Open Access | Research
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Older-aged C57BL/6 mice fed a diet high in saturated fat and sucrose for ten months show decreased resilience to aging
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 17 July 2023 / Revised: 17 August 2023 / Accepted: 26 September 2023 / Published: 28 September 2023
DOI: 10.31491/APT.2023.09.120
Abstract
The ability to respond to physical stress that disrupts normal physiological homeostasis at an older age embraces the concept of resilience to aging. A physical stressor could be used to induce physiological responses that are age-related, since resilience declines with increasing age. Increased fat and sugar intake is a nutritional stress with a high prevalence of obesity in older people. In order to determine the effect of this type of diet on resilience to aging, 18-month-old C57BL/6J male mice were fed a diet high in saturated fat (lard) and sucrose (HFS) for ten months. At the end of the 10-month study, mice fed the HFS diet showed increased cognitive impairment, decreased cardiac function, decreased strength and agility, and increased severity of renal pathology compared to mice fed a rodent chow diet low in saturated fat and sucrose (LFS). The degree of response aligned with decreased resilience to the long-term adverse effects of the diet with characteristics of accelerated aging. This observation suggests additional studies could be conducted to investigate the relationship between an accelerated decline in resilience to aging and enhanced resilience to aging under different dietary conditions.
Keywords
Resilience to aging, high-fat diet, physical stressor, C57BL/6J mice, cognition, cardiac function, kidney disease
Introduction
A decline in functionality with increasing age can be seen in changes in physical abilities [1], cognitive abilities [2], and overall morphological structures [3]. Basically, the ability to maintain normal function becomes impaired. However, not all people age at the same rate making some individuals more resilient to age-related changes compared to others [4]. The concept of physical resilience to aging builds on the heterogeneous response pattern to physical stress that disrupts normal physiological homeostasis and the rate of return to normal [5]. Therefore, a physical stressor could be used to induce physiological responses that are age-related for resilience measurement [6]. Resilience to aging could then be predicted by analyzing responses to a physical stressor that disrupts physiological functions correlated with the robustness of the response with aging endpoints.
One example of a physical stressor is nutritional stress. The prevalence of obesity in older people has dramatically increased in recent years. In the United States, more than 37 percent of men and women aged 60 years and over are obese, which puts the elderly at a much higher risk for developing disability and loss of function [7]. The potential of using a diet high in saturated fat and sucrose to predict resilience to aging can be studied in mice using behavioral and performance tests to measure phenotypic traits of aging. It is well established that C57BL/6J mice are sensitive to diets high in fat and sugar [8]. Changes can occur relatively quickly, but less is known about what happens in old C57BL/6 mice fed a high-fat diet over an extended period of time.
In this study, we show that older-aged (18 months) C57BL/6J male mice fed a diet high in saturated fat (lard) and table sugar (sucrose) for ten months have early divergent changes in body weight and body fat mass and later changes reflected in impaired cognition, cardiac dysfunction, decreased strength and agility, and increased severity of kidney pathology.
Materials and methods
Animals
C57BL/6J male mice were obtained from the United States National Institute on Aging aged rodent colony (contracted from Charles River, Inc.) at 18 months of age. Mice were housed 3 to 5 per cage in a specific pathogenfree facility at the University of Washington under a 12-hour light and 12-hour dark cycle with room temperature of 25 ± 4℃ and reverse osmosis water in an automated watering system. Mice were acclimated for two weeks and then randomly assigned to a high-fat and high sucrose (HFS) diet (N = 19) or a low-fat and low sucrose (LFS) rodent chow diet (N = 19).
Diet
The HFS diet (Bioserv, 3282, paste, gamma irradiated) has been described [9]. Briefly, it consisted of lard, sucrose, casein, maltodextrin, vitamins and minerals with 36 percent fat, 36 percent carbohydrate, and 20 percent protein. The level of kilocalories per gram of food was 5.54. The LFS diet (Picolab Rodent Diet 20, 5053) consisted of corn, soybean, wheat, fish meal, and vitamin-mineral mixture. The LFS diet contained 20% protein, 10.6% fat, 4.7% curd fiber, 52.9% nitrogen-free extract, and 10.3% mineral mass. The level of kilocalories per gram of food was 3.07. The diets were given ad lib and replaced weekly in each food holder over 10 months. Food consumption was calculated in the first week of each month by weighing the food placed in each food holder, and three days later weighing the remaining amount, including any fines in the bedding.
Physiological measurements
Body weight was measured weekly. Body fat mass and lean muscle mass were measured monthly by quantitative magnetic resonance imaging (QMR) (EchoMRI) with readouts in grams minus water content.
Functional performance tests
A spatial navigation learning task (box maze) was used to assess learning impairment [10]. Mice were placed in a square box with seven blocked exits and one escape hole leading to a dark non-stressful cage. On each trial, mice were allowed to explore the cage for 120 seconds. Mice were tested continuously for four trials in one day and their escape times were recorded.
Cardiac function was assessed by echocardiography. Echocardiography is a non-invasive procedure that allows the assessment of systolic and diastolic function in mice. A Seimans Acuson CV-70 system with standard imaging planes was used to measure myocardial performance index (MPI) and diastolic function (Ea/Aa ratio) as described [9].
Rotarod performance is a measure of balance and coordination, and was assessed as the ability of mice to maintain balance on a rotating rod using a Rotamex 4/8 (Columbus Instruments, Inc.) with an accelerating rod protocol as described [11]. Up to four mice were placed on the rod within their individual lanes in the rotarod enclosure. The software recorded photobeam breaks as the animal’s continuous participation in the task. Once an animal fell off the rotarod, there were no longer any beam breaks, and the final time was recorded. Three successive runs were performed.
Grip strength, which is one of the measures used to assess frailty in older people, was determined using a Grip Strength Meter (Columbus Instruments, Inc.) by measuring the amount of force the mouse can apply in grasping a specially designed pull bar assembly [11].
Geropathology
Tissues were collected in 10% buffered formalin, processed for H&E staining, and slides were blindly read by a pathologist (W. Ladiges). The histological lesions were graded based on the Geropathology Grading Platform [12,13]. Tissues including heart, lung, liver, kidney, and pancreas were graded.
Statistical analysis
Significance analysis was done by one- and two-way ANOVA. Mean values ± standard error of the mean (SEM) were presented in the figures. Statistical significance was established as P < 0.05.
Results and discussion
Mice consuming a diet high in fat and sucrose had biphasic changes in body weight and fat mass.
The body weight of mice fed the high-fat and highsucrose diet increased rapidly in the first three months (Figure 1A). On average, these mice had a body weight increase of 36% by the third month. After 10 months on the diet, when mice were 28 months of age, body weight had dropped to 38.3 ± 2.8 g with no significant difference from the starting baseline value of 35.3 ± 0.5 g. The body weight of mice fed the low-fat and low-sucrose (rodent chow) diet was relatively stable and dropped from 34.1 ± 0.8 g to 31.7 ± 0.8 g after 10 months. The amount of food consumed by the high-fat diet group decreased significantly after the first month (Figure 1D) to match the caloric intake of the low-fat diet group (Figure 1E). Body fat mass of mice on the high-fat diet increased from 4 gm to 17.6 gm in the first two months and then gradually decreased to 7.8 gm at 10 months (Figure 1B). Lean muscle mass trended upwards over the first 7 months in mice on the high-fat diet (Figure 1C). The body fat and lean muscle mass of mice on the low-fat diet were relatively stable through the 10-month period, but significant decreases were found between baseline and the 10-month end point for both parameters. Despite the change in body weight, fat mass, and lean muscle mass, the blood glucose was not affected by the high-fat diet (Figure 1F). However, both high-fat and low-fat diet groups had a significant decrease in blood glucose over the 10-month feeding period. There was no difference in amount of food ingested or caloric intake between the two groups, so any adverse effects can be attributed to dietary factors and not total calories consumed.
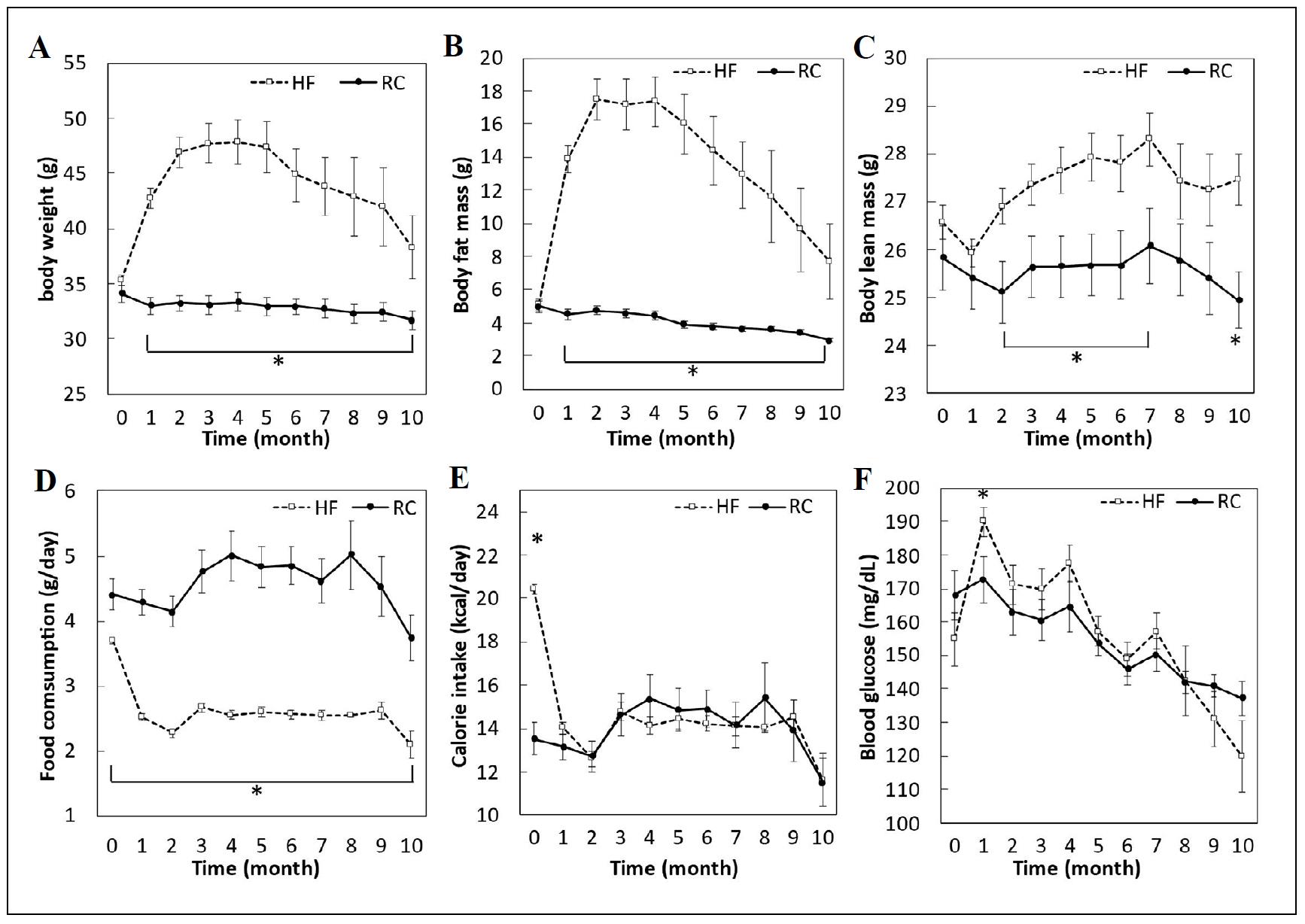
Figure 1. A diet high in fat and sucrose fed to mice starting at 18 months of age and continuing for 10 months induced biphasic physiological changes. (A). The body weight of mice fed the high-fat and high-sucrose (HF) diet increased rapidly in the first 3 months, then gradually decreased, while mice fed the low-fat and low-sucrose regular chow (RC) diet maintained a relatively stable body weight throughout the study. (B) Fat mass increased then decreased in mice fed the HF diet compared to mice fed the RC diet. (C) Lean muscle mass increased for the first 7 months in the HF diet groups compared to the low-fat (RC) diet group. (D) Mice fed the HF diet reduced their food consumption after the first month on the diet, and (E) decreased their caloric intake after an increase in the first 2 months to the same level as mice fed the low-fat RC diet. (F) Mice fed either diet had similar blood glucose levels with both decreasing over time. *P < 0.05, N = 12-19 per group.
A decrease in cardiac function, physical performance, and cognition occurred in mice fed a diet high in fat and sucrose.
Heart function was measured by echocardiography. Early to late ventricular filling velocities ratio (Ea/Aa) and myocardial performance index (MPI) were recorded at 0, 5, and 10 months (baseline, midpoint, and endpoint). Ea/Aa decreased significantly in mice fed the diet high in fat and sucrose (HFS) at both five months and 10 months compared to mice fed the LFS diet (Figure 2A). MPI values were not significantly different between the two diet groups (Figure 2B). The Ea/Aa ratio represents peak velocity flow of blood during relaxation of the left ventricle (E) compared to the peak velocity blood flow during contraction of the atria (A). Since the left ventricle pumps blood into the general circulatory system, a decrease in the Ea/Aa ratio suggests the left ventricle in mice fed the HFS diet was unable to maintain adequate blood flow consistent with good health, and resulted in a decrease in resilience to aging. The poor cardiac performance is of interest because it is well accepted that people ingesting diets high in fat and sugar are at increased risk for heart disease [14].
Mice were also assessed for physical performance in the rotarod and grip strength tests, and for cognitive behavior using a spatial navigation learning task (box maze) after 10 months on the diets. Significant differences were found between mice fed the HFS and LFS diets in all tests. Mice fed the HFS diet failed to maintain balance on the rotarod and their duration was 67% of the LFS group (Figure 2C). The mice in the HFS group had a 76% decrease in grip strength compared to the LFS group (Figure 2D). The learning behavior was measured by box maze escape times. Mice fed the HFS diet spent longer time in the maze than mice fed the LFS diet (Figure 2E). These assessments are routinely used to measure the degree of physical aging in mice, so increased impairment suggests accelerated aging in older mice.
Increased intake of fat and sugar is associated with cognitive impairment in older individuals [15]. Recent reports in mice fed long-term diets high in fat and sugar show similar findings of cognitive decline [16-18]. However, these studies used young mice or adult mice, while our study used old mice starting at 18 months of age and continuing for ten months when the mice were 28 months of age, which is equivalent to a person in the 80–90 year age range.
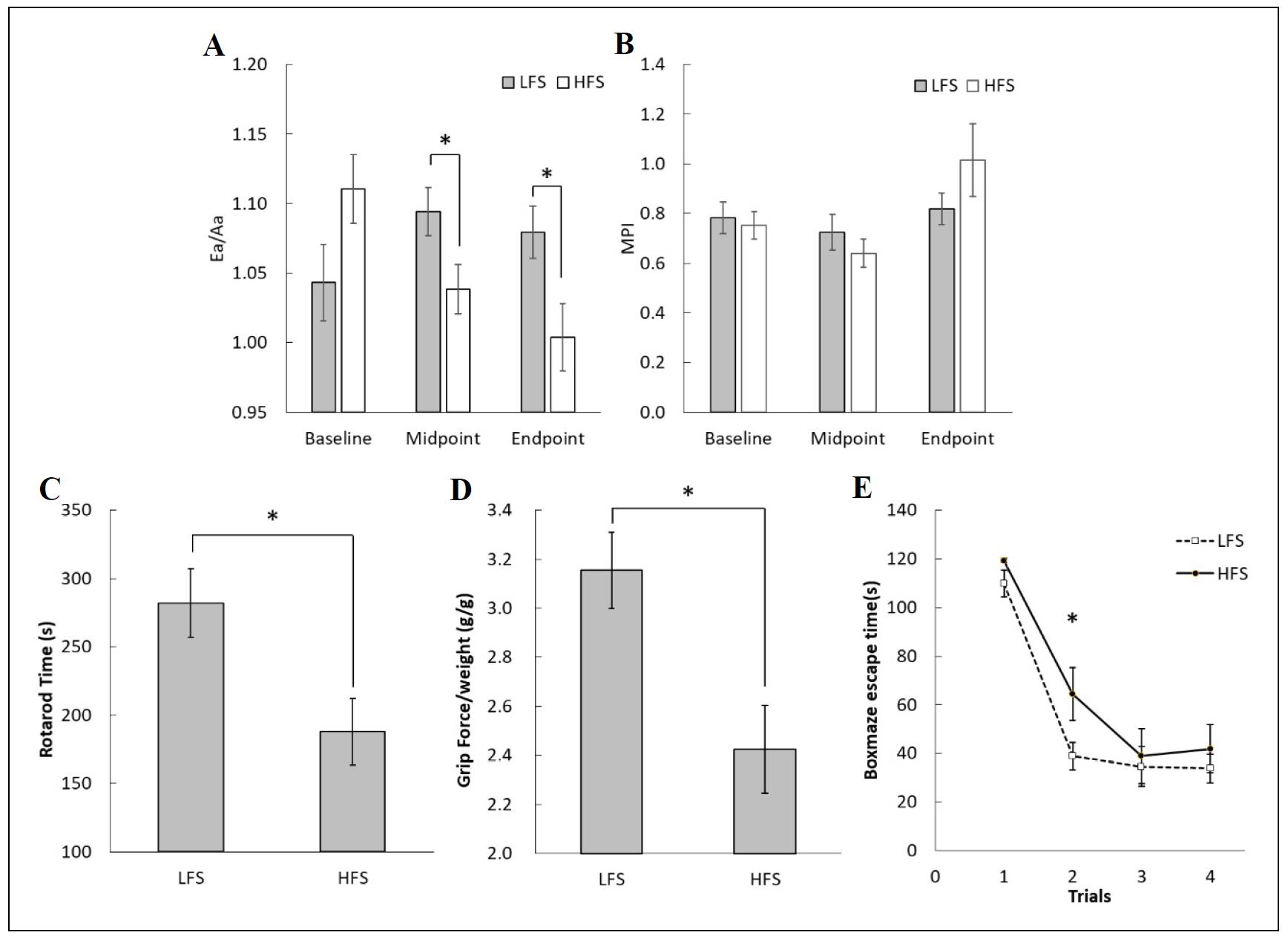
Figure 2. Cardiac function, physical performance, and cognition were impaired in mice fed a diet high in fat and sucrose (HFS). (A) Cardiac Ea/Aa decreased over time in mice fed the HFS diet compared to mice fed the low-fat and low-sucrose (LFS) diet. (B) There was no difference in MPI between the diet groups. (C) Mice fed the HFS diet were not able to stay on the rotarod as long as mice fed the LFS diet, (D) had decreased strength (standardized to body weight), and (E) required more time to find the escape hole in the box maze. *P < 0.05, N = 12-19 per group.
Mice fed a diet high in fat and sucrose had increased severity of kidney pathology but no difference in survival compared to mice fed a diet low in fat and sucrose.
The high-fat and sucrose (HFS) diet group had increased severity of age-related lesions in the kidney and a trend in the heart, but showed no effect in severity of age-related lesions in the liver, lung, or pancreas compared to the low-fat and sucrose (LFS) diet group (Figure 3A). Histology showed increased severity in specific lesions in the kidney, including nephropathy, lymphoid aggregates, infarcts, and amyloidosis. Despite the significant severity of renal pathology, the HFS and LFS diet groups had similar survival after the 10-month feeding trial when surviving mice were 28 months of age (Figure 3B). It is known that high-fat diets induce kidney disease in mice [19]. Our study confirms this observation and suggests that high-fat diet-induced nephropathy is a major disease complication in C57BL/6 mice at old age.
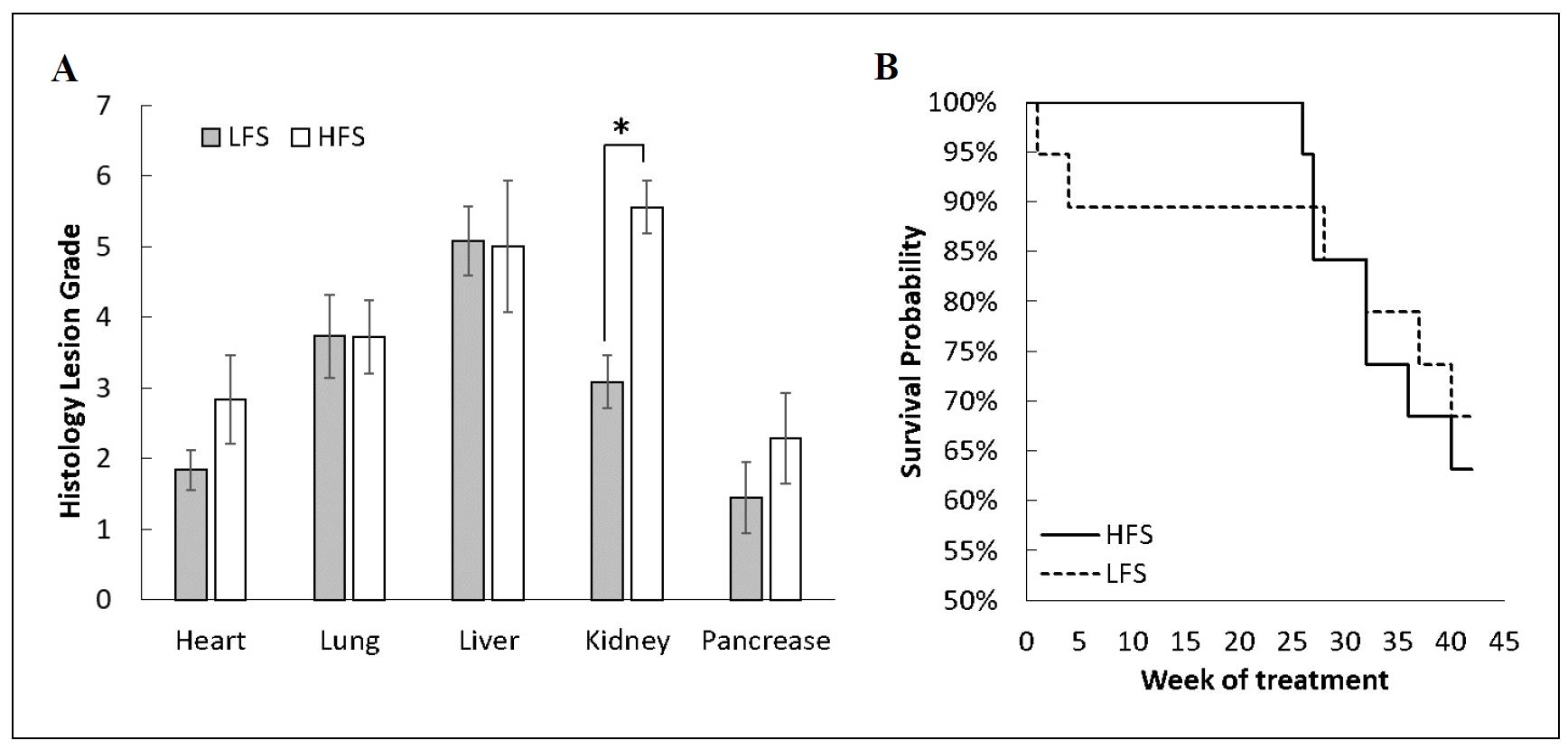
Figure 3. Mice fed a diet high in fat and sucrose had increased severity of kidney pathology but no difference in survival compared to mice fed a diet low in fat and sucrose. (A) Mice fed the high-fat and high-sucrose (HFS) diet had more severe kidney lesions and a trend in heart lesions, but no difference in lung, liver, or pancreatic lesion severity, compared to mice fed the low-fat and low-sucrose (LFS) diet. (B) Survival rate was similar in HFS and LFS diet groups over the 10-month feeding trial. *P < 0.05, N = 12-19 per group.
Conclusions
In conclusion, the observations suggest that a long-term (10 months) diet consisting of high levels of saturated fat and sucrose is detrimental to cardiac function, cognition, strength, and motor coordination, and increases the severity of age-related kidney lesions in C57BL/6J male mice starting at 18 months of age. This type of dietary stress in old-aged C57BL/6J mice will be useful to study physical resilience to aging and age-related diseases with relevance to clinical studies. Of particular interest will be the study of old-aged female C57BL/6J mice to determine differences and similarities attributed to sex.
Declarations
Financial support and sponsorship
This work was supported by National Institutes of Health (NIH) grant R01 AG057381 (Ladiges, PI).
Ethical approval
The study was approved by the University of Washington IACUC.
Availability of data and materials
The data supporting the study results are available from the corresponding author upon reasonable request.
Conflicts of interest
Warren Ladiges is a member of the editorial board of Aging Pathobiology and Therapeutics. The authors declare that they have no conflicts and were not involved in the journal’s review or decision regarding this manuscript.
References
1. Milanović Z, Pantelić S, Trajković N, Sporiš G, Kostić R, James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging, 2013, 8: 549-556. [Crossref]
2. Duffy CM, Hofmeister JJ, Nixon JP, Butterick TA. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol Learn Mem, 2019, 157: 41-47. [Crossref]
3. Laurentius T, Raffetseder U, Fellner C, Kob R, Nourbakhsh M, Floege J, et al. High-fat diet-induced obesity causes an inflammatory microenvironment in the kidneys of aging Long-Evans rats. J Inflamm (Lond), 2019, 16: 14. [Crossref]
4. Schorr A, Carter C, Ladiges W. The potential use of physical resilience to predict healthy aging. Pathobiol Aging Age Relat Dis, 2018, 8(1): 1403844. [Crossref]
5. Lei H, Huffman DM, Salmon AB, LeBrasseur NK, Carter C, Richardson A, et al. Resilience to aging is a heterogeneous characteristic defined by physical stressors. Aging Pathobiol Ther, 2022, 4(1): 19-22. [Crossref]
6. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A: Biol Sci Med Sci, 2016, 71(11): 1407-1414. [Crossref]
7. Batsis JA, Zagaria AB. Addressing obesity in aging patients. Med Clin North Am, 2018, 102(1): 65-85. [Crossref]
8. Schreyer SA, Wilson DL, LeBoeuf RC. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis, 1998, 136(1): 17-24. [Crossref]
9. Nickel K, Zhu L, Mangalindan R, Snyder JM, Tucker M, Whitson J, et al. Long-term treatment with Elamipretide enhances healthy aging phenotypes in mice. Aging Pathobiol Ther, 2022, 4(3): 76-83. [Crossref]
10. Darvas M, Mukherjee K, Lee A, Ladiges W. A novel oneday learning procedure for mice. Curr Protoc Mouse Biol, 2020, 10(1): e68. [Crossref]
11. Jiang Z, Wang J, Imai D, Snider T, Klug J, Mangalindan R, et al. Short term treatment with a cocktail of rapamycin, acarbose and phenylbutyrate delays aging phenotypes in mice. Sci Rep, 2022, 12(1): 7300. [Crossref]
12. Ladiges W, Ikeno Y, Niedernhofer L, McIndoe RA, Ciol MA, Ritchey J, et al. The geropathology research network: an interdisciplinary approach for integrating pathology into research on aging. J Gerontol A Biol Sci Med Sci, 2016, 71(4): 431-434. [Crossref]
13. Ladiges W, Snyder JM, Wilkinson E, Imai DM, Snider T, Ge X, et al. A new preclinical paradigm for testing anti-aging therapeutics. J Gerontol A Biol Sci Med Sci, 2017, 72(6): 760-762. [Crossref]
14. Abu Bakar NAF, Ahmad A, Wan Musa WZ, Shahril MR, Wan-Arfah N, Abdul Majid H, et al. Association between a dietary pattern high in saturated fatty acids, dietary energy density, and sodium with coronary heart disease. Sci Rep, 2022, 12(1): 13049. [Crossref]
15. Yeomans MR, Armitage R, Atkinson R, Francis H, Stevenson RJ. Habitual intake of fat and sugar is associated with poorer memory and greater impulsivity in humans. PLoS One, 2023, 18(8): e0290308. [Crossref]
16. Liang Z, Gong X, Ye R, Zhao Y, Yu J, Zhao Y, et al. Longterm high-fat diet consumption induces cognitive decline accompanied by Tau hyper-phosphorylation and microglial activation in aging. Nutrients, 2023, 15(1). [Crossref]
17. Lu Z, Wang H, Zhang X, Huang X, Jiang S, Li Y, et al. High fat diet induces brain injury and neuronal apoptosis via down-regulating 3-β hydroxycholesterol 24 reductase (DHCR24). Cell Tissue Res, 2023, 393(3): 471-487. [Crossref]
18. Wen J, Wang Y, Wang C, Yuan M, Chen F, Zou Q, et al. Dietary high-fat promotes cognitive impairment by suppressing mitophagy. Oxid Med Cell Longev, 2023, 2023: 4822767. [Crossref]
19. Qilu F, Qinglin L, Like Z, Yajun Q, Wenxiu X, Luo F. Paeony attenuates high fat diet-induced kidney injury via inflammation inhibition. Pak J Pharm Sci, 2023, 36(4): 1217-1225.