Open Access | Therapeutic Brief
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
GHK peptide prevents sleep-deprived learning impairment in aging mice
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School
of Medicine, University of Washington, Seattle, WA, USA.
Email: wladiges@uw.edu
Received: 07 Feburary 2023 / Revised: 02 March 2023 / Accepted: 22 March 2023 / Published: 29 March 2023
DOI: 10.31491/APT.2023.03.109
Abstract
Sleep deprivation is known to cause memory impairment and is associated with inflammation and cell damage linked to neurodegenerative diseases. GHK (glycyl-L-histidyl-L-lysine) is a naturally occurring tripeptide found in mammalian plasma. GHK has anti-inflammatory activity and can pass through the blood-brain barrier suggesting the potential to prevent neuroinflammation associated with sleep deprivation. In this study, mice were injected with 15 mg/kg GHK per day for five days and sleep deprived on the last two days of treatment. Sleepdeprived mice treated with GHK did not show the acute learning impairment seen in sleep-deprived mice treated with saline. GHK prevented an increase in MCP-1 and nitrotyrosine levels in the hippocampus of sleepdeprived mice suggesting that inflammatory and reactive nitrogen/oxygen species activity could be therapeutic targets for learning impairment associated with short-term sleep deprivation.
Keywords
GHK peptide, sleep deprivation, learning impairment, neuroinflammation, nitrotyrosine
Sleep deprivation is an increasing problem in modern society, with sleep duration among American adults decreasing over the past 25 years. As sleep deprivation becomes
the norm of the American working world, researchers are
finding that a decrease in overall sleep can cause serious
health issues. Both acute and chronic habitual sleep loss is
related to negative impacts on mental health, cardiometabolic conditions, and pain [1]. Insufficient sleep can cause
increased cytokine secretion provoking an inflammatory
response in various tissues including the brain which is
associated with cognitive impairment [2].
GHK (glycyl-L-histidyl-L-lysine) is a naturally occurring
human plasma copper-binding peptide known to possess
wound healing, antioxidant, anti-inflammatory, and antiaging effects [3]. Because of its antioxidant and antiinflammatory properties, GHK is a promising peptide
for use in the treatment of age-related neurodegenerative
conditions. This therapeutic brief describes preliminary
observations on the ability of GHK to prevent the adverse
neuropathological effects of short-term sleep deprivation
in aging mice. The rationale for using GHK without copper was that it is important to know if GHK can be effective without its copper complex as a therapeutic parenteral
injection for this type of cognitive impairment.
CB6F1 female mice were obtained from the National Institute on Aging aged rodent colony at 15 months of age.
Mice were housed in 3-4 per cage in an SPF facility at
the University of Washington under a 12-hour light-dark
cycle starting at 6 am. The room temperature was 25℃
± 4. Reverse osmosis water and irradiated food (Picolab
Rodent Diet 20, 5053) were supplied. All studies were approved by the University of Washington IACUC.
Mice were started on intraperitoneal injection (ip) of
copper-free GHK (Peptide Sciences, Henderson, NV ) at a
dose of 7.5 mg/kg (n = 8), or ip saline (n = 12), twice daily for five days. On days 4 and 5 of treatment, the GHK
group and 6 of the saline group were sleep deprived for 4
hours as described [4]. Mice were sleep deprived in their
home cage by light cage tapping and gentle stroking of
the back using a small paintbrush. Six of the saline group
were not sleep deprived. On the fifth day, following sleep
deprivation, each group was tested in a box maze spatial
navigation learning task [5]. Each mouse was given 120
seconds to find an escape hole and tested continuously for
4 trials with the escape time for each trial recorded.
Mice were euthanized by CO2 and brain fixed in formalin.
Sagittal 4 µm paraffin sections of the brain were mounted
on slides, which were rehydrated and incubated in a citrate buffer at 95℃ for 30 minutes for antigen retrieval.
Slides were then stained for MCP-1 and nitrotyrosine to
assess inflammation and oxidative stress, respectively,using specific antibodies and an HRP-DAB cell and tissue staining kit (R&D Systems). Digital images of CA3
and dentate gyrus (DG) sections of the hippocampus were
taken at 20x magnifications for stain intensity analysis and
processed through Image J with the IHC toolbox and IHC
profile plugin [6].
Mice treated with GHK showed little learning impairment
after sleep deprivation in line with saline-treated mice that
were not sleep deprived, and significantly less than sleepdeprived mice treated with saline (Figure 1). The learning ability in the Box maze was calculated as the slope
of escape times of each mouse. More negative numbers
indicated faster escape times and less learning impairment. The negative slope value of sleep-deprived mice
treated with GHK (SD + GHK) was similar to the negative slope value of non-sleep-deprived mice treated with
saline (Control). In contrast, sleep-deprived mice treated
with saline (SD) had a significant decrease in the learning
curve slope indicating learning impairment without GHK.
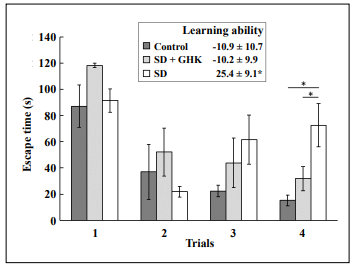
Figure 1. GHK treatment prevented learning impairment caused by sleep deprivation. The SD + GHK mouse group had escape times and a learning curve slope similar to the nonSD + saline (control) group but significantly less than the sleep-deprived saline-treated mice (SD) *: p < 0.05. Data from the nonSD + GHK cohort are not shown because there was no effect.
Inflammation and oxidative stress in sleep-deprived mice
were measured by MCP-1 and nitrotyrosine staining,
respectively, and quantitated by ImageJ digital analysis.
MCP-1 levels in sleep-deprived mice treated with GHK
were significantly lower in the CA3 region of the hippocampus compared to sleep-deprived mice treated with
saline, indicating GHK’s anti-inflammatory effect (Figure 2A). Notably, sleep-deprived mice treated with GHK had
lower inflammation compared to both sleep-deprived and
control mice in the CA3 region of the hippocampus. Sleep
deprivation increased nitrotyrosine levels in both the CA3
and DG regions of the hippocampus, and GHK treatment
successfully prevented such an effect (Figure 2B). GHK
is known to work as an anti-inflammatory and antioxidant
agent by decreasing inflammatory cytokines and reducing
reactive oxygen species (ROS) levels [7]. Nitrotyrosine is
considered a biomarker for oxidative stress as the result of the nitration of protein-bound and free tyrosine residues
by reactive peroxy nitrate molecules formed when nitric
oxide reacts with superoxide [8].
These results indicate that GHK can prevent sleepdeprived learning impairment associated with suppression
of increased inflammation and nitrotyrosine production in
the hippocampus, suggesting that GHK could be further
studied as a way to prevent adverse neuropathological effects of acute sleep disruption.
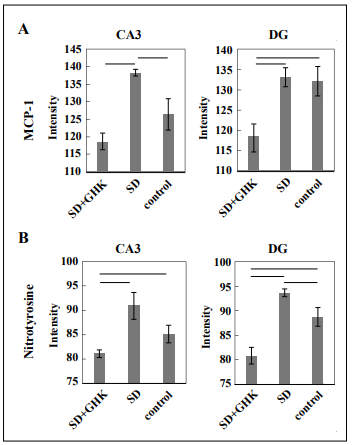
Figure 2. Immunohistochemistry images were quantified by staining intensity using ImageJ. Sleep-deprived mice treated with GHK had (A) decreased MCP-1 and (B) decreased nitrotyrosine staining intensity in the hippocampus. Results connected by horizontal lines are significantly different (p < 0.05). Control = nonSD + saline.
Declarations
Authors’ contributions
All authors made contributions to the generation of data and/or writing the manuscript.
Financial support and sponsorship
Supported in part by NIH grant R01 AG057381.
Conflicts of interest
Warren Ladiges is a member of the Editorial Board of Aging Pathobiology and Therapeutics. The author declares that there are no conflicts.
References
1. Conklin AI, Yao CA, & Richardson CG. Chronic sleep disturbance, not chronic sleep deprivation, is associated with self-rated health in adolescents. Prev Med, 2019, 124: 11-16. [Crossref]
2. Periasamy S, Hsu DZ, Fu YH, & Liu MY. Sleep deprivationinduced multi-organ injury: role of oxidative stress and inflammation. Excli j, 2015, 14: 672-683. [Crossref]
3. Pickart L, Vasquez-Soltero JM, & Margolina A. GHK-Cu may prevent oxidative stress in skin by regulating copper and modifying expression of numerous antioxidant genes. Cosmetics, 2015, 2(3), 236–247. [Crossref]
4. Mukherjee KK, Lee AY, Zhu L, Darvas M, & Ladiges W. Sleep-deprived cognitive impairment in aging mice is alleviated by rapamycin. Aging Pathobiol Ther, 2019, 1(1): 5-9. [Crossref]
5. Darvas M, Mukherjee K, Lee A, & Ladiges W. A Novel One-Day Learning Procedure for Mice. Curr Protoc Mouse Biol, 2020, 10(1): e68. [Crossref]
6. Varghese F, Bukhari AB, Malhotra R, & De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One, 2014, 9(5): e96801. [Crossref]
7. Dou Y, Lee A, Zhu L, Morton J, & Ladiges W. The potential of GHK as an anti-aging peptide. Aging Pathobiol Ther, 2020, 2(1): 58-61. [Crossref]
8. Bandookwala M, & Sengupta P. 3-Nitrotyrosine: a versatile oxidative stress biomarker for major neurodegenerative diseases. Int J Neurosci, 2020, 130(10): 1047-1062. [Crossref]