Open Access | Commentary
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
The house cricket is an unrecognized but potentially powerful model for aging intervention studies
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA, USA.
Email: wladiges@uw.edu
Received: 13 March 2024 / Accepted: 14 March 2024 / Published: 28 March 2024
DOI: 10.31491/APT.2024.03.135
Abstract
Human-based research on the biology of aging poses challenges due to ethical, social, and cost considerations. Animal models offer a pragmatic alternative, although no single model fully replicates all aspects of human aging. One unexplored model for studying aging is the house cricket (Acheta domesticus). House crickets present advantages for aging intervention research, such as consuming an omnivorous diet, availability on a heterogeneous genetic background, short lifespan, and simple but well-defined organ systems. In a preliminary experiment, the effects of flax oil, which is rich in omega-3 fatty acids, on the lifespan of house crickets were investigated. Cold-pressed flax oil was added as a 10% mixture to moist guinea pig chow mash and fed to crickets starting at 8 weeks of age and continuing for 11 weeks until the last cricket died. Results demonstrated a significant extension in survival of crickets fed the flax oil diet, further emphasizing the low cost, simplicity, and short time required to conduct dietary intervention studies in house crickets. Using this observation as a prototype, the house cricket is a promising and deserving model for interventional drug testing based on an aging platform.
Keywords
Aging, house cricket, lifespan, aging intervention, flax oil
Aging is a complex process characterized by functional decline and increasing morbidity, and stands as a predominant risk
factor for numerous diseases. Therefore, a better understanding of the underlying mechanisms associated with aging may facilitate
the development of new treatments [1]. The complexities of human-based research,
including ethical, social, and cost considerations, coupled with a long lifespan, pose significant challenges. Consequently,
exploring aging through animal models emerges as a pragmatic alternative. While no single animal model can fully replicate all
aspects of aging in humans, a comprehensive understanding of the characteristics of the specific model and a judicious
interpretation of the results within its defined limitations can facilitate focused investigations into critical facets of
age-related diseases and their associated treatments [2].
Criteria for the selection of effective animal models largely revolve around the translational value to humans in terms of
physiological and/or pathological aspects [3]. Rodents, non-human primates, flies
(Drosophila melanogaster), and worms (Caenorhabditis elegans) have been extensively used in aging research
[4]. Recognition of the challenges associated with vertebrate models, including long
lifespan, size, ethical constraints, and cost [5,
6], underscores the importance of integrating translational invertebrate models,
particularly in lifespan studies. A significant amount of new knowledge about aging and agerelated diseases has been generated
with these models.
Mammalian models of aging have some of the same constraints as humans, while flies and worms have technical constraints. Flies are
holometabolous (i.e., have multiple developmental life stages) and most studies are restricted to the adult stage only
[5], while worms feed on bacteria and lack a vascular system along with organs that
target it (i.e., brain, heart, kidney) [7]. As a result, test materials
and accurate dosing are technically demanding. Acheta domesticus, or more commonly known as the "house cricket", is a
promising model for the study of aging and age-related diseases for several reasons. 1) They are omnivorous with rudimentary but
well-defined organ systems (Figure 1); 2) They are relatively easy to rear at 30-32 ℃
in large quantities, small areas, and at low cost [8-10];
3) Crickets are hemimetabolous with an average lifespan of 4 months, with juveniles sharing the same diet as adults; 4) Crickets
have a visually distinguishable sexual maturity between developmental stages and adults
[9-11]; 5) Neurogenesis occurs in the cricket
brain in an area analogous to the mammalian hippocampus [12-14];
6) Cognitive and non-cognitive behavioral tests are easily performed in crickets [15].
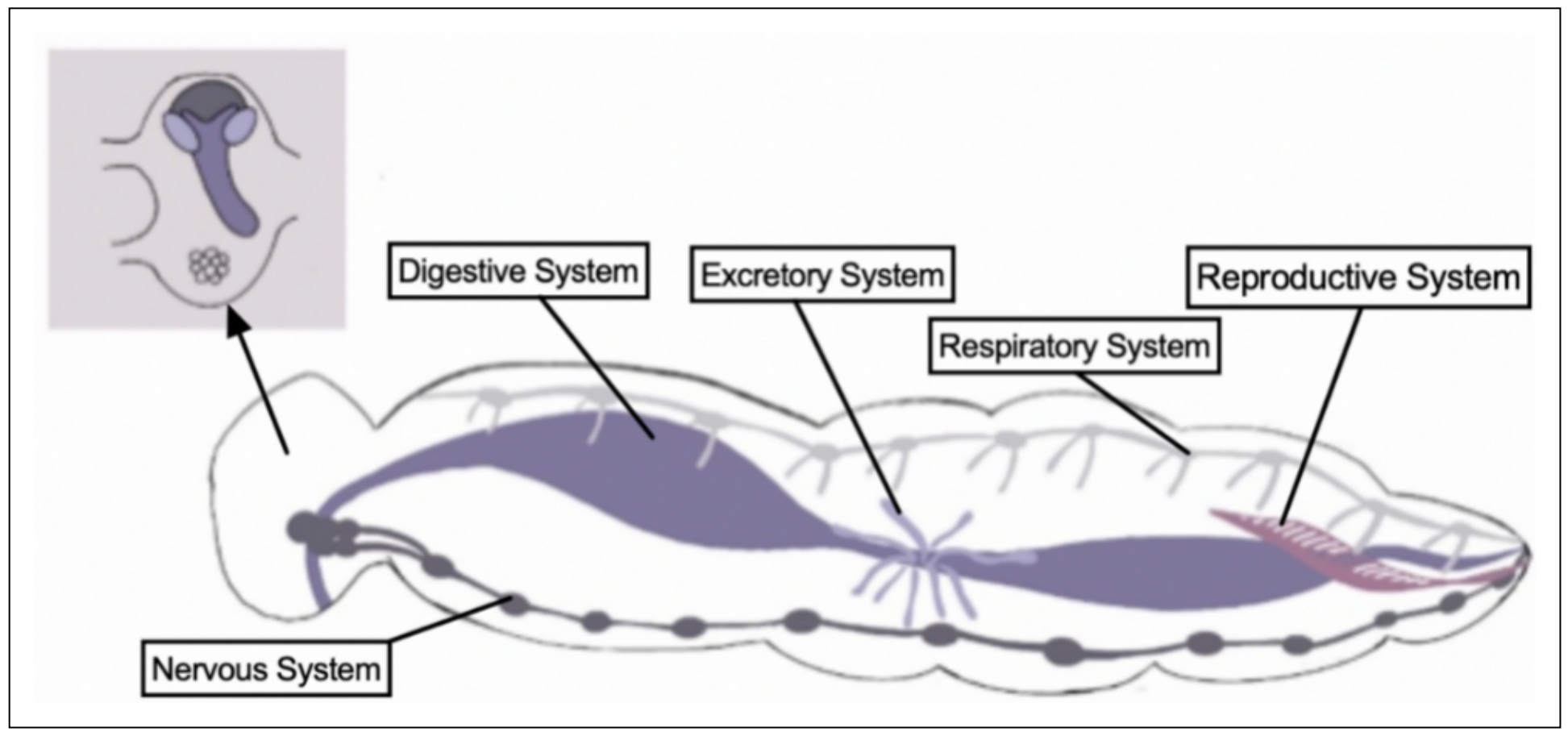
Figure 1. Internal structures of the domestic house cricket.
These characteristics make house crickets ideal for aging studies. For example, our laboratory has observed an increase in
lifespan when crickets were fed flax oil added to their diet. Genetically heterogeneous house crickets were obtained from Fluker
Farms (Louisiana) at 8 weeks of age and fed guinea pig chow or guinea chow supplemented with 10% cold-pressed flax oil (Barlean's).
They were housed in a transparent and aerated plastic box (36 × 36 × 20 cm) containing an egg carton shelter, a tray with a
moistened sponge, and a food tray at an environmental temperature of 20-21℃ and a 12 h on/12 h off light cycle. The guinea pig
chow contained protein 16%, fat 3.5%, fiber 12.5%, ash 6%, calcium 0.8%, phosphorus 0.6%, copper sulfate 15 mg/kg, vitamin A 6000
IU/kg, vitamin C 300 mg/kg, vitamin D3 520 IU/kg, vitamin E 24 IU/kg and was prepared as a mash with 30 g of chow and 300 mL of
sterilized distilled water to provide a consistency readily ingested by the crickets.
Crickets fed guinea chow with flax oil had a significant increase in lifespan compared to crickets fed just guinea pig chow
(Figure 2). The 50 percent survival rate was 82 and 94 days, respectively and the 10
percent survival rate was 109 and 136 days, respectively. Flax oil is rich in polyunsaturated omega-3 fatty acids (PUFA),
particularly alpha-linolenic acid, which has been shown to exhibit anti-inflammatory and antioxidant effects
[16]. Crickets have a simplified gut structure consisting of a single layer of epithelium
with various cells such as enterocytes responsible for nutrient absorption [17],
including dietary lipids such as PUFAs [18]. While the effects of PUFAs are not
well-documented in the insect digestive system, they are known to improve the integrity of the intestinal epithelial barrier in
mammals by preventing changes in epithelial permeability, inhibiting the production of proinflammatory molecules, and enhancing
the production of anti-inflammatory molecules
[19-21].
The optimal advantages of crickets as an animal model of aging, along with our preliminary observations, provide a strong rationale
for conducting more in-depth studies using house crickets to test a variety of intervention strategies using both lifespan and
cross-sectional approaches.
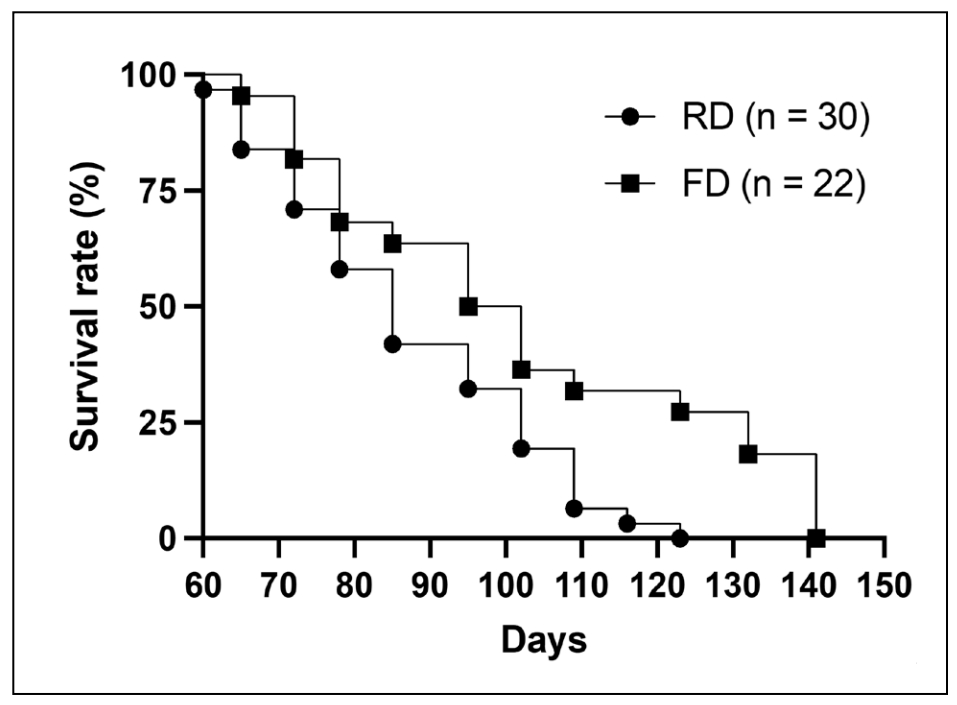
Figure 2. Beginning at 8 weeks of age, house crickets were fed guinea pig chow with 10% flax oil versus crickets fed just guinea pig chow. Lifespan in the two cohorts was assessed using Kaplan-Meier (logrank) survival analysis. Time points were assigned equal weight using a Mantel-Cox test. P < 0.05. N = 29-30 per cohort.
Declarations
Financial support and sponsorship
Supported by the National Institute on Aging grant R01 AG057381 (Ladiges, PI).
Conflicts of interest
Warren Ladiges is a member of the editorial board of Aging Pathobiology and Therapeutics. The authors declare that they have no conflicts and were not involved in the journal's review or decision regarding this manuscript.
References
1. Mitchell SJ, Scheibye-Knudsen M, Longo DL, & de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci, 2015, 3: 283-303. [Crossref]
2. Matute-Bello G, Frevert CW, & Martin TR. Animal models of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2008, 295(3): L379- L399. [Crossref]
3. Mukherjee P, Roy S, Ghosh D, & Nandi SK. Role of animal models in biomedical research: a review. Lab Anim Res, 2022, 38(1): 18-28. [Crossref]
4. Andersen ML, & Winter LMF. Animal models in biological and biomedical research—experimental and ethical concerns. An Acad Bras Cienc, 2019, 91(suppl 1): e20170238. [Crossref]
5. Lyn JC, Naikkhwah W, Aksenov V, & Rollo CD. Influence of two methods of dietary restriction on life history features and aging of the cricket Acheta domesticus. Age (Dordr), 2011, 33(4): 509-522. [Crossref]
6. Holtze S, Gorshkova E, Braude S, Cellerino A, Dammann P, Hildebrandt TB, et al. Alternative animal models of aging research. Front Mol Biosci, 2021, 8: 660959. [Crossref]
7. Litke R, Boulanger É, & Fradin C. Caenorhabditis elegans as a model organism for aging: relevance, limitations and future. Med Sci (Paris), 2018, 34(6-7): 571-579. [Crossref]
8. Patton R. Growth and development parameters for Acheta domesticus. Annals of the Entomological Society of America, 1978, 71(1): 40-42. [Crossref]
9. Clifford CW, Roe RM, & Woodring J. Rearing methods for obtaining house crickets, Acheta domesticus, of known age, sex, and instar. Annals of the Entomological Society of America, 1977, 70(1): 69-74. [Crossref]
10. Lyn J, Aksenov V, LeBlanc Z, & Rollo CD. Life history features and aging rates: insights from intra-specific patterns in the cricket Acheta domesticus. Evolutionary Biology, 2012, 39(3): 371-387. [Crossref]
11. Fuciarelli TM, & Rollo CD. Radiation exposure causes developmental alterations in size and shape of wings and structures associated with song production in male crickets (Acheta domesticus). Entomologia Experimentalis et Applicata, 2021, 169(2): 227-234. [Crossref]
12. Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell, 2018, 22(4): 589- 599.e585. [Crossref]
13. Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell, 2018, 23(1): 25-30. [Crossref]
14. Kumar A, Pareek V, Faiq MA, Ghosh SK, & Kumari C. Adult neurogenesis in human: a review of basic concepts, history, current research, and clinical implications. Innov Clin Neurosci, 2019, 16(5-6): 30-37.
15. Cayre M, Scotto-Lomassese S, Malaterre J, Strambi C, & Strambi A. Understanding the regulation and function of adult neurogenesis: contribution from an insect model, the house cricket. Chem Senses, 2007, 32(4): 385-395. [Crossref]
16. Al-Madhagy S, Ashmawy NS, Mamdouh A, Eldahshan OA, & Farag MA. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. Eur J Med Res, 2023, 28(1): 240-250. [Crossref]
17. Huang JH, Jing X, & Douglas AE. The multi-tasking gut epithelium of insects. Insect Biochem Mol Biol, 2015, 67: 15-20. [Crossref]
18. Chapman RF: The Insects: Structure and Function, 5 edn. Cambridge: Cambridge University Press; 2012. [Crossref]
19. Seethaler B, Lehnert K, Yahiaoui-Doktor M, Basrai M, Vetter W, Kiechle M, et al. Omega-3 polyunsaturated fatty acids improve intestinal barrier integrity-albeit to a lesser degree than short-chain fatty acids: an exploratory analysis of the randomized controlled LIBRE trial. Eur J Nutr, 2023, 62(7): 2779-2791. [Crossref]
20. Durkin LA, Childs CE, & Calder PC. Omega-3 polyunsaturated fatty acids and the intestinal epithelium-a review. Foods, 2021, 10(1): 1-35. [Crossref]
21. Fu Y, Wang Y, Gao H, Li D, Jiang R, Ge L, et al. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm, 2021, 2021: 8879227. [Crossref]