Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
A review of variable risk factors affecting the development of mild cognitive impairment in older adults
* Corresponding author: Hong Fan
Mailing address: Department of Geriatrics, Shaoxing People's Hospital (Shaoxing Hospital of Zhejiang University), Shaoxing 312000, Zhejiang, China.
Email: fhrainbow@163.com
Received: 30 August 2023 / Revised: 12 September 2023 / Accepted: 07 October 2023 / Published: 26 December 2023
DOI: 10.31491/APT.2023.12.123
Abstract
Mild cognitive impairment (MCI) is considered to be a transitional state in which cognitive function gradually deteriorates from normal to dementia, which is mainly characterized by reduced functioning in several cognitive domains, such as executive function, memory, language, processing speed, and attention. Currently, the clinical treatment of dementia is unsatisfactory, so we shifted our research focus to MCI to find variable risk factors, potential mechanisms, and effective preventive measures for the occurrence of MCI in older adults, to reduce the incidence of dementia and alleviate the health and economic burden on the family and society. Currently, we found that the variable risk factors affecting the occurrence and development of MCI in older adults are cardiovascular and respiratory diseases, endocrine-metabolic diseases, social life factors, and psychological factors. We mainly review the effects of these risk factors on cognitive function and the potential mechanisms of action, and propose interventions to improve cognitive function.
Keywords
Mild cognitive impairment, risk factors, type 2 diabetes, hypertension, intervention
Introduction
Mild cognitive impairment (MCI) is an excess stage between normal cognition and dementia and a precursor to Alzheimer's disease (AD). It is characterized primarily by impaired subjective memory and moderate deficits in at least one of the cognitive domains, such as executive function, memory, language, processing speed, or attention [1]. Neurobiological features are reflected in hypoperfusion and hypometabolism in the temporoparietal cortex, atrophy of the medial temporal lobe, elevated tau and phosphorylated tau, and decreased Aβ42 in the cerebrospinal fluid, and cerebral Aβ42 deposition [2]. MCI is currently divided into amnestic MCI (aMCI) and non-amnestic MCI (naMCI) [3]. aMCI is the most common subtype of MCI, manifesting as situational memory impairment, and is more likely to progress to typical AD, whereas naMCI affects impairment of cognitive domains other than memory and is more likely to progress to atypical AD (including vascular dementia or other types of dementia) [3]. The transition from normal cognition to MCI to AD is a continuous process in the development of cognitive impairment, and thus patients with MCI are considered to be at high risk of developing AD [4]. In China, 15.2%-15.9% of the elderly population over the age of 60 suffer from MCI, and the incidence of the disease is around 6.36% per year [5], with aMCI accounting for more than 80% of all MCI patients [3]. Meanwhile, the average annual conversion rate from MCI to AD is as high as 18.4% [6]. In terms of gender, the prevalence of MCI is higher in women than in men [7], and the gender difference is attributed to differences in socioeconomic status (e.g., education and occupation) and health status between men and women [3]. In addition, regarding social development, the prevalence of MCI is significantly lower in most developed countries than in developing countries [8]. Cognitive impairment causes a serious economic burden to individuals, families, and society, but the current treatment for AD patients is still unsatisfactory, and MCI as a precursor of AD has become a new direction of current research. Multiple risk factors can affect the development and transformation of MCI; therefore, this review mainly lists the risk factors (Figure 1), pathogenesis, and interventions of MCI to reduce the incidence of MCI and block its transformation to AD.
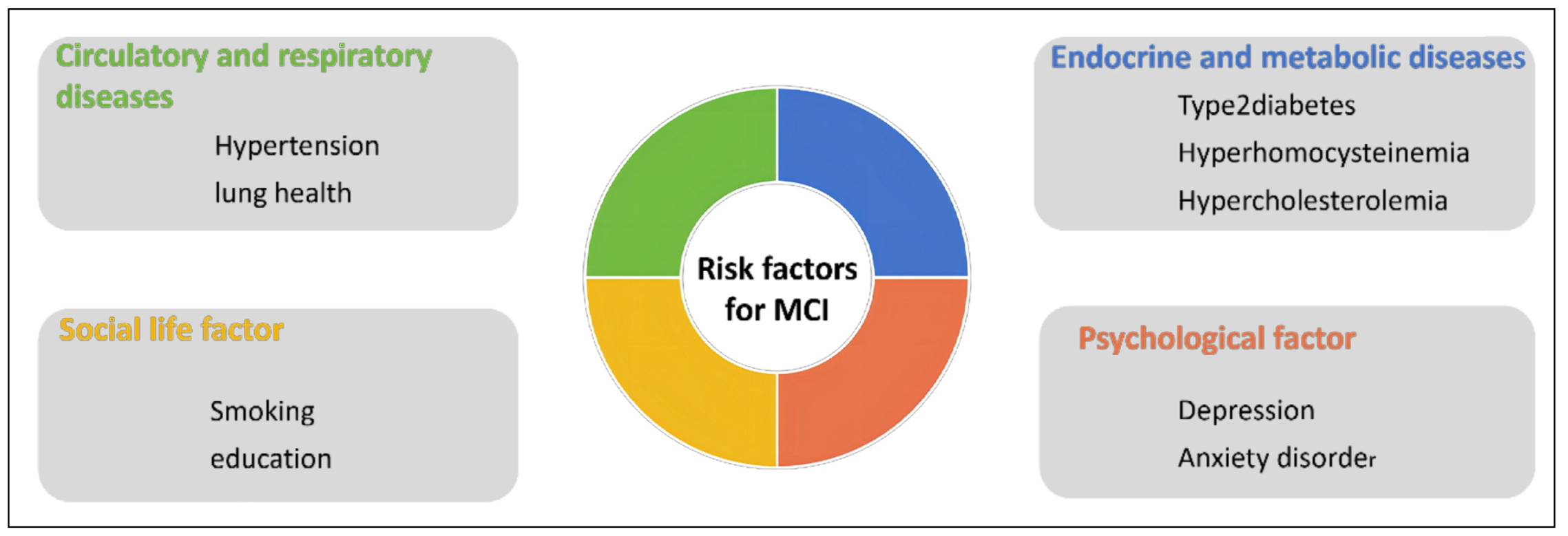
Figure 1. Risk factors affecting MCI.
Risk factors, mechanisms, and interventions
Type 2 diabetes
Diabetes mellitus is a group of metabolic disorders characterized by chronic hyperglycemia caused by multiple
etiologies, which can lead to chronic progressive lesions in multiple organs and systems. An estimated 537 million people will already have diabetes in 2021, and this
number is expected to reach 643 million by 2030 and 783 million by 2045 [9]. This means that in the next 20 years,
an average of 1 in 10 people worldwide will have diabetes, and more than 90% of them will have type 2 diabetes.
It is now well established that type 2 diabetes is one of the modifiable risk factors for the development of MCI
[5, 10, 11]. In a follow-up study of
diabetic patients for more than 10 years [10], the mean MCI prevalence in the
diabetic group was found to be 30.66 ± 3.09%, which was significantly higher than that in the non-diabetic group,
which was 22.32 ± 2.75%. In a meta-analysis, the prevalence of MCI in patients with type 2 diabetes mellitus was
even 45% worldwide [12]. There are two conclusions regarding whether mild cognitive functioning progresses to
dementia in patients with type 2 diabetes: a few researchers have suggested that although diabetes raises the prevalence of MCI, it seems to have no effect on its progression
[10, 13]; however, the majority of scholars have suggested
that the prolonged presence of diabetes may increase the
risk of conversion of MCI to dementia [14-16]. There is
a relatively significant difference between the two findings, which may be related to the sample size, age of the
participants, and duration of follow-up, and needs to be demonstrated by further study follow-up. The incidence
of MCI may be higher when type 2 diabetes is combined with other conditions than type 2 diabetes alone. In addition, hyperlipidemia, hypertension, diabetic nephropathy,
and macrovascular and microvascular lesions are thought to significantly increase the risk of MCI in patients with
diabetes [11, 17].
There may be multiple causes of MCI due to type 2 diabetes: cerebrovascular disease is the primary cause, and
chronic hyperglycemia may lead to thickening of the basement membrane of the cerebrovascular muscle, which
reduces cerebral blood circulation, leading to inadequate cerebral perfusion and white matter disease, and ultimately to MCI or
dementia [11, 18]. Second, galactose lectin 3
(Gal3) and inflammatory responses play an important role in the progression of cognitive impairment in diabetes.
Elevated circulating levels of Gal3 and some inflammatory markers, such as serum soluble vascular adhesion
molecule (svcAM-1) and high-sensitivity C-reactive protein (Hs-CRP), may be associated with the development
of MCI in type 2 diabetes patients [19, 20]. Third, type 2
diabetic patients with MCI exhibit significantly reduced amplitude of diffuse low-frequency fluctuations (ALFF) in
various brain regions significantly associated with cognitive performance, such as the bilateral insula, the left middle frontal gyrus, the left precuneus, and
significantly elevated ALFF in the temporal gyrus and the fusiform gyrus [21]. Fourth, dipeptidyl peptidase IV (DPP-IV) has been
shown to affect cognitive function through both enzymatic and non-enzymatic pathways. DPP4 may contribute to
cognitive dysfunction in diabetic patients by combining with PAR2 in the hippocampus, activating GSK-3β, and downregulating peroxisome proliferator-activated receptor
gamma coactivator 1α expression, leading to mitochondrial dysfunction [22]. In addition, advanced glycosylation
end products (AGEs), vascular endothelial dysfunction, abnormal insulin regulation, and neuroinflammation may also be engaged in the pathogenesis of MCI in diabetic
patients [17].
Some glucose-lowering drugs have been shown to improve cognitive function in patients. Recent studies have found that glucagon-like peptide-1 receptor agonists
(GLP-1 RAs) and DPP-IV inhibitors are effective in ameliorating cognitive impairment in diabetic patients [23].
GLP-1 acts as a physiological modulator of the central
nervous system and enhances learning and memory function by restoring insulin signaling [24]. DPP-IV inhibitors
ameliorate diabetes-mediated cerebrovascular dysfunction by lowering plasma endothelin-1 (ET-1) levels and
decreasing cerebrovascular hyperreactivity [25]. DPPIV inhibitors may also improve cognitive performance by
inhibiting Aβ aggregation [26]. Some other drugs, such as epalrestat, donepezil, and empagliflozin, prevent cognitive
impairment through antioxidant and anti-inflammatory effects [27, 28]. The
traditional Chinese exercise Tai Chi is an increasingly popular multimodal mind-body exercise that combines physical, cognitive, social, and meditative
activities to promote brain health while controlling blood glucose [29].
Hypertension
According to authoritative epidemiological surveys, the
number of hypertensive patients aged 30–79 years worldwide increased from 648 million between 1990 and 2019
to 1,278 million in 2019, but the global rate of hypertension control by 2019 was only 23% in women and 18% in
men [30]. Hypertension impairs cerebral blood supply by
impairing the structure and function of the cerebral microcirculation, promoting microvascular thinning, cerebral
microvascular endothelial dysfunction, and neurovascular uncoupling. At the same time, hypertension disrupts the blood-brain barrier, contributing to neuroinflammation
and exacerbating amyloidosis [31, 32]. Hypertensioninduced endothelial dysfunction
is associated with cognitive decline [33]. Vascular endothelial cells are subjected
to a combination of intravascular shear stress, turbulence, increased free radicals, and decreased NO signaling, ultimately leading to the development of atherosclerosis.
Intracranial and extracranial atherosclerosis promotes cognitive decline [32]. Proteomic studies have identified
reduced synaptic regulation and plasticity as well as abnormal myelination as potential signaling mechanisms
leading to cognitive decline in patients with hypertension combined with atherosclerosis [34]. Cerebrovascular
self-regulation ensures adequate cerebral perfusion during fluctuations in blood pressure by maintaining relatively constant cerebral blood flow. The mechanisms of
hypertension-induced alterations in cerebrovascular selfregulation involve structural changes in the cerebral vasculature (sclerosis and remodeling) and alterations in the
responsiveness of vascular smooth muscle cells to increases in transmural pressure [35]. Activation of AngII type
1 receptors (AT1R) in brain endothelial cells by chronic
hypertension via AngII is necessary for blood-brain barrier disruption [36]. A recent study showed that damage
to endothelial Kir2.1 channels, involving endothelial hyperpolarization and vasodilation under neural activity,
mediates neurovascular coupling defects in BPH mice
[37, 38]. In animal models, hypertension has been found
to mediate Aβ deposition, disruption of the blood-brain barrier, and cognitive function through activation of AGE
receptors and modulation of β- and γ-secretase activity. In addition, neuroradiological markers such as white matter
hyperintensities, lacunar infarcts, micro hemorrhages, and enlarged perivascular gaps are associated with the development of cognitive
impairment [31, 39].
Thus, hypertension has been identified as a variable risk factor for cognitive dysfunction, including
MCI [40, 41].
A multicenter study shows that the prevalence of cognitive impairment is higher in hypertensive patients than in the general population, with executive function and
semantic memory being the cognitive domains most affected [42]. In a meta-analysis of 47,179 participants,
Qin et al. showed that the overall prevalence of MCI in hypertensive patients was in the range of 30% and was
significantly higher than in non-hypertensive older adults [43]. Wang et al. further found in a 7-year retrospective
cohort study that different grades and durations of hypertension had different outcomes on the occurrence of MCI:
subjects with hypertension grade 1 or duration [40]. This result may be due to a compensatory effect of early-stage
hypertension on vascular lesions and inadequate cerebral perfusion. Also, hypertension is a risk factor for the conversion of cognitive function to AD in patients with MCI
[44].
Hypertension treatment reduces cognitive decline to some
extent [45-47]. A recent randomized clinical trial showed
that intensive blood pressure control (systolic blood pressure target < 120 mmHg) versus standard blood pressure
control (systolic blood pressure target < 140 mmHg) in patients found that control of blood pressure reduced the
risk of MCI, with less increase in cerebral white matter
lesion volume in the intensive treatment group [48, 49].
Although decreased renal function, as measured by eGFR, may be a factor in the increased risk of MCI, it is not
related to the presence or absence of intensive treatment of hypertension, i.e., intensive treatment of hypertension
does not lead to a decrease in eGFR [50]. Antihypertensive drugs, especially calcium channel blockers and reninangiotensin
system blockers (ACEIs and ARBs), may help to prevent cognitive decline by lowering blood pressure
and through neuroprotective mechanisms [46].
Hypercholesterolemia
Elevated plasma cholesterol is associated with a variety of
health conditions, and it may be related to the pathogenesis of MCI. A large study based on a Chinese population (n
= 46,011) demonstrated that hyperlipidemia is a risk factor for MCI [5]. The accumulation of cholesterol in neurons
contributes to amyloid deposition in the brain by accelerating the cleavage of amyloid precursor proteins into amyloid-like components, whereas keeping cholesterol low
in neurons may inhibit Aβ accumulation. Another metaanalysis reported that elevated cholesterol levels in midlife
may increase the risk of cognitive impairment in later life,
while elevated cholesterol levels in later life were not associated with dementia or cognitive impairment [51]. The
possible reasons for this phenomenon may be: cholesterol is essential for synaptic maturation and maintenance of
synaptic plasticity, and as the body ages, the cholesterol level in the brain gradually decreases, causing a decrease
in the role of cholesterol in the maintenance of synaptic plasticity, leading to a decline in cognitive function, while
those patients with normal cognition have cholesterol levels that are still sufficient to maintain synaptic plasticity;
Meanwhile, cholesterol is also an important component of the cell membrane, playing a role as an ion-permeable
regulator and a regulator of signal transduction, which may also impair this function to some extent [52]. At the
same time, cholesterol is an important component of cell membranes, acting as an ion-permeable regulator and a
regulator of signal transduction, which may be impaired to some extent by lower cholesterol levels.
Although statins are the most commonly used cholesterollowering drugs, it is not established whether they can be
used to prevent cognitive decline. It has been suggested that individuals taking statins, especially purist ApoE4 carriers, have a slower progression of cognitive decline
[53]. Simvastatin was found to be beneficial in maintaining white matter microstructure in cognitively normal
middle-aged adults, suggesting that simvastatin has the potential to prevent MCI. However, another prospective
study reported no difference in the rate of decline on cognitive tests between the pravastatin group and the control
group during 3 years of follow-up [54]. These conflicting
results result from the lack of harmonized biomarkers and adequate intervention time. Addressing these issues may
help us to further our understanding of the effects of lipidlowering drugs on cognitive function in older adults.
Hyperhomocysteinemia (HHcy)
Homocysteine (Hcy) is a non-essential sulfur-containing amino acid that is produced in vivo by the demethylation
of methionine and plays a central role in the methionine and folate cycles. HHcy is associated with a variety of
cognitive dysfunctions, including MCI [55, 56]. In a
follow-up study of 592 patients with acute stroke, patients with higher Hcy levels were found to be more likely to be
cognitively impaired 1 month after stroke than those with lower Hcy levels [57]. A dose-response meta-analysis of
29 prospective cohort studies found that for every 5 mol/L increase in blood Hcy levels, the relative risk of AD
increased by 15% [58]. It has been found that Hcy is capable of autoxidation in the presence of oxygen molecules
and promotes the formation of reactive oxygen species (e.g., hydrogen peroxide, hydroxyl, and thiol radicals),
which may have cytotoxic effects when the concentration of reactive oxygen species in the body is elevated
[59]. Endothelial inflammation under HHcy conditions
can impair vascular endothelial function, promote vascular injury, lead to cerebral small vessel disease, and alter the permeability of the blood-brain barrier, which in turn
leads to cognitive dysfunction [60]. Meanwhile, Hcy can have direct toxic effects on neurons by promoting DNA
damage and altering NMDA receptor expression, leading to dysregulation of calcium homeostasis, mitochondrial
function, neuronal autophagy, and apoptosis [61].
The study found that supplementation with B vitamins can significantly reduce Hcy levels, which may reduce agerelated cognitive decline and the risk of AD and overall
dementia [62]. Hcy produced by the human body is metabolized by three main pathways [63]:
(1) remethylation elimination, in which Hcy is remethylated to methionine with the aid of vitamins B2 and B12; (2) transsulfuration,
in which Hcy is first converted to cystathionine (with vitamin B6 acting as a cofactor), which is metabolized to
cysteine and α-keto-butyric acid, and ultimately excreted from the body; and (3) release into the extracellular fluid.
Abnormalities in any of these metabolic pathways elevate plasma Hcy concentrations. In contrast, Hcy metabolism
is heavily dependent on B vitamins, including folate, vitamin B12, and vitamin B6. Folic acid provides methyl for
Hcy methylation, while vitamin B12 functions as a coenzyme for methionine synthase in the process of Hcy methylation. Vitamin B6 activates the transsulfuration pathway,
transferring sulfur from homocysteine to cysteine, which can use the transferred sulfur to synthesize glutathione
with itself, and the reduction of glutathione enhances the antioxidant activity of the organism [64]. Clinically, older
adults are more likely to be deficient in B vitamins and show an increasing trend with age. Supplementation with folic acid, vitamin B6, and vitamin B12 not only reduces
plasma Hcy concentrations but also prevents cognitive
decline in patients with MCI [65]. In a 24-month randomized trial, Gong et al. found that in MCI patients with left
frontal lobe atrophy, B vitamins may be more effective in slowing cognitive decline [66]. And the combination of
folic acid and vitamin B12 demonstrated better efficacy than their use alone [67].
Smoking
Smoking is bad for brain health and increases the risk of cognitive impairment [53]. In elderly patients with MCI, functional performance declines more rapidly in smokers than in nonsmokers, which is associated with a more rapid decline in endonasal cortical mass over time [68]. Sleep plays an important mediating role in the relationship between smoking and MCI [69]. One of the sleep problems is sleep deprivation, which leads to short sleep duration, and fatigue, causing cognitive decline and reduced sleep duration [70, 71]. The study found an inverted U-shaped relationship between sleep duration and cognitive scores, and a positive linear relationship between sleep quality and cognitive scores, so too much or too little sleep and sleep quality may lead to cognitive decline [72]. Longterm smoking is associated with elevated brain oxidative stress, which plays an important role in reducing sleep duration [73], as well as decreasing melatonin secretion by the pineal gland, which can persistently impair cognitive function in animals and humans [74]. Smoking also alters the composition of the nicotinic acetylcholine receptor subunit, increases the expression of the glutamate receptor subunit GluR2, reduces neurogenesis, and alters Akt and ERK1/2 activity, causing mitochondrial dysfunction in the hippocampus and cortex [75]. In addition, smokinginduced inefficiencies in Aβ removal may also contribute to the development of MCI. Smoking affects the function of cortico-striatal circuits, with increased functional connectivity between dorsal striatal and parietal regions in cognitively normal smokers compared to non-smokers. Compared with cognitively normal smokers and MCI nonsmokers, MCI smokers showed reduced functional connectivity between the dorsal striatum and parieto-occipital regions and increased functional connectivity between the ventral striatum and frontal cortex, suggesting that smoking affects the functioning of cortico-striatal circuits in patients with MCI and that such an effect may disrupt the functioning of the cortico-striatal circuits by exacerbating Aβ pathology. Smoking also affects visual attention through cortico-striatal circuits, further contributing to memory loss in MCI patients [76]. The cortico-striatal circuit may be a potential therapeutic target for smoking [77]. It has also been suggested that chronic nicotine exposure may lead to disruption of functional connectivity between the Meynert's basal ganglia and the precuneus in patients with MCI, and it has been hypothesized that the precuneus may also be an important target for the cognitive effects of smoking in MCI [78]. Therefore, smoking cessation can reduce the damage to cognitive function caused by smoking through multiple pathways and improve cognitive status.
Lung health
There is growing evidence of an association between lung function decline, chronic obstructive pulmonary disease (COPD) and cognitive function [79, 80]. One of the most common causes of impaired lung function is COPD. COPD is a progressive disease, but treatable and preventable. The global prevalence of COPD among people aged 30–79 years is 10.3% (391 million people), with the majority of cases occurring in low- and middle-income countries and a slightly higher prevalence in highincome countries [81]. COPD is expected to be the third leading cause of death by 2030 [82]. A recent systematic review and meta-analysis found a strong correlation between COPD and increased incidence of MCI (OR = 2.11, 95%CI: 1.32-3.38) [83]. Other researchers have suggested that the prevalence of cognitive dysfunction in COPD patients is about 56.7%, four times higher than in non-COPD patients, including attention, learning, processing speed, visuospatial memory, language, and executive functioning [84]. COPD is characterized by partially irreversible chronic obstruction of pulmonary airflow, resulting in abnormally low blood oxygen levels, which may lead to cognitive dysfunction [85]. COPD-induced pulmonary dysfunction reduces cerebral oxygen supply, affects cerebral energy metabolism, and thus promotes cerebral ischemia-induced oxidative stress, leading to oxidative stress-mediated injury and accelerated vascular damage and degenerative disease. Long-term chronic hypoxia leads to impaired neurovascular coupling, apoptosis, transcription factor-mediated inflammation as well as Aβ accumulation and tau phosphorylation as causes of neurocognitive deficits. In addition, structural brain changes of hippocampal and cortical atrophy, ventricular enlargement, senile plaques, and neurogenic fiber tangle deposition can be observed under chronic hypoxia [86]. Olaparib [87] and dimethyl fumarate [88] have been found to inhibit the systemic inflammatory response to protect cognitive function in animal experimental models of acute lung injury, and thus inflammation may be able to be a target for the treatment of cognitive deficits induced by lung health problems. Long-term physical activity not only exercises respiratory muscle movement and improves ventilation, but also achieves systemic anti-inflammatory effects [89, 90].
Education
Educational attainment is an important factor influencing the occurrence of MCI, with healthy older adults with higher education having better cognitive performance than those with lower levels of education [91, 92]. Even very low levels of education (1–4 years) are associated with reduced odds of cognitive impairment compared with no formal education [92]. Cognitive reserve (CR) is the accumulation of neural resources, influenced by genetic and/or environmental factors, that mitigate cognitive function impairments caused by aging or disease [93]. Highly educated people are better able to withstand cognitive decline caused by pathological changes in the brain than those with low levels of education. That is, highly educated people have better CR. High levels of CR were found to be neuroprotective during EEG activity, possibly by enhancing rsEEGα source activation [94]. However, another study found that education was only associated with baseline levels of cognitive function. It is not associated with slow cognitive decline, late cognitive decline, and residual cognitive decline. This contradicts most CR theories [95]. The fact that education can prevent MCI can also be explained by the brain reserve (BR) theory. BR is a passive threshold model that emphasizes support for the structural integrity of cognitive performance and volumetric quantitative measures [7]. In other words, the brain responds to the pathology of cognitive impairment by enriching neurons and synapses [96]. Grey matter volume, cortical thickness, and surface area of the hippocampal and amygdala subregions of the brain increased after receiving higher education. Higher levels of education are associated with higher white matter integrity in several brain regions associated with AD [97]. Education also improves neural resources in childhood and young adulthood by increasing synaptic density, which then mitigates the effects of neurodegeneration caused by aging or age-related diseases [98]. This predicts that investing in education at an early stage of life may reduce the risk of cognitive impairment and dementia in later life, lowering the associated personal and societal costs and enabling them to withstand more cognitive decline [99]. In conclusion, in the majority of researchers' opinion, education not only reduces the incidence of MCI but also reduces the rate of conversion of MCI to dementia and protects against further deterioration of cognitive function in the early stages of cognitive impairment [92, 100, 101]. Thus, early education can benefit our cognitive function to some extent.
Depression
Depression is a common psychiatric symptom in people with MCI and dementia, with a prevalence of 32 percent
in MCI and 37 percent in dementia [102]. Studies have
shown that depression increases the risk of MCI in cognitively normal people [103, 104].
The Lancet Dementia Commission further reported that if late-life depression were eliminated, there would be a corresponding 4 percent reduction in the prevalence of
dementia [105]. After performing diffusion tensor imaging (DTI) in patients with early MCI (EMCI) without depression, patients with
mild depression in EMCI (EMCID), and patients with late MCI (LMCI) without depression, Feng et al. found that the mean controllability of the DMN was significantly
higher in the EMCI group and the LMCI group than in the EMCID group [106]. On the imaging side, patients
with depression and MCI have reduced volumes in areas such as the insula, superior temporal gyrus, inferior frontal gyrus, amygdala, hippocampus, and
thalamus [103]. Cognitive decline in old age was greater in people with depression than in those without depression, and memory
loss, executive function, and information processing speed were found to decline over time in these individuals
[107]. On cognitive tests, patients with MCI and depression scored lower than those with
MCI [108]. In addition, MCI participants with depression showed greater deficits in immediate and delayed memory than MCI participants
without depression [109].
Depression increases the risk of conversion of MCI to dementia, while causing severe impairment of functioning and quality of life and placing a significant burden on
caregivers [102]. The meta-analysis found that the group of MCI participants with depressive symptoms was prone
to progression to dementia compared to MCI participants without depressive symptoms (OR = 1.28) [110]. This
may be related to the fact that depressed patients have more amyloid abnormalities and an increased risk of neuropsychiatric symptoms due to Aβ load in
the brain [111, 112]. In addition, people with MCI are more likely to suffer from
depression [113].
Therapeutically, exercise interventions such as aerobic exercise [114], dance [115], and
Baduanjin [116] can improve the level of cognitive function in elderly patients with MCI. Adding cognitive behavioral therapy to
routine care may slightly reduce depressive symptoms in people with dementia and MCI and may increase remission rates
of depression [117]. There is little research on the psychological effects of game training in older adults with
MCI, which is based on cortical stimulation designed to improve patients' cognitive function and reduce the risk of
MCI and depression [118]. Xue et al. [119] found that 8
weeks of game training for people with MCI and depression significantly improved participants' cognitive and depression scores. However, the potential treatment with
antidepressants remains uncertain at this time [120, 121].
Anxiety
Current research has determined that anxiety can increase the incidence of MCI [122, 123]. Smith et al. [122] investigated 32,715 individuals aged ≥ 50 years from six countries and found a positive correlation between anxiety and MCI in all of them (OR 1.35-14.33), which suggests a high incidence of MCI in older people with anxiety disorders. The reason for this may be that anxiety may reduce intellectually stimulating activities, increase sleep problems, and increase the risk of cognitive decline with the use of benzodiazepines. Anna et al. [123] found that Aβ deposition and anxiety synergistically increased the risk of MCI (joint effect HR 6.77). The co-occurrence of MCI and anxiety increases the likelihood that MCI will turn into dementia, especially AD [124, 125]. A systematic review and meta-analysis determined that the group of MCI patients with anxiety were more likely to progress to dementia (OR=1.18) [126]. At the same time, people with MCI can also promote anxiety symptoms, and the two influence each other [127, 128]. One longitudinal study showed a significant bidirectional longitudinal association between anxiety and MCI, with a total effect of 37.1% for anxiety and 27.1% for MCI [129]. Healthy lifestyle habits such as physical activity [130], healthy diet [131], psychological interventions [132], as well as arts-based interventions (dance, theater, music, etc.) [133] can reduce anxiety and decrease the incidence of MCI.
Conclusions
The cardiovascular and respiratory systems, endocrine metabolism, social life factors, and psychological factors contribute to the development of MCI in the elderly. It is not difficult to find out that by targeting the mechanism of action of these factors, their effects on cognitive function can be mitigated and the progression of MCI to dementia can be reduced by appropriate pharmacological or non-pharmacological treatments. The interventions we reviewed are not too difficult to implement, implying a high degree of practical practicability, low requirements for medical conditions, and low investment in medical resources. However, there may be more variable risk factors than those mentioned above, which need to be further identified and effective interventions.
Declarations
Funding
This study was funded by the Public Welfare Technology Application Research Project of Zhejiang Province (LGF20H170005), the Traditional Chinese Medicine Scientific Research Fund Project of Zhejiang Province (2020ZB270), and the Health Science and Technology Program of Zhejiang Province (2022KY1285).
Conflicts of interest
The authors have declared that no conflicts interest exists.
Availability of date and material
No applicable.
Ethical approval and consent to participate
No applicable.
Consent for publication
Not applicable.
References
1. Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov, 2020, 19(9): 609-633. [Crossref]
2. Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectr, 2019, 24(1): 78-87. [Crossref]
3. Cong L, Ren Y, Wang Y, Hou T, Dong Y, Han X, et al. Mild cognitive impairment among rural-dwelling older adults in China: a community-based study. Alzheimers Dement, 2023, 19(1): 56-66. [Crossref]
4. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology, 2018, 90(3): 126-135. [Crossref]
5. Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health, 2020, 5(12): e661-e671. [Crossref]
6. Thaipisuttikul P, Jaikla K, Satthong S, & Wisajun P. Rate of conversion from mild cognitive impairment to dementia in a Thai hospital-based population: a retrospective cohort. Alzheimers Dement, 2022, 8(1): e12272. [Crossref]
7. Subramaniapillai S, Almey A, Natasha Rajah M, & Einstein G. Sex and gender differences in cognitive and brain reserve: implications for Alzheimer's disease in women. Front Neuroendocrinol, 2021, 60: 100879. [Crossref]
8. Deng Y, Zhao S, Cheng G, Yang J, Li B, Xu K, et al. The prevalence of mild cognitive impairment among Chinese people: a meta-analysis. Neuroepidemiology, 2021, 55(2): 79-91. [Crossref]
9. Magliano DJ, Boyko EJ, & committee IDFDAtes: IDF diabetes atlas. In., edn. Brussels: International Diabetes Federation; 2021.
10. Wang G, & Li W. Diabetes as a risk factor for abnormal cognition development. J Alzheimers Dis Rep, 2020, 4(1): 237-242. [Crossref]
11. Sun L, Diao X, Gang X, Lv Y, Zhao X, Yang S, et al. Risk factors for cognitive impairment in patients with type 2 diabetes. J Diabetes Res, 2020, 2020: 4591938. [Crossref]
12. You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol, 2021, 58(6): 671-685. [Crossref]
13. McCrimmon RJ, Ryan CM, & Frier BM. Diabetes and cognitive dysfunction. Lancet, 2012, 379(9833): 2291-2299. [Crossref]
14. Albai O, Frandes M, Timar R, Roman D, & Timar B. Risk factors for developing dementia in type 2 diabetes mellitus patients with mild cognitive impairment. Neuropsychiatr Dis Treat, 2019, 15: 167-175. [Crossref]
15. Pal K, Mukadam N, Petersen I, & Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol, 2018, 53(11): 1149-1160. [Crossref]
16. Dove A, Shang Y, Xu W, Grande G, Laukka EJ, Fratiglioni L, et al. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement, 2021, 17(11): 1769-1778. [Crossref]
17. Ehtewish H, Arredouani A, & El-Agnaf O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int J Mol Sci, 2022, 23(11): 6144. [Crossref]
18. Andrews RM, Shpitser I, Lopez O, Longstreth WT, Chaves PHM, Kuller L, et al. Examining the causal mediating role of brain pathology on the relationship between diabetes and cognitive impairment: the cardiovascular health study. J R Stat Soc Ser A Stat Soc, 2020, 183(4): 1705- 1726. [Crossref]
19. Ma S, Li S, Lv R, Hou X, Nie S, & Yin Q. Prevalence of mild cognitive impairment in type 2 diabetes mellitus is associated with serum galectin-3 level. J Diabetes Investig, 2020, 11(5): 1295-1302. [Crossref]
20. Hosny SS, Bahaaeldin AM, Khater MS, Bekhet MM, Hebah HA, & Hasanin GA. Role of inflammatory markers in elderly type 2 diabetic patients with mild cognitive impairment. Curr Diabetes Rev, 2019, 15(3): 247-253. [Crossref]
21. Zhou X, Zhang J, Chen Y, Ma T, Wang Y, Wang J, et al. Aggravated cognitive and brain functional impairment in mild cognitive impairment patients with type 2 diabetes: a resting-state functional MRI study. J Alzheimers Dis, 2014, 41(3): 925-935. [Crossref]
22. Sun C, Xiao Y, Li J, Ge B, Chen X, Liu H, et al. Nonenzymatic function of DPP4 in diabetes-associated mitochondrial dysfunction and cognitive impairment. Alzheimers Dement, 2022, 18(5): 966-987. [Crossref]
23. Chai S, Liu F, Yu S, Yang Z, & Sun F. Cognitive protection of incretin-based therapies in patients with type 2 diabetes mellitus: a systematic review and meta-analysis based on clinical studies. J Diabetes Investig, 2023, 14(7): 864- 873. [Crossref]
24. Mousa SA, & Ayoub BM. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen Res, 2019, 14(5): 745-748. [Crossref]
25. Hardigan T, Abdul Y, & Ergul A. Linagliptin reduces effects of ET-1 and TLR2-mediated cerebrovascular hyperreactivity in diabetes. Life Sci, 2016, 159: 90-96. [Crossref]
26. Xue J, Wang C, Pan C, Xing H, Xu L, Chen X, et al. Effect of DPP-4 inhibitor on elderly patients with T2DM combined with MCI. Exp Ther Med, 2020, 19(2): 1356-1362. [Crossref]
27. Jaiswal S, Mishra S, Torgal SS, & Shengule S. Neuroprotective effect of epalrestat mediated through oxidative stress markers, cytokines and TAU protein levels in diabetic rats. Life Sci, 2018, 207: 364-371. [Crossref]
28. Khan T, Khan S, Akhtar M, Ali J, & Najmi AK. Empagliflozin nanoparticles attenuates type2 diabetes induced cognitive impairment via oxidative stress and inflammatory pathway in high fructose diet induced hyperglycemic mice. Neurochem Int, 2021, 150: 105158. [Crossref]
29. Chen Y, Qin J, Tao L, Liu Z, Huang J, Liu W, et al. Effects of Tai Chi Chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in China: a randomized clinical trial. JAMA Netw Open, 2023, 6(4): e237004. [Crossref]
30. Levine DA, Gross AL, Briceño EM, Tilton N, Kabeto MU, Hingtgen SM, et al. Association between blood pressureand later-life cognition among black and white individuals. JAMA Neurol, 2020, 77(7): 810-819. [Crossref]
31. Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol, 2021, 17(10): 639-654. [Crossref]
32. Santisteban MM, Iadecola C, & Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension, 2023, 80(1): 22-34. [Crossref]
33. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev, 2021, 73(3): 924-967. [Crossref]
34. Wingo AP, Fan W, Duong DM, Gerasimov ES, Dammer EB, Liu Y, et al. Shared proteomic effects of cerebral atherosclerosis and Alzheimer's disease on the human brain. Nat Neurosci, 2020, 23(6): 696-700. [Crossref]
35. Claassen J, Thijssen DHJ, Panerai RB, & Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev, 2021, 101(4): 1487-1559. [Crossref]
36. Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension, 2020, 76(3): 795-807. [Crossref]
37. Schaeffer S, & Iadecola C. Revisiting the neurovascular unit. Nat Neurosci, 2021, 24(9): 1198-1209. [Crossref]
38. Koide M, Harraz OF, Dabertrand F, Longden TA, Ferris HR, Wellman GC, et al. Differential restoration of functional hyperemia by antihypertensive drug classes in hypertension-related cerebral small vessel disease. J Clin Invest, 2021, 131(18): e149029. [Crossref]
39. Liu Y, Dong YH, Lyu PY, Chen WH, & Li R. Hypertensioninduced cerebral small vessel disease leading to cognitive impairment. Chin Med J, 2018, 131(5): 615-619. [Crossref]
40. Wang F, Li D, Wang L, Zhu J, Zhao M, & Lei P. Mild hypertension protects the elderly from cognitive impairment: a 7-year retrospective cohort study. Psychogeriatrics, 2020, 20(4): 412-418. [Crossref]
41. Iadecola C, & Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res, 2019, 124(7): 1025-1044. [Crossref]
42. Vicario A, Cerezo GH, Del Sueldo M, Zilberman J, Pawluk SM, Lódolo N, et al. Neurocognitive disorder in hypertensive patients. Heart-brain study. Hipertens Riesgo Vasc, 2018, 35(4): 169-176. [Crossref]
43. Qin J, He Z, Wu L, Wang W, Lin Q, Lin Y, et al. Prevalence of mild cognitive impairment in patients with hypertension: a systematic review and meta-analysis. Hypertens Res, 2021, 44(10): 1251-1260. [Crossref]
44. Li JQ, Tan L, Wang HF, Tan MS, Tan L, Xu W, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer's disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry, 2016, 87(5): 476-484.[Crossref]
45. Wu L, He Y, Jiang B, Liu M, Wang J, Yang S, et al. The association between the prevalence, treatment and control of hypertension and the risk of mild cognitive impairment in an elderly urban population in China. Hypertens Res, 2016, 39(5): 367-375. [Crossref]
46. Rouch L, Cestac P, Hanon O, Cool C, Helmer C, Bouhanick B, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and metaanalyses, with discussion of potential mechanisms. CNS Drugs, 2015, 29(2): 113-130. [Crossref]
47. Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. Jama, 2020, 323(19): 1934-1944. [Crossref]
48. Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. Jama, 2019, 321(6): 553-561. [Crossref]
49. Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. Jama, 2019, 322(6): 524-534. [Crossref]
50. Kurella Tamura M, Gaussoin SA, Pajewski NM, Chelune GJ, Freedman BI, Gure TR, et al. Kidney disease, intensive hypertension treatment, and risk for dementia and mild cognitive impairment: the systolic blood pressure intervention trial. J Am Soc Nephrol, 2020, 31(9): 2122-2132. [Crossref]
51. Anstey KJ, Ashby-Mitchell K, & Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis, 2017, 56(1): 215-228. [Crossref]
52. Koudinov AR, & Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. Faseb j, 2001, 15(10): 1858-1860. [Crossref]
53. Rundek T, Tolea M, Ariko T, Fagerli EA, & Camargo CJ. Vascular cognitive impairment (VCI). Neurotherapeutics, 2022, 19(1): 68-88. [Crossref]
54. Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol, 2010, 257(1): 85-90. [Crossref]
55. Gasecka A, Siwik D, Gajewska M, Jaguszewski MJ, Mazurek T, Filipiak KJ, et al. Early biomarkers of neurodegenerative and neurovascular disorders in diabetes. J Clin Med, 2020, 9(9): 2807. [Crossref]
56. Nalder L, Zheng B, Chiandet G, Middleton LT, & de Jager CA. Vitamin B12 and folate status in cognitively healthy older adults and associations with cognitive performance. J Nutr Health Aging, 2021, 25(3): 287-294. [Crossref]
57. Zhou S, Chen J, Cheng L, Fan K, Xu M, Ren W, et al. Agedependent association between elevated homocysteine and cognitive impairment in a post-stroke population: a prospective study. Front Nutr, 2021, 8: 691837. [Crossref]
58. Zhou F, & Chen S. Hyperhomocysteinemia and risk of incident cognitive outcomes: an updated dose-response meta-analysis of prospective cohort studies. Ageing ResRev, 2019, 51: 55-66. [Crossref]
59. Olaso-Gonzalez G, Inzitari M, Bellelli G, Morandi A, Barcons N, & Viña J. Impact of supplementation with vitamins B(6) , B(12) , and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: a systematic review. IUBMB Life, 2022, 74(1): 74-84. [Crossref]
60. Periñán MT, Macías-García D, Jesús S, Martín-Rodríguez JF, Muñoz-Delgado L, Jimenez-Jaraba MV, et al. Homocysteine levels, genetic background, and cognitive impairment in Parkinson's disease. J Neurol, 2023, 270(1): 477- 485. [Crossref]
61. Ji Y, Lyu P, Jin W, Li X, Li X, & Dong Y. Homocysteine: a modifiable culprit of cognitive impairment for us to conquer? J Neurol Sci, 2019, 404: 128-136. [Crossref]
62. Kim KY, Shin KY, & Chang KA. Potential biomarkers for post-stroke cognitive Impairment: a systematic review and meta-analysis. Int J Mol Sci, 2022, 23(2): 602. [Crossref]
63. Fan X, Zhang L, Li H, Chen G, Qi G, Ma X, et al. Role of homocysteine in the development and progression of Parkinson's disease. Ann Clin Transl Neurol, 2020, 7(11): 2332-2338. [Crossref]
64. Sbodio JI, Snyder SH, & Paul BD. Regulators of the transsulfuration pathway. Br J Pharmacol, 2019, 176(4): 583- 593. [Crossref]
65. McGrattan A, van Aller C, Narytnyk A, Reidpath D, Keage H, Mohan D, et al. Nutritional interventions for the prevention of cognitive impairment and dementia in developing economies in East-Asia: a systematic review and meta-analysis. Crit Rev Food Sci Nutr, 2022, 62(7): 1838- 1855. [Crossref]
66. Gong X, Shi L, Wu Y, Luo Y, & Kwok T. B Vitamin supplementation slows cognitive decline in mild cognitive impairment patients with frontal lobe atrophy. J Alzheimers Dis, 2022, 89(4): 1453-1461. [Crossref]
67. Ma F, Zhou X, Li Q, Zhao J, Song A, An P, et al. Effects of folic acid and vitamin B12, alone and in combination on cognitive function and inflammatory factors in the elderly with mild cognitive impairment: a single-blind experimental design. Curr Alzheimer Res, 2019, 16(7): 622-632. [Crossref]
68. Chen M, Hu C, Dong H, Yan H, & Wu P. A history of cigarette smoking is associated with faster functional decline and reduction of entorhinal cortex volume in mild cognitive impairment. Aging, 2021, 13(4): 6205-6213. [Crossref]
69. Hu M, Yin H, Shu X, Jia Y, Leng M, & Chen L. Multi-angles of smoking and mild cognitive impairment: is the association mediated by sleep duration? Neurol Sci, 2019, 40(5): 1019-1027. [Crossref]
70. Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, & Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep, 2011, 34(5): 565-573. [Crossref]
71. Ji K, Chen DY, Karunakaran KD, & Biswal BB. Altered brain hemodynamic response and cognitive function after sleep deprivation: a functional near-infrared spectroscopy study. Brain-Apparatus Communication, 2023, 2(1): 2169589. [Crossref]
72. Li M, Wang N, & Dupre ME. Association between the selfreported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord, 2022, 304: 20-27. [Crossref]
73. Durazzo TC, Korecka M, Trojanowski JQ, Weiner MW, R OH, Ashford JW, et al. Active cigarette smoking in cognitively-normal elders and probable Alzheimer's disease is associated with elevated cerebrospinal fluid oxidative stress biomarkers. J Alzheimers Dis, 2016, 54(1): 99-107. [Crossref]
74. Arendt J, & Aulinas A: Physiology of the pineal gland and melatonin. In: Endotext. edn. Edited by Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J et al. South Dartmouth (MA): MDText.com, Inc; 2000.
75. Alhowail AH. Mechanisms underlying cognitive impairment induced by prenatal nicotine exposure: a literature review. Eur Rev Med Pharmacol Sci, 2021, 25(19): 6057- 6064. [Crossref]
76. Qiu T, Xie F, Zeng Q, Shen Z, Du G, Xu X, et al. Interactions between cigarette smoking and cognitive status on functional connectivity of the cortico-striatal circuits in individuals without dementia: a resting-state functional MRI study. CNS Neurosci Ther, 2022, 28(8): 1195-1204. [Crossref]
77. Ghahremani DG, Pochon JB, Perez Diaz M, Tyndale RF, Dean AC, & London ED. Functional connectivity of the anterior insula during withdrawal from cigarette smoking. Neuropsychopharmacology, 2021, 46(12): 2083- 2089. [Crossref]
78. Qiu T, Zeng Q, Luo X, Xu T, Shen Z, Xu X, et al. Effects of cigarette smoking on resting-state functional connectivity of the nucleus basalis of meynert in mild cognitive impairment. Front Aging Neurosci, 2021, 13: 755630. [Crossref]
79. Gu C, Ma M, Xu J, Yuan W, Li R, Guo H, et al. Association between pulmonary ventilatory function and mild cognitive impairment: a population-based study in rural China. Front Public Health, 2022, 10: 1038576. [Crossref]
80. Higbee DH, Granell R, Hemani G, Smith GD, & Dodd JW. Lung function, COPD and cognitive function: a multivariable and two sample Mendelian randomization study. BMC Pulm Med, 2021, 21(1): 246. [Crossref]
81. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, & Rudan I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med, 2022, 10(5): 447-458. [Crossref]
82. Excellence NIfC. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2008.
83. Zhao LY, & Zhou XL. Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia risk: a systematic review and meta-analysis. World J Clin Cases, 2022, 10(11): 3449-3460. [Crossref]
84. Wang T, Mao L, Wang J, Li P, Liu X, & Wu W. Influencing factors and exercise intervention of cognitive impairment in elderly patients with chronic obstructive pul-monary disease. Clin Interv Aging, 2020, 15: 557-566. [Crossref]
85. Ranzini L, Schiavi M, Pierobon A, Granata N, & Giardini A. From mild cognitive impairment (MCI) to dementia in chronic obstructive pulmonary disease. implications for clinical practice and disease management: a mini-review. Front Psychol, 2020, 11: 337. [Crossref]
86. Wang X, Cui L, & Ji X. Cognitive impairment caused by hypoxia: from clinical evidences to molecular mechanisms. Metab Brain Dis, 2022, 37(1): 51-66. [Crossref]
87. Sahu B, Narota A, & Naura AS. Pharmacological inhibition of poly (ADP-ribose) polymerase by olaparib, prevents acute lung injury associated cognitive deficits potentially through suppression of inflammatory response. Eur J Pharmacol, 2020, 877: 173091. [Crossref]
88. Wang X, Wang Y, Pan H, & Yan C. Dimethyl fumarate prevents acute lung injury related cognitive impairment potentially via reducing inflammation. J Cardiothorac Surg, 2021, 16(1): 331. [Crossref]
89. Fuertes E, Carsin AE, Antó JM, Bono R, Corsico AG, Demoly P, et al. Leisure-time vigorous physical activity is associated with better lung function: the prospective ECRHS study. Thorax, 2018, 73(4): 376-384. [Crossref]
90. O'Donovan G, & Hamer M. The association between leisure-time physical activity and lung function in older adults: the English longitudinal study of ageing. Prev Med, 2018, 106: 145-149. [Crossref]
91. Guerra-Carrillo B, Katovich K, & Bunge SA. Does higher education hone cognitive functioning and learning efficacy? Findings from a large and diverse sample. PLoS One, 2017, 12(8): e0182276. [Crossref]
92. Gonçalves NG, Avila JC, Bertola L, Obregón AM, Ferri CP, Wong R, et al. Education and cognitive function among older adults in Brazil and Mexico. Alzheimers Dement, 2023, 15(3): e12470. [Crossref]
93. Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, et al. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement, 2020, 16(9): 1305- 1311. [Crossref]
94. Babiloni C, Ferri R, Noce G, Lizio R, Lopez S, Lorenzo I, et al. Abnormalities of cortical cources of resting state alpha electroencephalographic rhythms are related to education attainment in cognitively unimpaired seniors and patients with Alzheimer's Disease and amnesic mild cognitive impairment. Cereb Cortex, 2021, 31(4): 2220- 2237. [Crossref]
95. Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, & Bennett DA. Education and cognitive reserve in old age. Neurology, 2019, 92(10): e1041-e1050. [Crossref]
96. Wada M, Noda Y, Shinagawa S, Chung JK, Sawada K, Ogyu K, et al. Effect of education on Alzheimer's diseaserelated neuroimaging biomarkers in healthy controls, and participants with mild cognitive impairment and Alzheimer's disease: a cross-sectional study. J Alzheimers Dis, 2018, 63(2): 861-869. [Crossref]
97. Seyedsalehi A, Warrier V, Bethlehem RAI, Perry BI, Burgess S, & Murray GK. Educational attainment, structural brain reserve and Alzheimer's disease: a Mendelian randomization analysis. Brain, 2023, 146(5): 2059-2074. [Crossref]
98. Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci, 2018, 19(11): 701-710. [Crossref]
99. Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, & Tucker-Drob EM. Education and cognitive functioning across the life span. Psychol Sci Public Interest, 2020, 21(1): 6-41. [Crossref]
100.Göthlin M, Eckerström M, Rolstad S, Kettunen P, & Wallin A. Better prognostic accuracy in younger mild cognitive impairment patients with more years of education. Alzheimers Dement, 2018, 10: 402-412. [Crossref]
101.Ampuero I, Ros R, Royuela A, Abraira V, del Ser T, GarcíaRibas G, et al. Risk factors for dementia of Alzheimer type and aging-associated cognitive decline in a Spanish population based sample, and in brains with pathology confirmed Alzheimer's disease. J Alzheimers Dis, 2008, 14(2): 179-191. [Crossref]
102.Chan JYC, Chan TK, Kwok TCY, Wong SYS, Lee ATC, & Tsoi KKF. Cognitive training interventions and depression in mild cognitive impairment and dementia: a systematic review and meta-analysis of randomized controlled trials. Age Ageing, 2020, 49(5): 738-747. [Crossref]
103.Zacková L, Jáni M, Brázdil M, Nikolova YS, & Marečková K. Cognitive impairment and depression: Meta-analysis of structural magnetic resonance imaging studies. Neuroimage Clin, 2021, 32: 102830. [Crossref]
104.Chen X, Han P, Yu X, Zhang Y, Song P, Liu Y, et al. Relationships between sarcopenia, depressive symptoms, and mild cognitive impairment in Chinese communitydwelling older adults. J Affect Disord, 2021, 286: 71-77. [Crossref]
105.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet, 2020, 396(10248): 413-446. [Crossref]
106.Fang F, Gao Y, Schulz PE, Selvaraj S, & Zhang Y. Brain controllability distinctiveness between depression and cognitive impairment. J Affect Disord, 2021, 294: 847- 856. [Crossref]
107.John A, Patel U, Rusted J, Richards M, & Gaysina D. Affective problems and decline in cognitive state in older adults: a systematic review and meta-analysis. Psychol Med, 2019, 49(3): 353-365. [Crossref]
108.Larner AJ: Diagnosis of dementia and cognitive impairment. In., vol. 9: MDPI; 2019: 180.
109.Johnson LA, Mauer C, Jahn D, Song M, Wyshywaniuk L, Hall JR, et al. Cognitive differences among depressed and non-depressed MCI participants: a project frontier study. Int J Geriatr Psychiatry, 2013, 28(4): 377-382. [Crossref]
110.Mourao RJ, Mansur G, Malloy-Diniz LF, Castro Costa E, & Diniz BS. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int J Geriatr Psychiatry, 2016, 31(8): 905-911. [Crossref]
111.Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, et al. Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry, 2018, 175(6): 530-537. [Crossref]
112.Krell-Roesch J, Vassilaki M, Mielke MM, Kremers WK, Lowe VJ, Vemuri P, et al. Cortical β-amyloid burden, neuropsychiatric symptoms, and cognitive status: the mayo clinic study of aging. Transl Psychiatry, 2019, 9(1): 123. [Crossref]
113.Mirza SS, Ikram MA, Bos D, Mihaescu R, Hofman A, & Tiemeier H. Mild cognitive impairment and risk of depression and anxiety: a population-based study. Alzheimers Dement, 2017, 13(2): 130-139. [Crossref]
114.Ahn J, & Kim M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: a systematic review and meta-analysis. Arch Gerontol Geriatr, 2023, 106: 104855. [Crossref]
115.Wu VX, Chi Y, Lee JK, Goh HS, Chen DYM, Haugan G, et al. The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: a systematic review and meta-analysis. Int J Nurs Stud, 2021, 122: 104025. [Crossref]
116.Zheng G, Zheng Y, Xiong Z, & Ye B. Effect of Baduanjin exercise on cognitive function in patients with post-stroke cognitive impairment: a randomized controlled trial. Clin Rehabil, 2020, 34(8): 1028-1039. [Crossref]
117.Orgeta V, Leung P, Del-Pino-Casado R, Qazi A, Orrell M, Spector AE, et al. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev, 2022, 4(4): Cd009125. [Crossref]
118.Krell-Roesch J, Vemuri P, Pink A, Roberts RO, Stokin GB, Mielke MM, et al. Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the APOE ε4 genotype. JAMA Neurol, 2017, 74(3): 332-338. [Crossref]
119.Xue B, Xiao A, Luo X, & Li R. The effect of a game training intervention on cognitive functioning and depression symptoms in the elderly with mild cognitive impairment: a randomized controlled trial. Int J Methods Psychiatr Res, 2021, 30(4): e1887. [Crossref]
120.Bingham KS, Flint AJ, & Mulsant BH. Management of lateLife depression in the context of cognitive impairment: a review of the recent literature. Curr Psychiatry Rep, 2019, 21(8): 74. [Crossref]
121.Chow YY, Verdonschot M, McEvoy CT, & Peeters G. Associations between depression and cognition, mild cognitive impairment and dementia in persons with diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract, 2022, 185: 109227. [Crossref]
122.Smith L, Jacob L, López-Sánchez GF, Butler L, Barnett Y, Veronese N, et al. Anxiety symptoms and mild cognitive impairment among community-dwelling older adults from low- and middle-income countries. J Affect Disord, 2021, 291: 57-64. [Crossref]
123.Pink A, Krell-Roesch J, Syrjanen JA, Vassilaki M, Lowe VJ, Vemuri P, et al. A longitudinal investigation of Aβ, anxiety, depression, and mild cognitive impairment. Alzheimers Dement, 2022, 18(10): 1824-1831. [Crossref]
124.Gallagher D, Coen R, Kilroy D, Belinski K, Bruce I, Coakley D, et al. Anxiety and behavioural disturbance as markers of prodromal Alzheimer's disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry, 2011, 26(2): 166-172. [Crossref]
125.Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, & Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology, 2007, 68(19): 1596-1602. [Crossref]
126.Li XX, & Li Z. The impact of anxiety on the progression of mild cognitive impairment to dementia in Chinese and English data bases: a systematic review and metaanalysis. Int J Geriatr Psychiatry, 2018, 33(1): 131-140. [Crossref]
127.Chen C, Hu Z, Jiang Z, & Zhou F. Prevalence of anxiety in patients with mild cognitive impairment: a systematic review and meta-analysis. J Affect Disord, 2018, 236: 211-221. [Crossref]
128.Mirza S, Ikram M, Bos D, Mihaescu R, Hofman A, & Tiemeier H. Mild cognitive impairment and risk of depression and anxiety: a population-based study. Alzheimer's & Dementia, 2016, 13(2): 130-139. [Crossref]
129.Jain N, Wang Y, Zhang Y, Jacobsen E, Andreescu C, Snitz BE, et al. It goes both ways: the relationship between anxiety and mild cognitive impairment. Int J Geriatr Psychiatry, 2023, 38(3): e5899. [Crossref]
130.Kanaley JA, Colberg SR, Corcoran MH, Malin SK, Rodriguez NR, Crespo CJ, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the american college of sports medicine. Med Sci Sports Exerc, 2022, 54(2): 353-368. [Crossref]
131.Nguyen B, Ding D, & Mihrshahi S. Fruit and vegetable consumption and psychological distress: cross-sectional and longitudinal analyses based on a large Australian sample. BMJ Open, 2017, 7(3): e014201. [Crossref]
132.Orgeta V, Qazi A, Spector A, & Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and metaanalysis. Br J Psychiatry, 2015, 207(4): 293-298. [Crossref]
133.Lin R, Luo YT, Yan YJ, Huang CS, Chen LL, Chen MF, et al. Effects of an art-based intervention in older adults with mild cognitive impairment: a randomised controlled trial. Age Ageing, 2022, 51(7): afac144. [Crossref]