Open Access | Research
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Behavioral and neuropathological features of Alzheimer's disease are attenuated in 5xFAD mice treated with intranasal GHK peptide
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle WA, USA.
Email: wladiges@uw.edu
Received: 16 May 2024 / Revised: 03 June 2024 / Accepted: 14 August 2024 / Published: 30 September 2024
DOI: 10.31491/APT.2024.09.148
Abstract
Alzheimer's disease (AD) is a complex neurodegenerative disease and a leading cause of morbidity and mortality. Efforts to find disease modifying treatments have met with limited success. The naturally occurring peptide GHK (glycyl-L-histidyl-L-lysine), in its Cu-bound form, supports angiogenesis, remodeling, and tissue repair, has anti-inflammatory and antioxidant properties, and has been shown to improve cognitive performance in aging mice. These features raised the question of whether GHK-Cu could alleviate neurodegeneration observed in AD. Male and female 5xFAD transgenic mice on the C57BL/6J background at 4 months of age were given 15 mg/kg GHK-Cu intranasally 3 times per week for 3 months until 7 months of age. Results showed that intranasal GHK-Cu treatment delayed cognitive impairment, reduced amyloid plaques, and lowered MCP1-mediated inflammation levels in the frontal cortex and hippocampus. These observations provide the rationale for conducting additional studies to investigate the potential of GHK-Cu peptide as a promising treatment for AD.
Keywords
Alzheimer's disease, GHK-Cu, 5xFAD transgenic mouse, intranasal administration, amyloid plaques, neuroinflammation
Introduction
Alzheimer's disease (AD) is a complex disease that was the seventh leading cause of mortality in the US in 2022
[1]. Contrary to most other leading causes of mortality, there is no effective treatment
for AD and efforts to find effective treatments have met with limited success in part because the focus has been on testing drugs
that target a specific pathogenic mechanism. The probability of effectively targeting several mechanistic pathways would be
greatly increased by using a drug that individually targets more than one pathway [2,
3]. In addition, the Connectivity Map gene profiling software developed by the Broad
Institute, showed that out of several thousand biological molecules, the naturally occurring peptide glycyl-L-histidyl-L-lysine
(GHK), in its Cu-bound form prevented impaired TGFβ1 signaling [4], a pathway associated
with Aβ deposition and neurofibrillary tangle formation [5]. This correlation raised the
question of whether GHK-Cu could alleviate neurodegeneration observed in Alzheimer's disease (AD).
GHK is a naturally occurring peptide released from secreted protein acidic and rich in cysteine (SPARC) during proteolytic
breakdown [6]. In the event of an injury, GHK supports angiogenesis, remodeling, and
tissue repair as a copper complex (GHK-Cu) [7-9].
The peptide is clinically approved as a topical application for age-related skin conditions and promoted mainly as a skin
rejuvenation drug [10]. GHK has been shown to be an endogenous antioxidant by decreasing
hydroxyl and peroxyl radicals [11], presumptively involved in the neuropathogenesis of
AD [12] and improve cognitive performance in aging mice
[13].
Targeted neurotherapeutics face a challenge in penetrating the blood brain barrier (BBB)
[14-16]. This inherent restriction has
significantly limited the availability of effective AD treatments and has triggered investigation into methods or strategies to
bypass the BBB. In this regard, the intranasal route is a promising delivery method to obtain greater efficacy in maintaining
optimal drug concentrations in the brain compared to parenteral injections
[17-20]. The intranasal system uses olfactory
epithelium and its associated neural pathways to deliver drugs in therapeutically relevant concentrations.
The 5xFAD transgenic mouse line has been genetically engineered to model features of AD through the expression of human amyloid
precursor protein (APP) and presenilin-1 transgenes, which are associated with earlyonset AD
[21]. Mutations in these genes are linked to the regulation of amyloid-beta processing,
a crucial component of AD pathogenesis. Transgenic 5xFAD mice have amyloid neuropathology similar to characteristic amyloidplaque
pathology in human patients [21, 22], and
have been shown to develop impairment in cognitive behaviors that correspond to part of the dementia syndrome in AD.
Neuroinflammation plays a crucial role in the onset and progression of AD, with chemokines such as monocyte chemoattractant
protein-1 (MCP-1) being key mediators in these inflammatory processes. Elevated levels of MCP-1 have been linked to chronic
inflammation, which is a hallmark of neurodegenerative diseases. MCP-1 contributes to the recruitment of immune cells to the
central nervous system (CNS), potentially exacerbating neuroinflammation and promoting disease progression
[23-25]. Additionally, increased MCP-1 levels
are associated with heightened blood-brain barrier (BBB) permeability, further complicating disease pathology by allowing more
inflammatory cells to infiltrate the CNS [26]. Therefore, evaluating the extent of
inflammatory reactivity to amyloid plaques and microglial activity through MCP-1 staining would provide insights into the role of
MCP-1 in neuroinflammatory responses in AD.
The aim of this study was to determine if intranasal delivery of GHK-Cu as a neurotherapeutic would effectively delay the onset of
cognitive impairment and neuropathology seen in 5xFAD transgenic mice. The advantages of intranasal administration, including
reduced dosage loss, enhanced precision in drug delivery, and expedited brain access, were used to show that GHK-Cu was able to
mitigate cognitive impairment and features of AD neuropathology.
Materials and methods
Animals and experimental design
C57BL/6J mice with the transgenic 5xFAD genotype (JAX, Bar Harbor, Maine) of both sexes were used. The 5xFAD mouse line has
five mutations: the Swedish (K670N/M671L), Florida (I716V), and London (V717I) mutations in APP, as well as the M146L and L286V
mutations in PSEN1. Collectively, these mutations induce the formation of amyloid plaques. Mice were bred and genotyped following
standard procedures from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were grouphoused, up to five per cage, in a specific
pathogen free facility verified through viral and bacterial tests (IDEXX Bioanalytics). Nestlets (Ancare Corp, Bellmore, NY) were
provided for physical and mental stimulation. Mice were monitored for health daily and cages were changed biweekly. All
experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
The experiment started when mice were 4 months of age and ended when they were 7 months of age for a treatment period of three
months (12 weeks). Transgenic 5xFAD and wild type mice of both sexes were stratified across a peptide treatment cohort and a saline
control cohort.
GHK-Cu dose and delivery
GHK was used as a GHK-Cu complex (Active Peptide, Cambridge, MA) at a dose of 15 mg/kg body weight. Unpublished observations in
our lab suggested that GHKCu complex was more effective than unbound GHK while unbound Cu had no effect in several in vitro
and in vivo experiments. However, Cu ions can induce toxicity in mice if dosage exceeds levels of 35 mg/kg
[27]. Within the GHK-Cu complex, Cu made up 14% of the total molecular content, so a 15
mg/kg GHK-Cu dose had 2.1 mg/kg copper.
Drug administration occurred under 3% isoflurane anesthesia. Mice were then positioned at a 10-15° decline, and a hand grip was
applied to the back, tail, and neck. This lowered the head in relation to the body, which maximized the surface area of the
olfactory epithelium for efficient dosage uptake [8]. The drug was then drawn up using a
micropipette with a clean tip and applied carefully to the rim of the mouse nostril, one drop at a time. The timing of droplet
placement was synchronized with natural inhalation to allow for drops to settle on the olfactory and respiratory epithelium within
the nasal cavity [9]. Droplets were alternated between nostrils with each breath until a
20 μL volume was administered, typically requiring three to four droplets per mouse. Mice were then maintained in the declined
position for an additional minute, during which stimulation was applied to the sternum region to enhance volume uptake. Intranasal
GHK-Cu was administered three times per week using this procedure for the duration of the study. Saline was administered to the
control group using similar methods as stated above.
Behavioral Test
Cognitive function was assessed by an attentive working memory task (Y-maze) designed to assess working spatial memory. The Y-Maze was performed as previously described [28]. Briefly, it consisted of three equally spaced arms at 120° angles from each other, each enclosed by raised walls. There was no escape option within the maze, and mice were allowed to freely explore it for five minutes while the path they took through the three arms was tracked by recording their entries into each arm. After completing the Y-maze, the mice were temporarily removed and placed in a separate resting cage while the maze was sterilized. All littermates underwent the assessment in a similar manner and were collectively returned to their home cage at the end of the trial. The Y-Maze test assessed spontaneous alternation, an indicator of working memory, as it measures the tendency of mice to explore novel arms of the maze rather than revisitations. Data from the trials administered at weeks four, eight, and twelve of the study were recorded. Spontaneous alternation data were expressed as a percentage calculated as the number of times a mouse completed a triad or loop through all three arms during the trial divided by the number of entries minus two [29]. This percentage represents the number of triads over the number of possible triads for a particular mouse otherwise known as spontaneous alternation percentage.
Neuropathology
At the conclusion of the twelve-week (3 months) treatment period, mice were euthanized using CO2 and tissues were collected and processed. Sections of the brain and other major organs were rapidly frozen in liquid nitrogen and stored at -80°C. The remaining brain sections and systemic organs were fixed in 10% buffered formalin for 48 hours. Brain tissues were placed in PBS for 24 hours before being embedded in paraffin wax and sectioned onto histology slides. Brain section slides were stained with Congo red to evaluate amyloid plaques by averaging the number of plaques in ten different fields under 10x magnification in multiple areas of the brain blindly by two separate observers. Additional staining was performed using an Abcam immunohistochemistry kit for MCP-1 as a measure of general neuroinflammation. Stained slides were analyzed using Qu-Path digital imagining analysis to measure staining intensity as optical density on positively stained tissue as previously described [30].
Data analysis
Behavioral groups were compared using either a one-way analysis of variance (ANOVA) or two-way ANOVA test whenever appropriate and presented in the form of mean ± standard deviation. Main trial-number effects were analyzed using the Bonferroni post-hoc multiple comparisons test to quantify the extent of latency differences. A twotailed t-test was employed to compare results between cohorts for neuropathological data. All data were analyzed using GraphPad Prism software (version 10.0.3, GraphPad Software Inc., San Diego, CA, USA) as standard error of the mean with statistical significance at P < 0.05.
Results and Discussion
Intranasal GHK-Cu peptide improved cognitive performance in transgenic 5xFAD mice
This study showed that transgenic 5xFAD mice of both sexes exhibited improved cognitive performance after 8 weeks of intranasal
GHK-Cu treatment, compared to intranasal saline treated transgenic mice. This pattern continued through the 12-week treatment
duration, corresponding to when the mice were 7 months old.
Transgenic female mice treated with intranasal GHK-Cu had higher alternation percentages in the Y maze, indicating improved
cognitive performance, beginning as early as the second month of treatment (Week 8), and continuing through the third month
(Week 12) when the study ended, compared to transgenic mice treated with intranasal saline
(Figure 1A). For male mice, there were significant increases in alternation percentages
in transgenic male mice treated with intranasal GHK-Cu compared to transgenic male mice treated with intranasal saline starting
the first month and continuing for the next 2 months of the study (Figure 1B). Similar to
females, intranasal treatment with GHK-Cu in transgenic mice resulted in a cognitive performance level comparable to non-transgenic
wildtype mice.
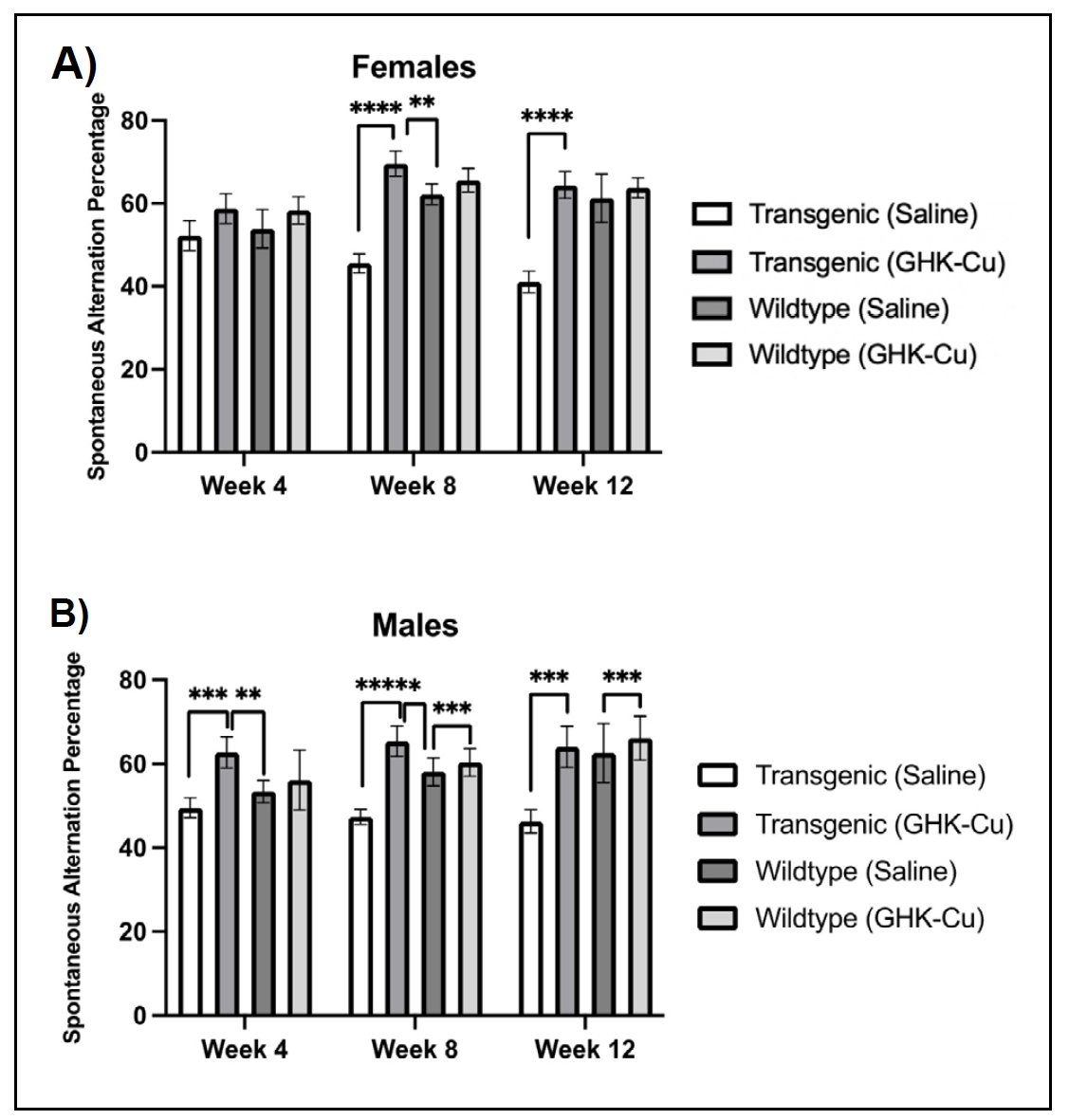
Figure 1. Y-maze percent alternation as a measure of cognitive function over 12 weeks of intranasal GHK-Cu treatment. Cognitive performance was evaluated relative to the 50% threshold. (A) A higher alternation percentage was observed for female transgenic mice treated with intranasal GHK-Cu compared to transgenic females treated with intranasal saline at weeks 8 and 12. (B) A similar pattern was observed for male transgenic mice treated with intranasal GHK-Cu compared to mice receiving intranasal saline at weeks 4 and 8. **P < 0.01, ***P < 0.001, ****P < 0.0001, N = 4-7/cohort, Transgenic = 5xFAD.
GHK-Cu-treated transgenic mice of both sexes exhibited more spontaneous alternations in the Y-maze test, indicative of rescued working memory and prefrontal cortical functions [31]. Similar levels of spontaneous alternations were observed in both sexes of GHK-Cu-treated mice compared to other studies involving AD-related drug intervention [32-35]. However, evidence supporting the long-term effects of intranasal GHK-Cu on cognitive performance of 5xFAD mice is limited [34, 36], emphasizing the need for additional studies.
Intranasal GHK-Cu peptide attenuated features of neuropathology in 5xFAD mice
The 5xFAD mouse genotype is characterized by the onset of amyloid plaques at 3 to 4 months of age, which progress to substantial and densely concentrated lesions in the brain [37]. Using a Congo Red stain, transgenic mice treated with intranasal GHK-Cu exhibited a reduction in amyloid plaques compared to transgenic mice treated with intranasal saline, irrespective of sex (Figure 2). Both male and female transgenic 5xFAD mice displayed the development of amyloid plaques in the midbrain and thalamus region, frontal cortex, and the hippocampus. Among the transgenic cohorts, those treated with intranasal GHKCu had significantly fewer detectable plaques or protein aggregates in comparison to those receiving intranasal saline. Visual observation suggested a pattern where the plaques in saline-treated mice were generally larger and more densely stained compared to the plaques in GHKCu-treated cohorts (Figure 3). Wild-type (control) littermates did not display any amyloid plaques, consistent with their genotype.
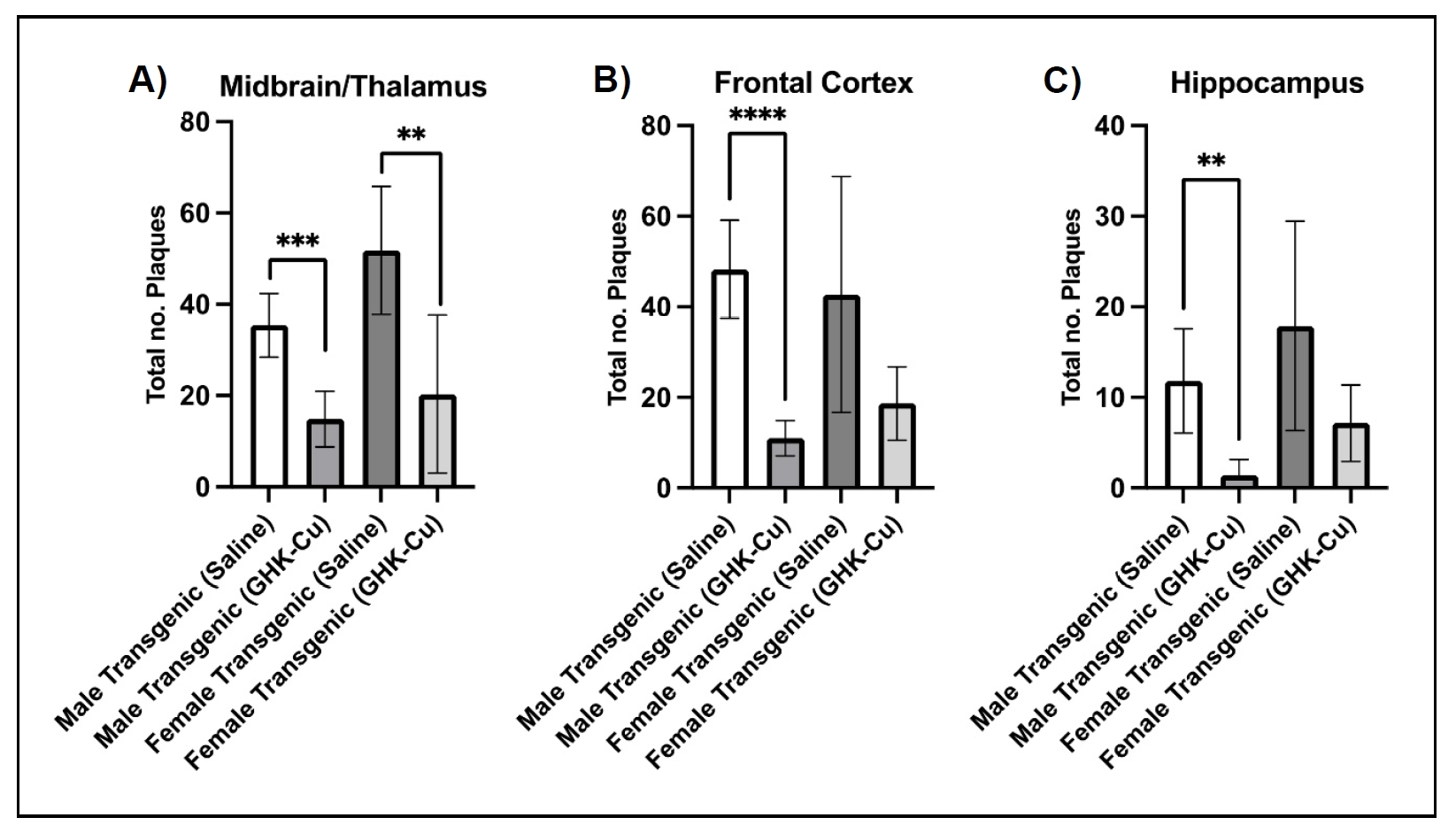
Figure 2. Amyloid plaques were reduced in multiple brain regions of 5xFAD mice following intranasal treatment with GHK-Cu. Quantitative visual count analysis of Congo Red-stained midbrain/thalamus (A), frontal cortex (B), and hippocampal (C) sections from male mice showed a reduction in amyloid plaques in intranasal GHK-Cu-treated transgenic mice compared to intranasal saline-treated transgenic mice while female mice showed a reduction in the midbrain/thalamus region. **P < 0.01, ***P < 0.001, ****P < 0.0001. N = 6-7/cohort. Transgenic = 5xFAD.
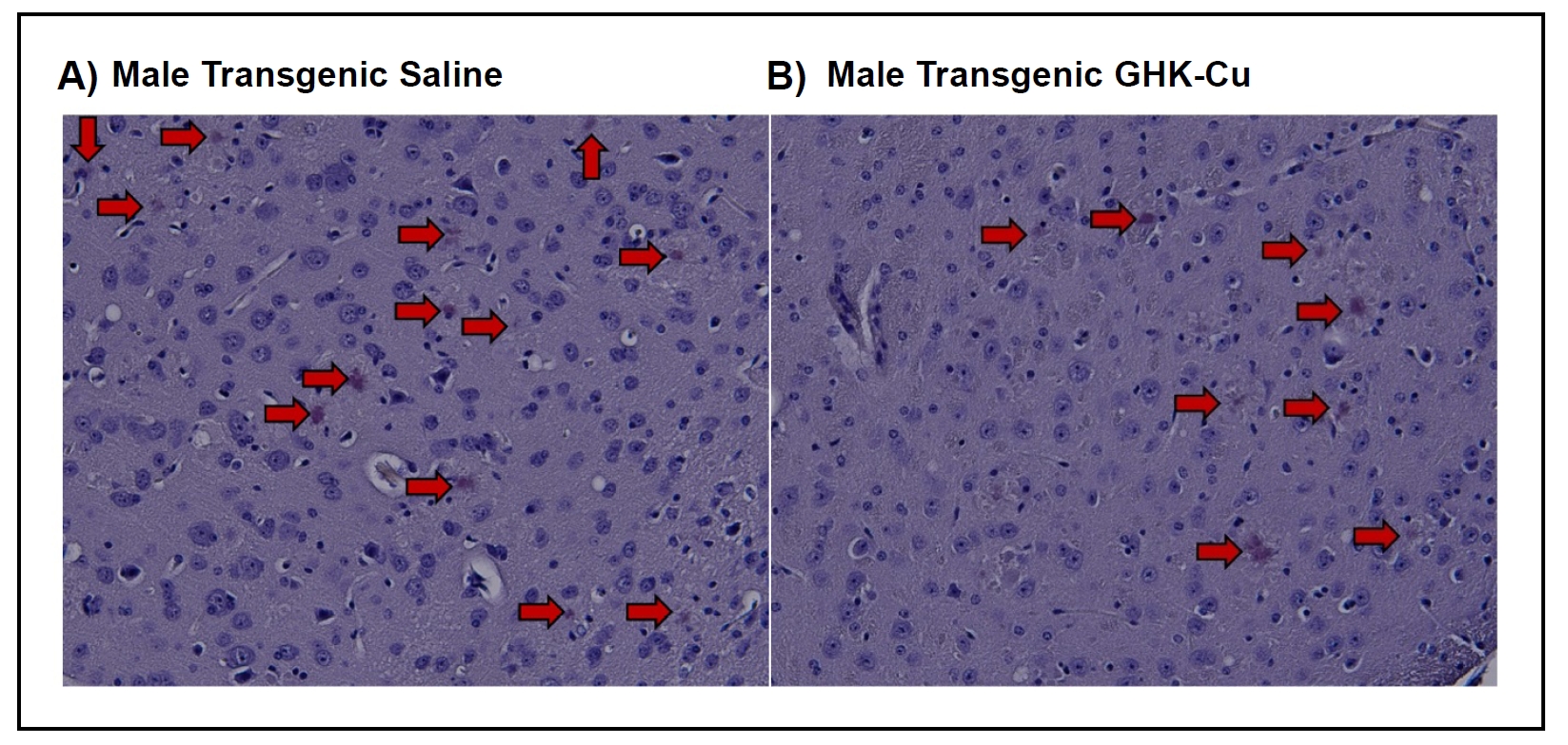
Figure 3. Representative Congo Red staining of frontal cortex sections from (A) male transgenic mouse treated with intranasal saline or (B) male transgenic mouse treated with intranasal GHK-Cu. Transgenic = 5xFAD. Magnification 200×.
The accumulation of amyloid plaques is a pivotal factor associated with subsequent neuronal toxicity in AD pathogenesis,
eventually associated with synaptic dysfunction and more severe cognitive deficits [38].
Our study revealed that GHK-Cu treated 5xFAD mice exhibited improved cognitive performance compared to saline-treated cohorts while
also demonstrating a reduction in amyloid plaques in the midbrain, frontal cortex and hippocampus. While the rescued cognitive
abilities in GHK-Cu treated 5xFAD mice may be linked to diminished amyloid plaque formation, the extent of associated
neurodegeneration in AD progression was not evaluated. Further investigations targeting this disparity are warranted based on a
study protocol designed to assess neurodegeneration and neuronal loss specifically in 5xFAD mice approaching one year of age
[39].
MCP-1 staining using immunohistochemistry and QuPath digital imaging showed that both male and female transgenic 5xFAD mice that
received intranasal GHK-Cu had decreased staining intensity for MCP-1 in the frontal cortex and hippocampus
(Figure 4) within tissues registering a positive stain for MCP-1. This correlation
indicates reduced neuroinflammation associated with MCP-1 compared to transgenic mice treated with intranasal saline. Heat maps
from the Qu-Path generated data show a visual representation of the staining intensity in the frontal cortex from mice treated with
intranasal GHK-Cu or saline (Figure 5). The same visual staining intensities were seen
in the hippocampus (not shown).
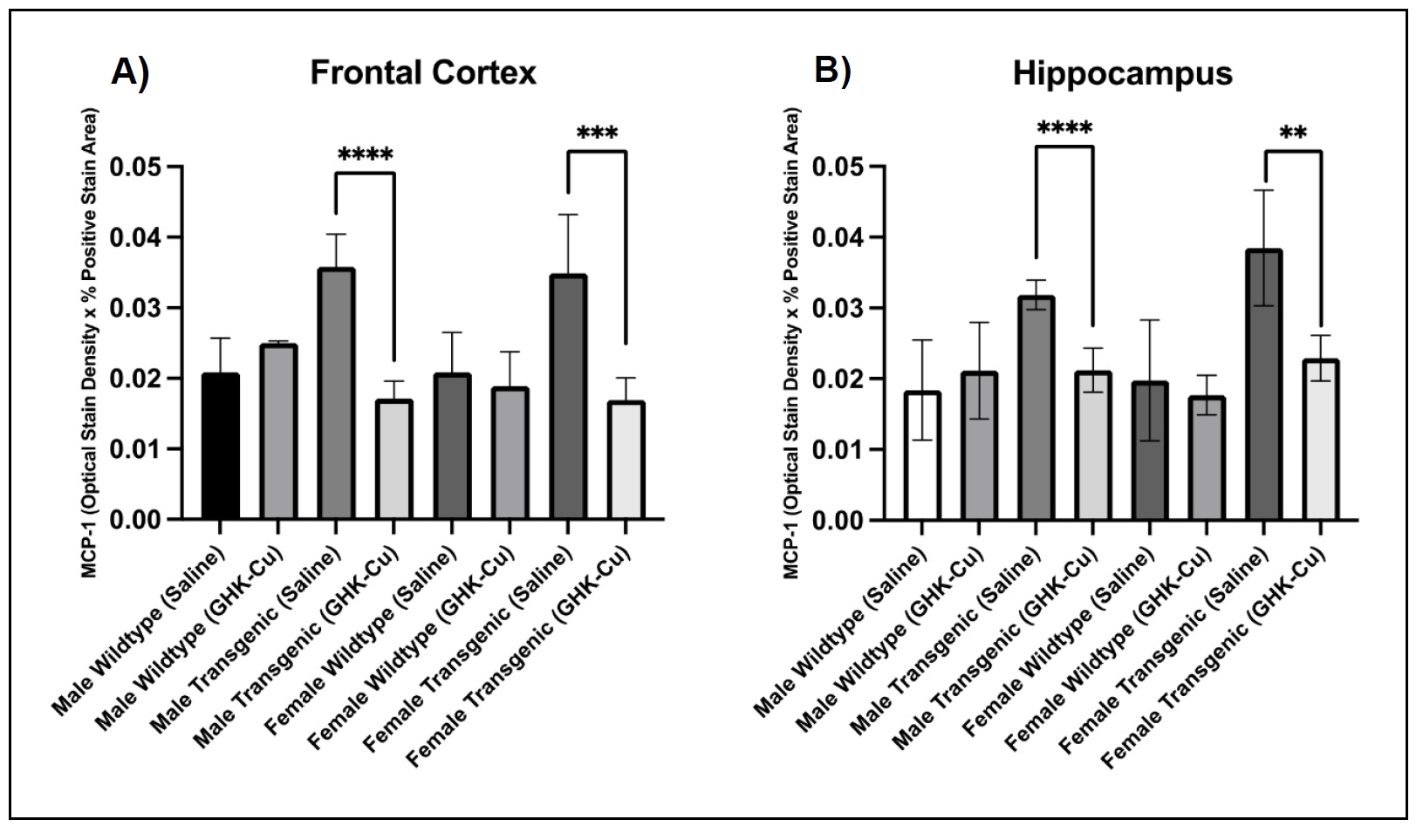
Figure 4. MCP-1 digital stain intensity of positively stained tissues in frontal cortex and hippocampus. (A) Transgenic male and female mice treated with GHK-Cu displayed reduced optical stain intensity in positive stained tissues within the frontal lobe when compared to saline-treated cohorts. (B) Transgenic male and female mice treated with GHK-Cu also exhibited lower optical stain density in positively stained tissues within the hippocampus in comparison to saline-treated counterparts. Data presented as mean ± standard deviation. **P < 0.01, ***P < 0.001, ****P < 0.0001. N = 6-10/cohort. Transgenic = 5xFAD.
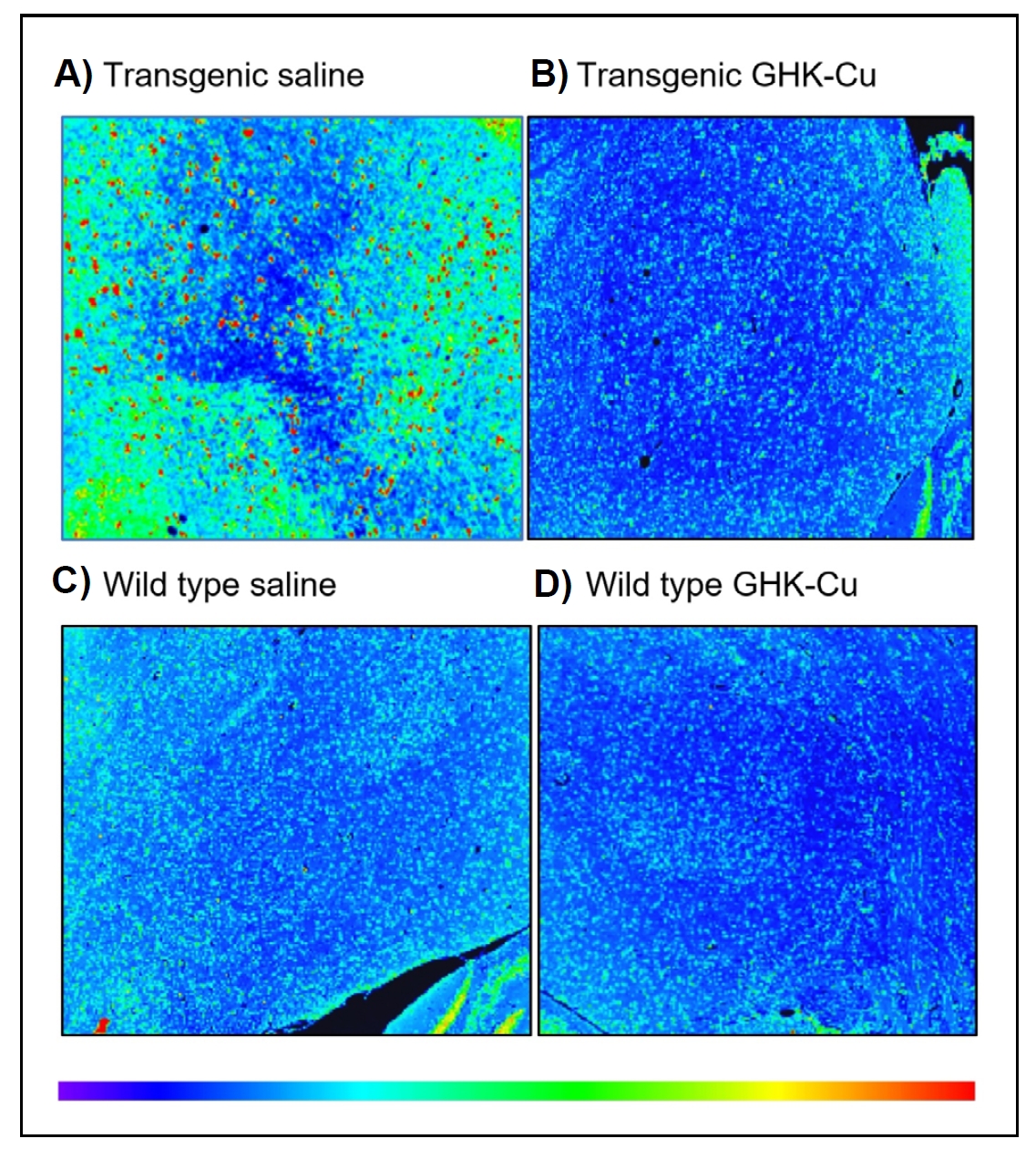
Figure 5. Q-Path generated heat map of an MCP-1 immunohistochemistry stain of frontal cortex from female mice after 12 weeks of treatment. (A) Transgenic mouse treated with intranasal saline. (B) Transgenic mouse treated with intranasal GHK-Cu. (C) Wild type mouse treated with intranasal saline. (D) Wild type mouse treated with intranasal GHK-Cu. The heat map legend indicates blue as low intensity staining all the way up to red as high intensity staining. Photos taken at ×40 magnification. Transgenic = 5xFAD; wild type = nontransgenic (age, strain, and sex matched controls).
Given that chemokine upregulation can result in chronic inflammation associated with onset and progression of age-related
neurodegenerative diseases such as AD, our study employed MCP-1 staining in the frontal cortex and the hippocampus to assess the
extent of possible inflammatory reactivity to amyloid plaques and microglial activity
[23-25]. Furthermore, elevated MCP-1 levels as
a result of chemokine upregulation associated with neuroinflammatory diseases have been shown to be associated with increased BBB
permeability, which can further exacerbate disease progression [26]. Our results
demonstrated a notable decrease in MCP-1 staining intensity in both brain regions in 5xFAD mice that received intranasal GHK-Cu
compared to saline-treated cohorts. The observed decrease in MCP-1 staining intensity suggests that intranasal administration of
GHK-Cu in transgenic 5xFAD mice had a reducing effect on the AD-induced inflammatory phenotype and could serve as a reliable
prototype to study drugs that slow or stop progression of AD [40].
The intranasal administration of GHK-Cu represents a novel approach aimed at overcoming the challenge of bypassing the blood-brain
barrier, a common hurdle in traditional targeted neurotherapeutics
[14-16]. By leveraging the olfactory epithelium
and its associated neural pathways, this method capitalizes on the large surface area, efficient blood flow, and neural connections
of the nasal mucosa to provide a relatively non-invasive approach towards efficient peptide delivery into the brain
[41-46]. The selection of a
three-times-weekly administration schedule accounted for preventing adverse effects of daily anesthesia, and potential localized
irritation and toxicity within the nasal mucosa [41,
47]. While systemic toxicity of copper ions was carefully addressed, a comprehensive
assessment of inflammation and inflammatory cell infiltration of the nasal epithelium in future studies would further validate the
safety and non-toxicity of intranasal GHK-Cu administration.
Conclusions
In conclusion, this study demonstrates the positive therapeutic effect of intranasal GHK-Cu in transgenic 5xFAD mice, a widely used model for AD. Cognitive improvement was observed after 8 weeks of treatment in both females and males, sustained through a 12-week study period, and accompanied by a concurrent reduction in amyloid plaques and neuroinflammation compared to intranasal saline-treated transgenic mice. Behavioral tests, including the Y-maze working memory task and a spatial navigation learning task, helped provide evidence that intranasal GHK-Cu treatment can rescue cognitive performance in a mouse model of AD. The intranasal delivery method allowed a non-invasive approach to efficient drug delivery. These findings suggest that intranasal GHKCu has the potential to attenuate features of AD, including cognitive decline, amyloid plaque accumulation, and neuroinflammation, and provide the rationale for further studies in aging mice, for example using an AAV vector containing sequences of Aβ42 and mutant tau [48].
Declarations
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by National Institutes of Health grants R01 AG057381 (Ladiges and Darvas, co-PI's) and R01 AG057381 (Ladiges PI).
Conflicts of interest
Warren Ladiges and Martin Darvas are members of the editorial board of Aging Pathobiology and Therapeutics. The authors declare that they have no conflicts and were not involved in the journal's review or decision regarding this manuscript.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. Ahmad FB, Cisewski JA, Xu J, & Anderson RN. Provisional mortality data - United States, 2022. MMWR Morb Mortal Wkly Rep, 2023, 72(18): 488-492. [Crossref]
2. Ladiges W. The quality control theory of aging. Pathobiol Aging Age Relat Dis, 2014, 4. [Crossref]
3. Rosenfeld M, & Ladiges W. Pharmaceutical interventions to slow human aging. Are we ready for cocktails? Aging Pathobiol Ther, 2022, 4(2): 51-52. [Crossref]
4. Pickart L, Vasquez-Soltero JM, & Margolina A. The effect of the human peptide GHK on gene expression relevant to nervous system function and cognitive decline. Brain Sci, 2017, 7(2). [Crossref]
5. Bosco P, Ferri R, Salluzzo MG, Castellano S, Signorelli M, Nicoletti F, et al. Role of the transforming-growthfactor-β1 gene in late-onset alzheimer's disease: implications for the treatment. Curr Genomics, 2013, 14(2): 147-156. [Crossref]
6. Lane TF, Iruela-Arispe ML, Johnson RS, & Sage EH. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol, 1994, 125(4): 929-943. [Crossref]
7. Pickart L, & Margolina A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int J Mol Sci, 2018, 19(7): 1987-1997. [Crossref]
8. Badenhorst T, Svirskis D, & Wu Z. Physicochemical characterization of native glycyl-l-histidyl-l-lysine tripeptide for wound healing and anti-aging: a preformulation study for dermal delivery. Pharm Dev Technol, 2016, 21(2): 152-160. [Crossref]
9. Zhang H, Wang Y, & He Z. Glycine-Histidine-Lysine (GHK) alleviates neuronal apoptosis due to intracerebral hemorrhage via the miR-339-5p/VEGFA pathway. Front Neurosci, 2018, 12: 644-654. [Crossref]
10. Dou Y, Lee A, Zhu L, Morton J, & Ladiges W. The potential of GHK as an anti-aging peptide. Aging Pathobiol Ther, 2020, 2(1): 58-61. [Crossref]
11. Sakuma S, Ishimura M, Yuba Y, Itoh Y, & Fujimoto Y. The peptide glycyl-ʟ-histidyl-ʟ-lysine is an endogenous antioxidant in living organisms, possibly by diminishing hydroxyl and peroxyl radicals. Int J Physiol Pathophysiol Pharmacol, 2018, 10(3): 132-138.
12. Miller MB, Huang AY, Kim J, Zhou Z, Kirkham SL, Maury EA, et al. Somatic genomic changes in single alzheimer's disease neurons. Nature, 2022, 604(7907): 714-722. [Crossref]
13. Rosenfeld M, Nickel K, & Ladiges W. GHK peptide prevents sleep-deprived learning impairment in aging mice. Aging Pathobiol Ther, 2023, 5(1): 33-35. [Crossref]
14. Ballabh P, Braun A, & Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis, 2004, 16(1): 1-13. [Crossref]
15. Hur GH, Han SC, Ryu AR, Eom Y, Kim JW, & Lee MY. Effect of oligoarginine conjugation on the antiwrinkle activity and transdermal delivery of GHK peptide. J Pept Sci, 2020, 26(2): e3234. [Crossref]
16. Uğurlu T, Türkoğlu M, & Özaydın T. In vitro evaluation of compression-coated glycyl-L-histidyl-L-lysine-Cu(II) (GHK-Cu2+)-loaded microparticles for colonic drug delivery. Drug Dev Ind Pharm, 2011, 37(11): 1282-1289. [Crossref]
17. Mittal D, Ali A, Md S, Baboota S, Sahni JK, & Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv, 2014, 21(2): 75-86. [Crossref]
18. Chauhan MB, & Chauhan NB. Brain uptake of neurotherapeutics after intranasal versus intraperitoneal delivery in mice. J Neurol Neurosurg, 2015, 2(1): 009.
19. Crowe TP, Greenlee MHW, Kanthasamy AG, & Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci, 2018, 195: 44-52. [Crossref]
20. Kumar H, Mishra G, Sharma AK, Gothwal A, Kesharwani P, & Gupta U. Intranasal drug delivery: a non-invasive approach for the better delivery of neurotherapeutics. Pharm Nanotechnol, 2017, 5(3): 203-214. [Crossref]
21. Brody DL, Jiang H, Wildburger N, & Esparza TJ. Noncanonical soluble amyloid-beta aggregates and plaque buffering: controversies and future directions for target discovery in Alzheimer's disease. Alzheimers Res Ther, 2017, 9(1): 62-72. [Crossref]
22. Younkin SG. The role of A beta 42 in Alzheimer's disease. J Physiol Paris, 1998, 92(3-4): 289-292. [Crossref]
23. Singh S, Anshita D, & Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol, 2021, 101(Pt B): 107598. [Crossref]
24. Chen WW, Zhang X, & Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep, 2016, 13(4): 3391-3396. [Crossref]
25. Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, & Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci, 1997, 51(3): 135-138. [Crossref]
26. Yao Y, & Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci, 2014, 71(4): 683-697. [Crossref]
27. Liu H, Guo H, Jian Z, Cui H, Fang J, Zuo Z, et al. Copper induces oxidative stress and apoptosis in the mouse liver. Oxid Med Cell Longev, 2020, 2020: 1359164. [Crossref]
28. Darvas M, Morsch M, Racz I, Ahmadi S, Swandulla D, & Zimmer A. Modulation of the Ca2+ conductance of nicotinic acetylcholine receptors by Lypd6. Eur Neuropsychopharmacol, 2009, 19(9): 670-681. [Crossref]
29. Miedel CJ, Patton JM, Miedel AN, Miedel ES, & Levenson JM. Assessment of Spontaneous alternation, novel object recognition and limb clasping in transgenic mouse models of Amyloid-β and tau neuropathology. J Vis Exp, 2017, (123): e55523. [Crossref]
30. Lee A, Jiang Z, Zhu L, & Ladiges W. QuPath. A new digital imaging tool for geropathology. Aging Pathobiol Ther, 2020, 2(2): 114-116. [Crossref]
31. Kraeuter AK, Guest PC, & Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol, 2019, 1916: 105-111. [Crossref]
32. Cai M, Lee JH, & Yang EJ. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer's disease animal model. J Neuroinflammation, 2019, 16(1): 264-274. [Crossref]
33. Jo KW, Lee D, Cha DG, Oh E, Choi YH, Kim S, et al. Gossypetin ameliorates 5xFAD spatial learning and memory through enhanced phagocytosis against Aβ. Alzheimers Res Ther, 2022, 14(1): 158-168. [Crossref]
34. Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F, et al. Vitamin D improves neurogenesis and cognition in a mouse model of alzheimer's disease. Mol Neurobiol, 2018, 55(8): 6463-6479. [Crossref]
35. Mukherjee KK, Lee AY, Zhu L, Darvas M, & Ladiges W. Sleep-deprived cognitive impairment in aging mice is alleviated by rapamycin. Aging Pathobiol Ther, 2019, 1(1): 5-9. [Crossref]
36. Sosna J, Philipp S, Albay R, 3rd, Reyes-Ruiz JM, BagliettoVargas D, LaFerla FM, et al. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer's disease. Mol Neurodegener, 2018, 13(1): 11-22. [Crossref]
37. Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci, 2006, 26(40): 10129-10140. [Crossref]
38. Zhu C, Ren X, Liu C, Liu Y, & Wang Y. Rbm8a regulates neurogenesis and reduces Alzheimer's disease-associated pathology in the dentate gyrus of 5×FAD mice. Neural Regen Res, 2024, 19(4): 863-871. [Crossref]
39. Zhang L, Li J, & Lin A. Assessment of neurodegeneration and neuronal loss in aged 5XFAD mice. STAR Protoc, 2021, 2(4): 100915. [Crossref]
40. Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, & Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol, 2009, 19(3): 392-398. [Crossref]
41. Tulbah AS, Elkomy MH, Zaki RM, Eid HM, Eissa EM, Ali AA, et al. Novel nasal niosomes loaded with lacosamide and coated with chitosan: a possible pathway to target the brain to control partial-onset seizures. Int J Pharm X, 2023, 6: 100206. [Crossref]
42. Elkomy MH, Zaki RM, Alsaidan OA, Elmowafy M, Zafar A, Shalaby K, et al. Intranasal nanotransferosomal gel for quercetin brain targeting: I. optimization, characterization, brain localization, and cytotoxic studies. Pharmaceutics, 2023, 15(7): 1805-1816. [Crossref]
43. Eissa EM, Elkomy MH, Eid HM, Ali AA, Abourehab MAS, Alsubaiyel AM, et al. Intranasal delivery of dranisetron to the brain via nanostructured cubosomes-based in situ gel for improved management of chemotherapy-induced emesis. Pharmaceutics, 2022, 14(7): 1374-1388. [Crossref]
44. Elkomy MH, Ali AA, & Eid HM. Chitosan on the surface of nanoparticles for enhanced drug delivery: a comprehensive review. J Control Release, 2022, 351: 923-940. [Crossref]
45. Abo El-Enin HA, Elkomy MH, Naguib IA, Ahmed MF, Alsaidan OA, Alsalahat I, et al. Lipid nanocarriers overlaid with chitosan for brain delivery of berberine via the nasal route. Pharmaceuticals (Basel), 2022, 15(3): 281-293. [Crossref]
46. Eid HM, Elkomy MH, El Menshawe SF, & Salem HF. Transfersomal nanovesicles for nose-to-brain delivery of ofloxacin for better management of bacterial meningitis: formulation, optimization by box-behnken design, characterization and in vivo pharmacokinetic study. Journal of drug delivery science and technology, 2019, 54: 101304. [Crossref]
47. Elsenosy FM, Abdelbary GA, Elshafeey AH, Elsayed I, & Fares AR. Brain targeting of duloxetine HCL via intranasal delivery of loaded cubosomal gel: in vitro characterization, ex vivo permeation, and in vivo biodistribution studies. Int J Nanomedicine, 2020, 15: 9517-9537. [Crossref]
48. Darvas M, Keene D, & Ladiges W. A geroscience mouse model for Alzheimer's disease. Pathobiol Aging Age Relat Dis, 2019, 9(1): 1616994. [Crossref]