Open Access | Therapeutic Brief
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Molecular hydrogen may activate the transcription factor Nrf2 to alleviate oxidative stress through the hydrogen-targeted porphyrin
* Corresponding author: Shigeo Ohta
Mailing address: Department of Neurology Medicine, Juntendo
University Graduate School of Medicine, 2-1-1 Hongo, Bunkyoku, Tokyo 113-8421, Japan.
Email: ohta@nms.ac.jp
This article belong to the Special Issue: Hormesis-Based Anti-Aging Strategies: The role of free radicals and antioxidants in neurodegenerative diseases
Received: 30 December 2022 / Revised: 31 January 2023 / Accepted: 06 March 2023 / Published: 29 March 2023
DOI: 10.31491/APT.2023.03.104
Abstract
Oxidative stress is one of the major causes of most age-dependent neurodegenerative disorders. Neurons accumulate oxidative damage over time due to post-mitotic cells. Thus, modulation of oxidative stress is essential to overcome these disorders. Molecular hydrogen (H2) has great potential for treating various diseases and improving quality of life by exerting multiple functions including anti-oxidation, anti-inflammation, and energy metabolism promotion. Among these functions, H2 activates a transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2) to enhance the transcription of transcribe a broad range of anti-stress enzymes, including antioxidant enzymes. There was an elusive contradiction between H2 and Nrf2 because Nrf2 is activated in response to oxidative stress, whereas H2 has a reducing potential. The target molecule for H2 has recently been identified as the oxidized form of Fe-porphyrin conjugated with the -OH group (PrP-Fe(III)-OH). As the initial step, the hydroxyl radical (•OH) oxidizes heme (PrP-Fe(II)) to form PrP-Fe(III)-OH. Then, H2 reacts with PrP-Fe(III)-OH to produce PrP-Fe(III)-H and H2O. In turn, Fe(III) with H has the potential to act as an electrophile to oxidize Kelch-like ECH-associated protein 1 (Keap1), resulting in activating Nrf2. Thus, when the original highly damaging electrophilicity of •OH is buffered by H2 and its target porphyrin, the electrophilicity provided by •OH can indirectly activate Nrf2 to reduce oxidative stress. Even without lowering the dosage, the effect of alleviated potent is considered to be hormesis-like. This “Therapeutic Brief” propose that the alleviated oxidative potent of •OH functions to activate Nrf2 as hormesis-like.
Keywords
Hematin, hydroxyl radical, molecular hydrogen, Nrf2, oxidative stress, porphyrin
Introduction
Molecular hydrogen (dihydrogen; H2) is an inert molecule
in the absence of a catalyst. It has long been believed
that H2 has no biological function in mammalian cells
because mammals lack the genes encoding hydrogenases
that catalyze reactions involving H2 [1, 2]. In 2007, this
concept was overturned by publishing the article entitled
“Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals” [3]. This paper
served as a trigger for the initiation of a new field of “hydrogen medicine and agriculture” [4, 5]. Subsequently, in
addition to its antioxidant action, H2 has been revealed to
exert multiple functions such as anti-inflammatory, antiallergic, anti-cell death, and metabolic stimulating effects
by modulating various intracellular signal transductions [5, 6]. H2 has no adverse effects, leading to extensive clinical
studies for various diseases [5, 6]. In addition, H2 not only
improves patients with various diseases, but also supports
the quality of life (QOL) of healthy people in various
fields such as healthcare, sports, and beauty [5]. In 2014,
the US Food Drug Administration (FDA) approved H2 as
generally recognized as safe (GRAS), allowing hydrogeninfused water to be marketed as a drink. In 2016, H2 gas inhalation therapy was approved by the Japanese government as an advanced treatment for post-cardiac arrest
syndrome [7]. Furthermore, H2 is beneficial not only to
animals and humans but also to higher plants. Therefore,
H2 can have a strong impact on agriculture [8]. Oxidative stress is derived by excessive generation of
reactive oxygen species (ROS) such as superoxide anion
radical (•O2-), hydrogen peroxide (H2O2), nitric oxide
(NO), and hydroxyl radical (•OH) [9-10]. As neurons are
post-mitotic cells, neurons accumulate oxidative damage
over many years. However, ROS such as H2O2 and •O2-
and NO play important physiological roles in signaling
cascades and biological processes such as cell proliferation, differentiation, apoptosis, and immunomodulation
[11-14], and thus, excessive antioxidant intake is not beneficial and induces mortality as published [15, 16]. Aging is associated with an increased incidence of neurodegenerative disorders. This is because neurons accumulate oxidative damage due to post-mitotic cells over a long
period [30]. Oxidative stress is one of the leading causes
of most neurodegenerative disorders [31, 32]. Several
animal studies indicate that H2-treatment is potentially
applicable to alleviate neurodegenerative disorders and
improve the quality of life in the elderly [33-37]. Thus, H2
is expected to ameliorate aging-related neurodegeneration.
In particular, overcoming Alzheimer’s disease (AD) is one
of the most important challenges in the world’s aging society [38]. Nrf2 transcribes the genes encoding several antioxidant
enzymes to protect cells against oxidative stress [50-51].
Moreover, Nrf2 contributes not only to the reduction of oxidative stress, but also to widespread fields, including
toxicology [52], oncology [53], inflammation [54], ischemia stroke [55], and the aging process [56]. Nrf2’s targets
are the genes encoding NAD (P) H quinone oxidoreductase 1 (Nqo1), thioredoxin, reductase 1 (TXNRD1), heme
oxygenase-1 (HO-1), glutathione S-transferase (GST),
and so on [50-51]. Nrf2 is maintained in an inactive form
in the cytosol when it forms a complex with the Kelchlike ECH-associated protein 1 (Keap1). Upon oxidation of
the essential cysteine residues of Keap1 by electrophiles,
Nrf2 is released from Keap1 and then translocated into the
nucleus, enabling the transcriptions [50-51]. A break-through paper entitled “Fe-porphyrins: redox-related biosensors of molecular hydrogen” has recently been
published [74], showing that the molecular target/biosensor for H2 is the oxidized form of Fe-porphyrins (designate
“hematin”). This paper has shown the discovery that addresses the fundamental questions about the mechanisms
in which H2 is involved. Figure 1. Fe-porphyrin catalyzes the reaction of H2 with the hydroxyl
radical.
However, the molecular mechanism by which H2 exerts
multiple functions remained unclear. The current Therapeutic Brief will discuss and propose a molecular mechanism by which H2 with the hydrogen-targeted porphyrin
activates the transcription factor, nuclear factor erythroid
2-related factor 2 (Nrf2) to alleviate oxidative stress, suggesting a hormesis-like effect.
H2 selectively reacts with hydroxyl radicals in
living cells
Molecular hydrogen (H2) selectively reduces highly toxic
ROS, •OH and peroxynitrite (ONOO-), but neither •O2-
, H2O2, nor NO [3]. In cell culture experiments, H2 decreased the fluorescence signal of 3’-p-(hydroxyphenyl)
fluorescein (HPF) when oxidative stress was induced in
various ways [3]. HPF is an intracellular marker for •OH
[17, 18]. Decrease in this fluorescent signal by H2 was not
only observed in cultured cells, but also in various tissues
as shown in testicular radioprotection [19], hematopoietic
stem cell damage by total body irradiation [20], and hyperoxia in cultured cells [21], lung hypoxia/reoxygenation
[22], retinal ischemia-reperfusion [23], and retinal sonication [24].
H2 can be infused into water (hydrogen water) up to a
maximum of 0.8 mM at atmospheric pressure. After drinking H2 water or inhaling H2 gas, measuring the H2 content
revealed that H2 is consumed in the human body [25, 26].
Deuterium gas (D2) was used in rats as a metabolic tracer
to monitor D2 oxidation, indicating that molecular hydrogen is indeed oxidized in vivo [27].
Thus, H2 was confirmed to decrease cellular •OH in a variety of ways across cell types and tissues although •OH
is the most oxidative molecule to damage the cell components in a chaotic manner [10].
By the way, in homogeneous aqueous kinetics, the reaction rate of •OH with H2 is much slower (the kinetic rate is
0.35 × 10-8 M-1s
-1) than those with other antioxidants [28].
For example, •OH reacts with glucose and glutathione
with kinetic rates (15 × 10-8 M-1s
-1), and (230 × 10-8 M-1s
-1
), respectively [29]. The other biomolecules also react
with •OH much faster than H2. The contradiction between
homogeneous aqueous solutions and living organisms has
been debated for a long time.
Although H2 cannot react with most molecules without
a metal catalyst, effective amounts of metals such as Cu,
Fe, Ni, and Pt are unlikely to be present in living cells.
In addition, there is no report indicating the discovery of
an organo-catalyst for H2. Despite extensive worldwide
research, it was hard to discover a catalyst that facilitates
the reaction of H2 with •OH. An H2-target molecule as
described below has recently been identified, providing a
clue to explain the underlying contradiction of H2.Aging is associated with an increased incidence
of neurodegenerative diseases
It has been shown that drinking H2 water reduces oxidative stress and ameliorated cognitive deficits in AD model
mice [39]. Subsequently, a randomized, placebo-controlled, double-blind clinical trial was conducted on subjects with mild cognitive impairment (MCI), who drank
0.6 mM H2 water (approximately 300 mL per day) for 1
year [39]. A sub-analysis showed that subjects with the
apolipoprotein E (APOE4) genotype, a well-known genetic factor for AD [40, 41], were significantly improved.
Improvement was assessed by the Alzheimer’s Disease
Assessment Scale-Cognitive Subscale (ADAS-cog), one
of the most reliable ways to assess cognition [42, 43].
H2 inhalation has been applied in several clinical areas [5,
7, 44-46]. The most important feature of H2 gas inhalation
therapy is that it is non-cytotoxic and safe for humans, as
approved in Phase I clinical trial [47].
A patient with severe Alzheimer’s continued to inhale 3%
hydrogen gas twice for one hour a day for two years. Diffusion tensor imaging (DTI) [48, 49] then visualized the
activation of neurons of the patient, and urinary and fecal
incontinence was improved [38]. This case report is of
value even for a single case, as it is commonly understood
that patients with severe AD are irreversible [38].H2 activates Nrf2 to function to reduce oxidative stress
H2 can induce the activity of Nrf2, as shown in many publications. In Nrf2 knockout mice, the effects of H2 were
at least partially attenuated, in protecting various cells
and tissues in response to various stressors [57]. These
findings are consistent with subsequent publications that
Nrf2-activation is one of the antioxidant effects of H2 [58-
69]. Therefore, it is concluded that the activation of Nrf2
is involved in one of the H2 functions.
As a molecular mechanism, it is unlikely that H2 directly
influences Nrf2. H2 must indirectly activate Nrf2 through
multiple steps. One idea was proposed that H2 enhances
mitochondrial respiratory activity to generate excess ROS,
which in turn oxidizes intracellular Keap1 to release Nrf2
[70]. Alternatively, H2 opens mitochondria-(ATP) K+
channels [71, 72] to induce ROS [73]. However, although
there is no doubt that H2 activates Nrf2, there is no direct
evidence that mitochondria-derived ROS can oxidize the
residues of cytosolic Keap1. Moreover, an elusive contradiction exists between Nrf2 activation and H2; activation
of Nrf2 requires Keap1 oxidation, whereas H2 has a reducing potential.Target discovery of hydrogen molecules
Hematin is an oxidized form of Fe(III)-containing porphyrin (PrP) converted from Fe(II)-containing porphyrin
(heme) [75, 76]. This breakthrough paper showed a novel
reaction showing that H2 replaces the hydroxy group (-OH)
conjugated to hematin Fe(III) with the hydrogen group
(-H). The atom H of this -H group should behave as a hydride (H-) and, due to its high reactivity, •OH was rapidly
converted to H2O by this catalyst (Figure 1).
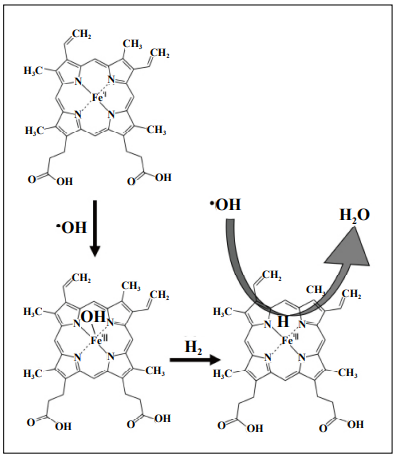
(Equation 1) PrP-Fe(II) + •OH → PrP-Fe(III)-OH
(Equation 2) PrP-Fe(III)-OH + H2 → PrP-Fe(III)-H + H2O
(Equation 3) PrP-Fe(III)-H + •OH → PrP-Fe(II) + H2O
The formal name of Hematin PrP-Fe(III)-OH is ferriprotoporphyrin IX
hydroxide.
(1) PrP-Fe(II) + •OH → PrP-Fe(III)-OH
(2) PrP-Fe(III)-OH + H2 → PrP-Fe(III)-H + H2O
(3) PrP-Fe(III)-H + •OH → PrP-Fe(II) + H2O
The overall equation (4) indicates that heme (PrP-Fe(II))
catalyzed the following reactions:
(4) 2 •OH + H2 → 2H2O
As noted above, the unresolved discrepancy between
aqueous and live-cell reactions can be explained by the
catalytic reaction according to the above equations (2) and
(3).
At the same time, H2 can reduce the oxidized porphyrin
with Fe(III) to restore heme, the reduced form of Fe(II).
Proposal of a mechanism to elucidate the mechanism by which reducing H2 activates Nrf2
Porphyrins are distributed everywhere inside and outside
the living cells in the body. Heme is present in hemoglobin
in the blood and myoglobin in muscles and is responsible for delivering molecular oxygen (O2) throughout the body
[77]. Thus, heme is frequently exposed to O2 or H2O2, and
thus, Fe(II) of heme can frequently catalyze the formation
of •OH by the Fenton reaction or its mimic reactions [78-80]. Porphyrins are located as cytochromes in the electron
transport chain of the mitochondrial inner membrane, and
play a role in electron transport by converting Fe(II) to/
from Fe(III) [81]. In the intracellular cytosol, the antioxidant enzymes such as catalase [82] and peroxidase [83],
P450 [84], and nitric oxide (NO) synthase [85] have porphyrins as an essential component [86]. These porphyrins with Fe(II)/(III) act as mediators of redox reactions and
are subject to oxidative stress.
Hematin (PrP-Fe(III)-OH) is converted from hemin (PrPFe(III)-Cl) [87, 88]. Notably, hemin (PrP-Fe(III)-Cl)
activate Nrf2 [89-91] (Figure 2). The Fe(III) of hemin
probably functions as an electrophile, oxidizing the residues of Keap1 and activating Nrf2. The electronegativities
of H and Cl are 2.2 and 3.16, respectively. Thus, Fe(III)
containing H should be more electrophilic than Fe(III)
containing Cl, and may be able to oxidize Keap1 more
efficiently according to the equation of PrP-Fe(III)-H + e-
→PrP-Fe(II) + 1/2 H2 (Figure 2).
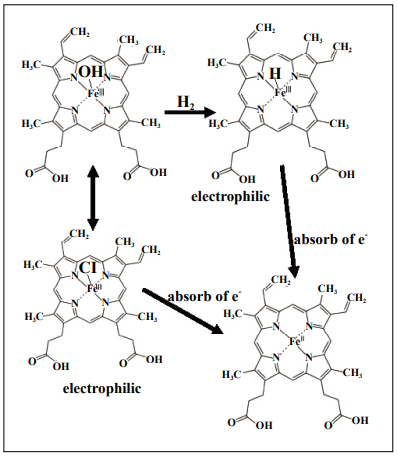
Figure 2. Fe(III) in hydride hematin can serve as an electrophile to
oxidize the residues of Keap1.
Hematin and hemin can mutually be converted, and hemin is known to
activate Nrf2 by oxidizing Keap1.
Fe(III) of hemin is electrophilic to activate Nrf2. Fe(III)-H may be more
electrophilic than Fe(III)-Cl to efficiently oxidize Keap1, resulting in
activating Nrf2.
The formal name of Hemin PrP-Fe(III) is ferriprotoporphyrin IX
chloride.
As mentioned in the above equation (1) PrP-Fe(II) + •OH
→ PrP-Fe(III)-OH, hematin (PrP-Fe(III)-OH) was originally formed by oxidizing heme (PrP-Fe(II)) by •OH. •OH
is the most oxidative molecule to damage biomolecules
indiscriminately [10], but the strong electrophilicity of
•OH can be buffered in the presence of H2 and Fe-PrP, and
resultant electrophilicity in Fe-PrP can contribute to activating Nrf2, resulting in reducing oxidative stress.
Hormesis is a term used by toxicologists to refer to a biphasic dose response to an environmental agent characterized by a low-dose stimulation or beneficial effect and a
high-dose inhibitory or toxic effect [92] or defined as the
paradoxical beneficial effects of low-dose stressors, which
can be better defined as the biphasic dose-effect or timeeffect relationship for any substance [93].
•OH is the most oxidative molecule that caused damage to
biomolecules [10], but, the strong electrophilicity of •OH
can be alleviated through stepwise reactions in the presence of H2 and porphyrin.
Lowering the concentration of a toxic substance is reducing its toxicity. It is proposed that even without lowering
the dosage, the effect of alleviated strong potent is considered to be hormesis-like.
Once the original strong electrophilicity of •OH is transferred to PrP-Fe(III)-OH and PrP-Fe(III)-H, it is possible
that the alleviated oxidative potent contributes to the activation of Nrf2 as a hormesis-like effect.
The current proposal needs to be examined in more detail
in the future.
Conclusion
H2 acts as a therapeutic antioxidant [3] and activates Nrf2, which transcribes antioxidant enzymes to reduce oxidative stress and protected cells against various stresses. There was an unresolved contradiction between H2’s reductive property and Nrf2’s requirement of oxidative stress for its activation. The target molecule for H2 has recently been identified as the oxidized form of Fe-porphyrin conjugated with the OH group (PrP-Fe(III)-OH) [74]. H2 and the H2-targeting porphyrin can buffer the highly oxidizing electrophilicity of •OH. When the original •OH’s oxidative and harmful electrophilicity is alleviated, the resultant electrophilic potent may contribute to the activation of Nrf2 as a hormesis-like effect.
Declarations
Authors’ contributions
The authors contributed equally to the article.
Availability of data and materials
Not applicable.
Conflicts of interest
The author is the director of H2 WATER JAPAN, Inc., (Tokyo, Japan) and H2 Global Group (Ostrava, Czech Republic), companies involved in H2.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
1. Yagi T, & Higuchi Y. Studies on hydrogenase. Proc Jpn Acad Ser B Phys Biol Sci, 2013, 89(1): 16-33. [Crossref]
2. Hansen M, & Perner M. Hydrogenase Gene Distribution and H2 Consumption Ability within the Thiomicrospira Lineage. Front Microbiol, 2016, 7: 99. [Crossref]
3. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med, 2007, 13(6): 688-694. [Crossref]
4. Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther, 2014, 144(1): 1-11. [Crossref]
5. Ohta S. Development of Hydrogen Medicine and Biology: Potential for Various Applications in Diverse Fields. Curr Pharm Des, 2021, 27(5): 583-584. [Crossref]
6. Nicolson GL, de Mattos GF, Settineri R, Costa C, Ellithorpe R, Rosenblatt S, et al. Clinical Effects of Hydrogen Administration: From Animal and Human Diseases to Exercise Medicine. International Journal of Clinical Medicine, 2016, 07(01): 32-76. [Crossref]
7. Katsumata Y, Sano F, Abe T, Tamura T, Fujisawa T, Shiraishi Y, et al. The Effects of Hydrogen Gas Inhalation on Adverse Left Ventricular Remodeling After Percutaneous Coronary Intervention for ST-Elevated Myocardial Infarction - First Pilot Study in Humans. Circ J, 2017, 81(7): 940-947. [Crossref]
8. Li C, Gong T, Bian B, & Liao W. Roles of hydrogen gas in plants: a review. Funct Plant Biol, 2018, 45(8): 783-792. [Crossref]
9. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol, 2003, 552(Pt 2): 335-344. [Crossref]
10. Chou DS, Lee JJ, Hsiao G, Hsieh CY, Tsai YJ, Chen TF, et al. Baicalein induction of hydroxyl radical formation via 12-lipoxygenase in human platelets: an ESR study. J Agric Food Chem, 2007, 55(3): 649-655. [Crossref]
11. Josephson RA, Silverman HS, Lakatta EG, Stern MD, & Zweier JL. Study of the mechanisms of hydrogen peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem, 1991, 266(4): 2354-2361.
12. Papa S, & Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem, 1997, 174(1-2): 305-319.
13. Sauer H, Wartenberg M, & Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem, 2001, 11(4): 173-186. [Crossref]
14. Thomas DT, DelCimmuto NR, Flack KD, Stec DE, & Hinds TD, Jr. Reactive Oxygen Species (ROS) and Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin. Antioxidants (Basel), 2022, 11(2). [Crossref]
15. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, & Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA, 2007, 297(8): 842-857. [Crossref]
16. Hackam DG. Review: antioxidant supplements for primary and secondary prevention do not decrease mortality. ACP J Club, 2007, 147(1): 4.
17. Setsukinai K, Urano Y, Kakinuma K, Majima HJ, & Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem, 2003, 278(5): 3170-3175. [Crossref]
18. Tomizawa S, Imai H, Tsukada S, Simizu T, Honda F, Nakamura M, et al. The detection and quantification of highly reactive oxygen species using the novel HPF fluorescence probe in a rat model of focal cerebral ischemia. Neurosci Res, 2005, 53(3): 304-313. [Crossref]
19. Chuai Y, Shen J, Qian L, Wang Y, Huang Y, Gao F, et al. Hydrogen-rich saline protects spermatogenesis and hematopoiesis in irradiated BALB/c mice. Med Sci Monit, 2012, 18(3): Br89-94. [Crossref]
20. Chuai Y, Qian L, Sun X, & Cai J. Molecular hydrogen and radiation protection. Free Radic Res, 2012, 46(9): 1061- 1067. [Crossref]
21. Yu J, Yu Q, Liu Y, Zhang R, & Xue L. Hydrogen gas alleviates oxygen toxicity by reducing hydroxyl radical levels in PC12 cells. PLoS One, 2017, 12(3): e0173645. [Crossref]
22. Chen M, Zhang J, Chen Y, Qiu Y, Luo Z, Zhao S, et al. Hydrogen protects lung from hypoxia/re-oxygenation injury by reducing hydroxyl radical production and inhibiting inflammatory responses. Sci Rep, 2018, 8(1): 8004. [Crossref]
23. Oharazawa H, Igarashi T, Yokota T, Fujii H, Suzuki H, Machide M, et al. Protection of the retina by rapid diffusion of hydrogen: administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci, 2010, 51(1): 487-492. [Crossref]
24. Igarashi T, Ohsawa I, Kobayashi M, Igarashi T, Suzuki H, Iketani M, et al. Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci Rep, 2016, 6: 31190. [Crossref]
25. Shimouchi A, Nose K, Shirai M, & Kondo T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv Exp Med Biol, 2012, 737: 245-250. [Crossref]
26. Shimouchi A, Nose K, Mizukami T, Che DC, & Shirai M. Molecular hydrogen consumption in the human body during the inhalation of hydrogen gas. Adv Exp Med Biol, 2013, 789: 315-321. [Crossref]
27. Hyspler R, Ticha A, Schierbeek H, Galkin A, & Zadak Z. The Evaluation and Quantitation of Dihydrogen Metabolism Using Deuterium Isotope in Rats. PLoS One, 2015, 10(6): e0130687. [Crossref]
28. Buxton GV, Greenstock, C.L., Helman, W.P., Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−) in aqueous solution. J Phys Chem Ref Data, 1988, 17(2): 513-886. [Crossref]
29. Wood KC, & Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med, 2007, 13(6): 673-674. [Crossref]
30. Espinós C, Galindo MI, García-Gimeno MA, IbáñezCabellos JS, Martínez-Rubio D, Millán JM, et al. Oxidative Stress, a Crossroad Between Rare Diseases and Neurodegeneration. Antioxidants (Basel), 2020, 9(4). [Crossref]
31. Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, & Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci, 2012, 322(1-2): 254-262. [Crossref]
32. Chalke SD, & Kale PP. Combinational Approaches Targeting Neurodegeneration, Oxidative Stress, and Inflammation in the Treatment of Diabetic Retinopathy. Curr Drug Targets, 2021, 22(16): 1810-1824. [Crossref]
33. Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, & Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology, 2009, 34(2): 501-508. [Crossref]
34. Li J, Wang C, Zhang JH, Cai JM, Cao YP, & Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res, 2010, 1328: 152-161. [Crossref]
35. Tan X, Shen F, Dong WL, Yang Y, & Chen G. The role of hydrogen in Alzheimer’s disease. Med Gas Res, 2018, 8(4): 176-180. [Crossref]
36. Zheng ZG, Sun WZ, Hu JY, Jie ZJ, Xu JF, Cao J, et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir Res, 2021, 22(1): 149. [Crossref]
37. Lin YT, Shi QQ, Zhang L, Yue CP, He ZJ, Li XX, et al. Hydrogen-rich water ameliorates neuropathological impairments in a mouse model of Alzheimer’s disease through reducing neuroinflammation and modulating intestinal microbiota. Neural Regen Res, 2022, 17(2): 409-417. [Crossref]
38. Ono H, Nishijima Y, Sakamoto M, Kitamura S, Naitoh Y, Suzuki K, et al. Long-Term Inhalation of Hydrogen Gas for Patients with Advanced Alzheimer’s Disease: A Case Report Showing Improvement in Fecal Incontinence. Med Res Arch, 2022, 10(7). [Crossref]
39. Nishimaki K, Asada T, Ohsawa I, Nakajima E, Ikejima C, Yokota T, et al. Effects of Molecular Hydrogen Assessed by an Animal Model and a Randomized Clinical Study on Mild Cognitive Impairment. Curr Alzheimer Res, 2018, 15(5): 482-492. [Crossref]
40. Bookheimer S, & Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer’s and cognitive aging. Annu Rev Clin Psychol, 2009, 5: 343-362. [Crossref]
41. Uddin MS, Kabir MT, Al Mamun A, Abdel-Daim MM, Barreto GE, & Ashraf GM. APOE and Alzheimer’s Disease: Evidence Mounts that Targeting APOE4 may Combat Alzheimer’s Pathogenesis. Mol Neurobiol, 2019, 56(4): 2450-2465. [Crossref]
42. Grossberg GT, Schmitt FA, Meng X, Tekin S, & Olin J. Reviews: Effects of transdermal rivastigmine on ADAS-cog items in mild-to-moderate Alzheimer’s disease. Am J Alzheimers Dis Other Demen, 2010, 25(8): 627-633. [Crossref]
43. Kueper JK, Speechley M, & Montero-Odasso M. The Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog): Modifications and Responsiveness in PreDementia Populations. A Narrative Review. J Alzheimers Dis, 2018, 63(2): 423-444. [Crossref]
44. Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen Gas Inhalation Treatment in Acute Cerebral Infarction: A Randomized Controlled Clinical Study on Safety and Neuroprotection. J Stroke Cerebrovasc Dis, 2017, 26(11): 2587-2594. [Crossref]
45. Botek M, Krejčí J, Valenta M, McKune A, Sládečková B, Konečný P, et al. Molecular Hydrogen Positively Affects Physical and Respiratory Function in Acute Post-COVID-19 Patients: A New Perspective in Rehabilitation. Int J Environ Res Public Health, 2022, 19(4). [Crossref]
46. Pluta R, Januszewski S, & Czuczwar SJ. Molecular Hydrogen Neuroprotection in Post-Ischemic Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Underlying Mechanisms and Potential for Clinical Implementation-Fantasy or Reality? Int J Mol Sci, 2022, 23(12) . [Crossref]
47. Cole AR, Sperotto F, DiNardo JA, Carlisle S, Rivkin MJ, Sleeper LA, et al. Safety of Prolonged Inhalation of Hydrogen Gas in Air in Healthy Adults. Crit Care Explor, 2021, 3(10): e543. [Crossref]
48. Toniolo S, Serra L, Olivito G, Caltagirone C, Mercuri NB, Marra C, et al. Cerebellar White Matter Disruption in Alzheimer’s Disease Patients: A Diffusion Tensor Imaging Study. J Alzheimers Dis, 2020, 74(2): 615-624. [Crossref]
49. Torso M, Bozzali M, Zamboni G, Jenkinson M, & Chance SA. Detection of Alzheimer’s Disease using cortical diffusion tensor imaging. Hum Brain Mapp, 2021, 42(4): 967- 977. [Crossref]
50. Baird L, & Yamamoto M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol Cell Biol, 2020, 40(13). [Crossref]
51. Bellezza I, Giambanco I, Minelli A, & Donato R. Nrf2- Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res, 2018, 1865(5): 721-733. [Crossref]
52. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol, 2013, 53: 401-426. [Crossref]
53. Taguchi K, & Yamamoto M. The KEAP1-NRF2 System as a Molecular Target of Cancer Treatment. Cancers (Basel), 2020, 13(1). [Crossref]
54. Saha S, Buttari B, Panieri E, Profumo E, & Saso L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules, 2020, 25(22). [Crossref]
55. Farina M, Vieira LE, Buttari B, Profumo E, & Saso L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules, 2021, 26(16). [Crossref]
56. Yu C, & Xiao JH. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid Med Cell Longev, 2021, 2021: 6635460. [Crossref]
57. Kawamura T, Wakabayashi N, Shigemura N, Huang CS, Masutani K, Tanaka Y, et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol, 2013, 304(10): L646-656. [Crossref]
58. Zhai X, Chen X, Shi J, Shi D, Ye Z, Liu W, et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic Biol Med, 2013, 65: 731-741. [Crossref]
59. Chen H, Xie K, Han H, Li Y, Liu L, Yang T, et al. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol, 2015, 28(1): 643-654. [Crossref]
60. Yu J, Zhang W, Zhang R, Jiang G, Tang H, Ruan X, et al. Molecular hydrogen attenuates hypoxia/reoxygenation injury of intrahepatic cholangiocytes by activating Nrf2 expression. Toxicol Lett, 2015, 238(3): 11-19. [Crossref]
61. Tamaki N, Orihuela-Campos RC, Fukui M, & Ito HO. Hydrogen-Rich Water Intake Accelerates Oral Palatal Wound Healing via Activation of the Nrf2/Antioxidant Defense Pathways in a Rat Model. Oxid Med Cell Longev, 2016, 2016: 5679040. [Crossref]
62. Diao M, Zhang S, Wu L, Huan L, Huang F, Cui Y, et al. Hydrogen Gas Inhalation Attenuates Seawater InstillationInduced Acute Lung Injury via the Nrf2 Pathway in Rabbits. Inflammation, 2016, 39(6): 2029-2039. [Crossref]
63. Liu Y, Dong F, Guo R, Zhang Y, Qu X, Wu X, et al. Hydrogen-Rich Saline Ameliorates Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice Via the Nrf2-ARE Signaling Pathway. Inflammation, 2019, 42(2): 586-597. [Crossref]
64. Yu Y, Yang Y, Yang M, Wang C, Xie K, & Yu Y. Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/HO-1-dependent pathway. Int Immunopharmacol, 2019, 69: 11-18. [Crossref]
65. Lu Y, Li CF, Ping NN, Sun YY, Wang Z, Zhao GX, et al. Hydrogen-rich water alleviates cyclosporine A-induced nephrotoxicity via the Keap1/Nrf2 signaling pathway. J Biochem Mol Toxicol, 2020, 34(5): e22467. [Crossref]
66. Xie K, Zhang Y, Wang Y, Meng X, Wang Y, Yu Y, et al. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm Res, 2020, 69(7): 697-710. [Crossref]
67. Yu Y, Feng J, Lian N, Yang M, Xie K, Wang G, et al. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. Int Immunopharmacol, 2020, 85: 106585. [Crossref]
68. Hu Y, Wang P, & Han K. Hydrogen Attenuated Inflammation Response and Oxidative in Hypoxic Ischemic Encephalopathy via Nrf2 Mediated the Inhibition of NLRP3 and NF-κB. Neuroscience, 2022, 485: 23-36. [Crossref]
69. Peng J, He Q, Li S, Liu T, & Zhang J. Hydrogen-Rich Water Mitigates LPS-Induced Chronic Intestinal Inflammatory Response in Rats via Nrf-2 and NF-κB Signaling Pathways. Vet Sci, 2022, 9(11). [Crossref]
70. Murakami Y, Ito M, & Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One, 2017, 12(5): e0176992. [Crossref]
71. Yoshida A, Asanuma H, Sasaki H, Sanada S, Yamazaki S, Asano Y, et al. H(2) mediates cardioprotection via involvements of K(ATP) channels and permeability transition pores of mitochondria in dogs. Cardiovasc Drugs Ther, 2012, 26(3): 217-226. [Crossref]
72. Jiao Y, Yu Y, Li B, Gu X, Xie K, Wang G, et al. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuropathy via activation of the mitochondrial ATP‑sensitive potassium channel channels in rats. Mol Med Rep, 2020, 21(1): 282-290. [Crossref]
73. Andrukhiv A, Costa AD, West IC, & Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol, 2006, 291(5): H2067-2074. [Crossref]
74. Jin Z, Zhao P, Gong W, Ding W, He Q. Fe-porphyrin: A redox-related biosensor of hydrogen molecule. Nano Research, 2022, 16 (2): 2020-2025. [Crossref]
75. Green D, & Ts’ao CH. Hematin: effects on hemostasis. J Lab Clin Med, 1990, 115(2): 144-147.
76. Siegert SW, & Holt RJ. Physicochemical properties, pharmacokinetics, and pharmacodynamics of intravenous hematin: a literature review. Adv Ther, 2008, 25(9): 842- 857. [Crossref]
77. Gunsalus IC, Sligar SG, Nordlund T, Frauenfelder H. Oxygen sensing heme proteins: monoxygenases, myoglobin and hemoglobin. Adv Exp Med Bio, 1977, 78: 37-50. [Crossref]
78. Nagababu E, & Rifkind JM. Heme degradation by reactive oxygen species. Antioxid Redox Signal, 2004, 6(6): 967- 978. [Crossref]
79. Liu Y, Zhao Y, & Wang J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J Hazard Mater, 2021, 404(Pt B): 124191. [Crossref]
80. Liochev SL. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic Res, 1996, 25(5): 369-384. [Crossref]
81. Piel RB, 3rd, Dailey HA, Jr., & Medlock AE. The mitochondrial heme metabolon: Insights into the complex(ity) of heme synthesis and distribution. Mol Genet Metab, 2019, 128(3): 198-203. [Crossref]
82. Umezawa N, Matsumoto N, Iwama S, Kato N, & Higuchi T. Facile synthesis of peptide-porphyrin conjugates: Towards artificial catalase. Bioorg Med Chem, 2010, 18(17): 6340-6350. [Crossref]
83. Damos FS, Sotomayor Mdel P, Kubota LT, Tanaka SM, & Tanaka AA. Iron(III) tetra-(N-methyl-4-pyridyl)-porphyrin as a biomimetic catalyst of horseradish peroxidase on the electrode surface: an amperometric sensor for phenolic compound determinations. Analyst, 2003, 128(3): 255-259. [Crossref]
84. Watanabe Y. Alternatives to the oxoferryl porphyrin cation radical as the proposed reactive intermediate of cytochrome P450: two-electron oxidized Fe(III) porphyrin derivatives. J Biol Inorg Chem, 2001, 6(8): 846-856. [Crossref]
85. Pfeiffer S, Schrammel A, Koesling D, Schmidt K, & Mayer B. Molecular actions of a Mn(III)Porphyrin superoxide dismutase mimetic and peroxynitrite scavenger: reaction with nitric oxide and direct inhibition of NO synthase and soluble guanylyl cyclase. Mol Pharmacol, 1998, 53(4): 795-800. [Crossref]
86. Di Venosa G, Batlle A, Fukuda H, Macrobert A, & Casas A. Distribution of 5-aminolevulinic acid derivatives and induced porphyrin kinetics in mice tissues. Cancer Chemother Pharmacol, 2006, 58(4): 478-486. [Crossref]
87. Bohle DS, & Helms JB. Synthesis of beta-hematin by dehydrohalogenation of hemin. Biochem Biophys Res Commun, 1993, 193(2): 504-508. [Crossref]
88. Grenoble DC, & Drickamer HG. The effect of pressure on the oxidation state of iron. 3. Hemin and hematin. Proc Natl Acad Sci U S A, 1968, 61(4): 1177-1182. [Crossref]
89. Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, & Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem, 2001, 276(21): 18399-18406. [Crossref]
90. Nagai T, Kikuchi S, Ohmine K, Miyoshi T, Nakamura M, Kondo T, et al. Hemin reduces cellular sensitivity to imatinib and anthracyclins via Nrf2. J Cell Biochem, 2008, 104(2): 680-691. [Crossref]
91. Georgiou-Siafis SK, & Tsiftsoglou AS. Activation of KEAP1/NRF2 stress signaling involved in the molecular basis of hemin-induced cytotoxicity in human proerythroid K562 cells. Biochem Pharmacol, 2020, 175: 113900. [Crossref]
92. Mattson MP. Hormesis defined. Ageing Res Rev, 2008, 7(1): 1-7. [Crossref]
93. Li X, Yang T, & Sun Z. Hormesis in Health and Chronic Diseases. Trends Endocrinol Metab, 2019, 30(12): 944- 958. [Crossref]