otero A, & Coutinho P. Application of microalgae and microalgal bioactive compounds in skin regeneration. Algal Research, 2021, 58: 102395.