Open Access | Mini Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Modeling resilience to sleep disruption to study resistance to Alzheimer's disease
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 12 November 2023 / Revised: 29 November 2023 / Accepted: 04 December 2023 / Published: 26 December 2023
DOI: 10.31491/APT.2023.12.128
Abstract
Alzheimer's disease (AD) is a devastating neurodegenerative condition with unknown etiology and no cure. Therefore, it is imperative to learn more about the underlying risk factors. Since AD is an age-related disease, one approach is to look at factors associated with aging. One example is sleep disruption, which increases with age and accelerates the progression of cognitive decline. However, some people with sleep loss experience little or no cognitive impairment and are considered resilient. The concept that resilience to sleep disruption increases resistance to AD can be modeled in aging mice with or without cognitive impairment to determine resistance or susceptibility to AD. Given that sleep disruption is a relevant and rising health concern, it is essential to gain a better understanding of resilience, and factors associated with resistance to AD, in order to develop successful intervention strategies.
Keywords
Aging, sleep disruption, resilience, cognitive impairment, Alzheimer's disease, neuropathology, resistance
Introduction
The prevalence of neurodegenerative diseases such as Alzheimer's disease (AD) is expected to soar with the number of elderly individuals in both developed
and developing countries rising dramatically. Efforts to find diseasemodifying treatments have been largely unsuccessful, in
part due to the inability to assess early signs of disease and the lack of knowledge about risk factors associated
with increasing age. One approach to investigating risk factors for AD is to look at attributes that oppose risk, i.e.,
resilience and resistance. Physical resilience to aging is the ability of an organism to respond to physical stressors
without causing long-term adverse effects [1, 2]. Resistance to AD is defined as
the failure to develop significant cognitive impairment and neuropathology associated with
amyloid beta and phosphorylated tau [3, 4].
Sleep disturbances, such as sleep fragmentation and sleep loss, occur in humans with increasing age. It has recently
been recognized as an important risk factor for AD [5-7].
There is growing evidence that poor sleep leads to acceleration in the progression of neurodegenerative disorders
and may play a role in their pathogenesis [8-10]. Research
has shown that some individuals are resilient, and others
are sensitive to sleep disruption [11-13]. A study by
Dennis et al. [14] suggested that this phenotypic feature is
maintained with increasing age. Approximately two-thirds of healthy older adults showed performance deficits with
moderate to severe sleep loss, while one-third showed few or no performance deficits even with severe sleep loss.
Therefore, sleep disruption is a universal issue that affects individuals in different ways. While some individuals experience a deficit in daily performance, others
experience resiliency as they can maintain high levels of physical and mental function despite sleep loss. Although sleep loss
causes cognitive dysfunction in areas such as learning and memory, it is not well understood how resilience to sleep
disruption may be associated with a slowdown in aging, improved overall health, and decreased risk (resistance)
of developing AD. The expectation is that those with enhanced resilience to sleep disruption would have an
increased resilience to aging and the ability to adapt successfully without developing age-related diseases such as AD.
A mouse model of resilience to acute sleep disruption
Over the past decade, several studies have shown that people who are vulnerable versus resilient to the effects of sleep loss differ in their brain activation and behavioral performance when they are well rested [15, 16]. Observations in sleep-deprived mice are similar, as evidenced by our recent publication showing that learning impairment is an adverse effect of acute sleep disruption [17]. In this study, we utilized a novel spatial navigation task designated as the Box Maze [18, 19] in sleep-disrupted mice in order to identify resilience or sensitivity to learning impairment. Parameters consisted of keeping mice awake for a four-hour period of time each day for four days (Figure 1). The mice showed a consistent learning impairment pattern that was not seen in non-sleep-deprived mice of the same age. Interestingly, the Box Maze task showed variable levels of performance within the sleep-disrupted mice, indicating that certain mice were resilient (fast learners) and other mice were sensitive (slow learners) to the development of impaired learning.
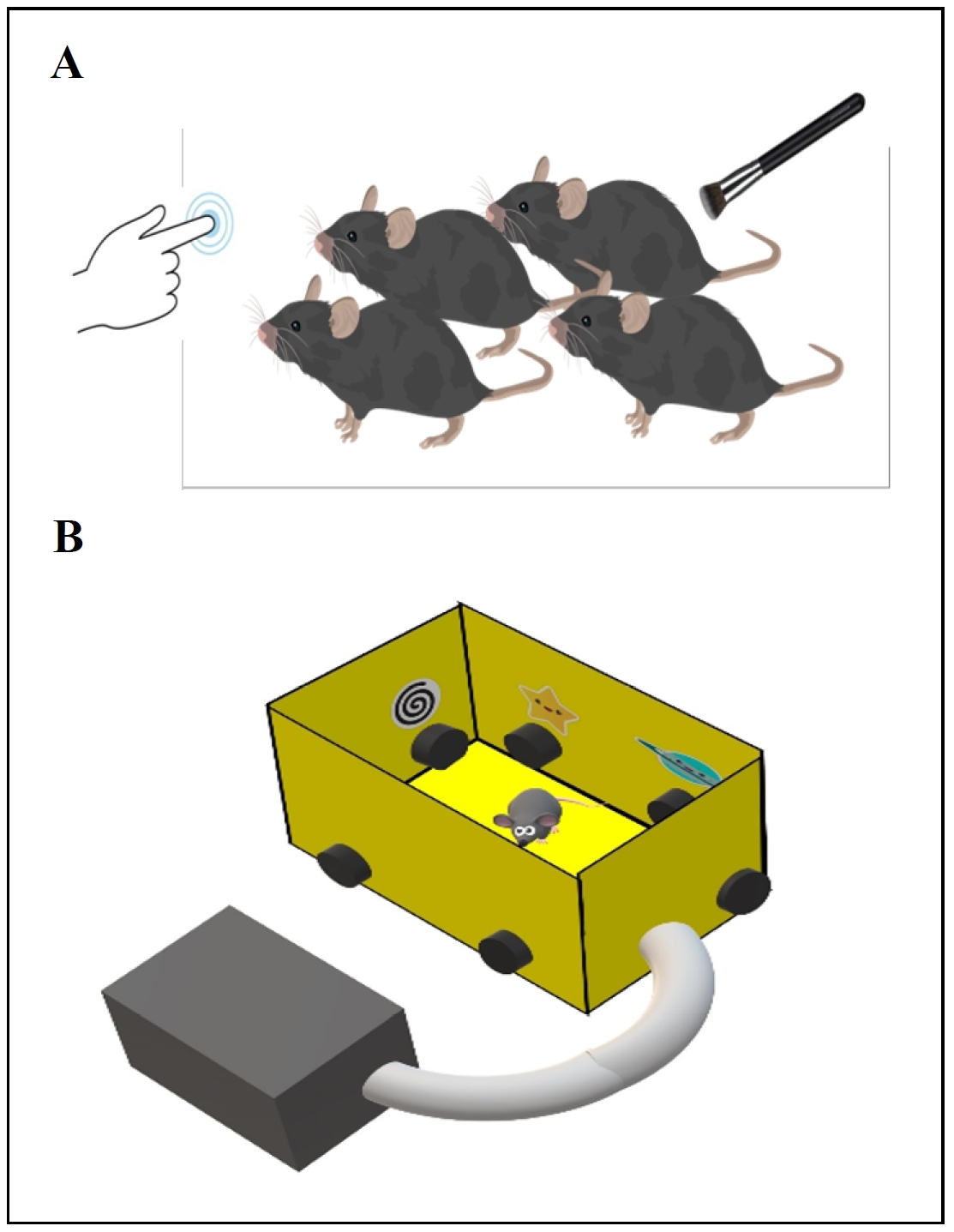
Figure 1. Protocol for identifying mice resilient to sleep disruption. (A) Four hours after lights come on, mice are placed in a new cage and maintained in an awake state by gentle cage tapping and brush stroking for four hours daily for up to four days. (B) Mice are then tested in a spatial navigation learning task (Box Maze) to identify those that rapidly find the escape hole (resilient and classified as fast learners) versus those that are slow in finding the escape hole (sensitive and classified as slow learners).
Are aging mice resilient to sleep disruption also resistant to AD?
In a preliminary experiment, we identified 23-month-old male and female C57BL/6J mice as fast learners (resilient) or slow learners (sensitive) after short-term sleep disruption. Since AD is an age-related disease, it was imperative to use an aging mouse model of AD instead of a transgenic model that expresses neuropathology at a young age [20]. We therefore elected to use an AAV PHP.eB vector [21] containing sequences to Aβ1-42 and P301L tau [22] to induce AD in our resilient and sensitive aging cohorts. Three months later, when the mice were 26 months of age, brains were examined for neuropathology using immunohistochemistry and Qu-Path digital imaging as previously described [23]. As shown in Figure 2, sleep-disrupted resilient mice had significantly less evidence of the presence of Aβ1-42/P301L tau, less inflammation, and better synaptic integrity compared to sleep-disrupted sensitive mice. While these observations are very preliminary, they do provide increased interest in the concept of resilience to sleep disruption as a way to investigate resistance to agerelated neurodegenerative diseases such as AD. Given that sleep disruption is a relevant and rising health concern, the novel mouse model we describe could be useful in helping gain a better understanding of resilience to aging and factors associated with resistance to AD.
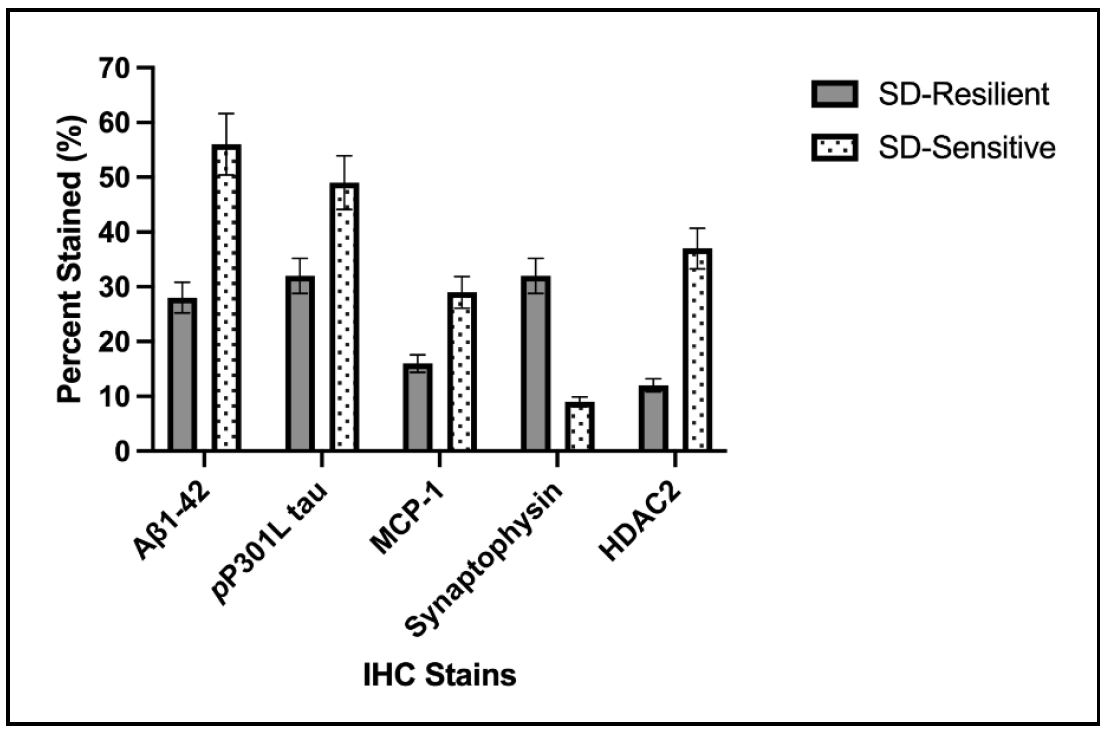
Figure 2. Sleep disrupted resilient C57BL/6 mice (SD-Resilient), 26 months of age, developed less neuropathology than sleep-disrupted sensitive mice following intravenous injection of AAV Aβ1-42/P301L tau at 23 months of age. Data are presented as percent superpixel staining using immunohistochemistry and Qu-Path digital imaging. P ≤ 0.05 for all stains. N = 8-10/cohort. Similar results were seen in males and females. Antibodies were obtained from Abcam, Inc.
Declarations
Financial support and sponsorship
Supported by National Institutes of Health grants R01 AG057381 and R01 AG067193 (Ladiges PI).
Conflicts of interest
Warren Ladiges is a member of the editorial board of Aging Pathobiology and Therapeutics. The authors declare that they have no conflicts and were not involved in the journal’s review or decision regarding this manuscript.
References
1.Kirkland JL, Stout MB, & Sierra F. Resilience in aging mice. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2016, 71(11): 1407-1414. [Crossref]
2. Schorr A, Carter C, & Ladiges W. The potential use of physical resilience to predict healthy aging. Pathobiol Aging Age Relat Dis, 2018, 8(1): 1403844. [Crossref]
3. Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR, et al. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the Nun and Honolulu-Asia aging studies. J Neuropathol Exp Neurol, 2017, 76(6): 458-466. [Crossref]
4. Arenaza-Urquijo EM, & Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology, 2018, 90(15): 695-703. [Crossref]
5. Kang DW, Lee CU, & Lim HK. Role of sleep disturbance in the trajectory of Alzheimer’s disease. Clin Psychopharmacol Neurosci, 2017, 15(2): 89-99. [Crossref]
6. Spira AP, & Gottesman RF. Sleep disturbance: an emerging opportunity for Alzheimer’s disease prevention? International psychogeriatrics, 2017, 29(4): 529-531. [Crossref]
7. Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev, 2018, 40: 4-16. [Crossref]
8. Malhotra RK. Neurodegenerative disorders and sleep. Sleep medicine clinics, 2018, 13(1): 63-70. [Crossref]
9. Zdanys KF, & Steffens DC. Sleep disturbances in the elderly. Psychiatric Clinics, 2015, 38(4): 723-741. [Crossref]
10. Frau R, Traccis F, & Bortolato M. Neurobehavioral complications of sleep deprivation: shedding light on the emerging role of neuroactive steroids. Journal of neuroendocrinology, 2020, 32(1): e12792. [Crossref]
11. Van Dongen HP, Maislin G, & Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med, 2004, 75(3 Suppl): A147-154.
12. Goel N, & Dinges DF. Behavioral and genetic markers of sleepiness. J Clin Sleep Med, 2011, 7(5 Suppl): S19-21. [Crossref]
13. Goel N, & Dinges DF. Predicting risk in space: genetic markers for differential vulnerability to sleep restriction. Acta Astronaut, 2012, 77: 207-213. [Crossref]
14. Dennis LE, Wohl RJ, Selame LA, & Goel N. Healthy adults display long-term trait-like neurobehavioral resilience and vulnerability to sleep loss. Sci Rep, 2017, 7(1): 14889. [Crossref]
15. Chuah YM, Venkatraman V, Dinges DF, & Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci ,2006, 26(27): 7156-7162. [Crossref]
16. Patanaik A, Kwoh CK, Chua EC, Gooley JJ, & Chee MW. Classifying vulnerability to sleep deprivation using baseline measures of psychomotor vigilance. Sleep, 2015, 38(5): 723-734. [Crossref]
17. Lee A, Lei H, Zhu L, Jiang Z, & Ladiges W. Resilience to acute sleep deprivation is associated with attenuation of hippocampal mediated learning impairment. Aging Pathobiol Ther, 2020, 2(4): 195-202. [Crossref]
18. Mukherjee KK, Lee AY, Zhu L, Darvas M, & Ladiges W. Sleep-deprived cognitive impairment in aging mice is alleviated by rapamycin. Aging Pathobiol Ther, 2019, 1(1): 5-9. [Crossref]
19. Darvas M, Mukherjee K, Lee A, & Ladiges W. A novel oneday learning procedure for mice. Curr Protoc Mouse Biol, 2020, 10(1): e68. [Crossref]
20. Qiu H, Zhong R, Liu H, Zhang F, Li S, & Le W. Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer’s disease-like pathologies in AβPP(swe)/ PS1(ΔE9) mice. J Alzheimers Dis, 2016, 50(3): 669-685. [Crossref]
21. Kawabata H, Konno A, Matsuzaki Y, & Hirai H. A bloodbrain barrier-penetrating AAV2 mutant created by a brain microvasculature endothelial cell-targeted AAV2 variant. Mol Ther Methods Clin Dev, 2023, 29: 81-92.