Open Access | Review
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Senescence of mesenchymal stem cells: implications in extracel- lular vesicles, miRNAs and their functional and therapeutic po- tentials
* Corresponding author: Guangqian Zhou
Mailing address: Department of Medical Cell Biology and Genetics, Shenzhen University, 1066 Xueyuan Avenue, Shenzhen
518060, China.
Email: gqzhou@szu.edu.cn
This article belongs to the Special Issue: Age-linked Stem Cell-Mediated dysfunction
Received: 29 November 2022 / Revised: 31 January 2023 / Accepted: 16 February 2023 / Published: 29 March 2023
DOI: 10.31491/APT.2023.03.107
Abstract
Senescence is seen as the cellular counterpart of tissue and biological aging, with irreversible stagnation of cell growth, and changes in function and behavior. Mesenchymal stem cells (MSCs) are one of the key therapeutic tools in regenerative medicine, and their regenerative and therapeutic potential declines significantly with the increasing age of cell donors and prolonged continuous culture in vitro. MicroRNAs (miRNAs) are regarded as important players in regulating the expression and function of multiple genes and pathways. Emerging evidence suggests that extracellular vesicles (EVs) participate in a complex cell senescence network, at least partially by providing certain miRNAs. Therefore, MSC EVs and miRNAs are implicated in not only contributing to but also influenced by MSC senescence. Here, we will provide an overview of the recent results on roles and mechanisms of miRNAs, particularly EV-miRNAs, involved in MSC senescence, and discuss their implications in functional properties and therapeutic efficacy of MSCs and their EVs.
Keywords
Extracellular vesicles, microRNAs, mesenchymal stem cells, senescence
Introduction
Stem cells offer the foundation of regenerative medicine.
Based on the plasticity of stem cells, in vitro and in vivo
induction or gene modification methods can make them
transdifferentiate into therapeutic cells to achieve therapeutic purposes. Mesenchymal stem cells (MSCs) are
one of the most accepted therapeutic cells in regenerative medicine and tissue engineering [1]. MSCs can be
obtained from a rather wide range of adult tissues (e.g.,
muscles, bone marrow (BM), and adipose tissue) and
neonatal tissues (e.g., umbilical cord (UC), placenta, and
amnion), and amplified by in vitro expansion [2], easily
reaching the manufacturing levels. MSCs have the potential for self-renewal and multi-lineage differentiation and
exert pro-angiogenesis, pro-proliferation, anti-apoptotic,
anti-fibrosis, and anti-inflammatory functions through
the interaction between cells and the secretion of many
soluble factors [3].
Cellular senescence refers to the irreversible stagnation of
cell growth under the action of various stress factors and
may be important to prevent the proliferation of damaged
cells and acts as a barrier to tumor lesions [4]. However,
cells that undergo permanent proliferation arrest may be
detrimental to the entire individual, and senescent cells are
present in aging tissues and accumulate in an age-dependent manner that accelerates the decline of tissue function
and contributes to the development of age-related diseases
[5]. The regenerative and therapeutic potential of MSCs
decreased significantly with the increasing age of the cell
donor. In cell-based therapy and tissue engineering, MSCs
require prolonged and large-scale in vitro manufacture, in
which continuous expansion may lead to replicative senescence [1], likely constraining the manufacturing quantity in return. Senescent MSCs usually show decreased
regenerative ability, reduced differentiation ability, and
weakened immune-regulatory functions, and thus possibly
fail to achieve optimal therapeutic outcomes. In order to
manufacture the highest quantity of MSCs with optimal
functional properties, there is an urgency to develop technologies to easily assess and delay the replicative senescence of MSCs.
MicroRNAs (miRNAs) are a class of special small RNAs
composed of about 22 nucleotides that selectively bind
to the 3’-untranslated region (3’-UTR) of the mRNA sequence and regulating the translation and stability of the
targeted mRNA, thus altering gene expression without
changing the genetic code [6, 7]. MiRNAs are important
regulators of senescence-related gene expression. Most
miRNAs that regulate stem cell senescence have been
shown in MSCs and hematopoietic stem cells (HSCs) by
targeting genes associated with metabolism, epigenetics,
and DNA damage [8, 9]. Extracellular vesicles (EVs) are
heterogeneous vesicles induced by stimuli such as cell differentiation, activation, senescence, and transformation.
They are formed by lipid bilayer membranes and contain
proteins, nucleic acids, lipids, and their derivatives. EVs
are an important participant in cell-to-cell communication
and can precisely regulate receptor cell senescence and
inflammation under various physiological and pathological conditions [10, 11]. It has been reported that miRNAs
released in the extracellular environment by cell-secreted
EVs can influence the senescence of surrounding cells.
In this review, we will focus on the latest advances in the
regulatory role of miRNAs, especially those in EVs, in
MSC senescence, and their application potentials.
MSC senescence
Although MSCs originate from the mesoderm, they can
differentiate into mesodermal tissues (e.g., adipose, bone,
cartilage, and hematopoietic tissues) and non-mesodermal
tissues (e.g., neurons and glial cells) [12, 13]. Due to their
self-renewal, multipotent differentiation, and immunomodulatory properties, MSCs are considered ideal candidates to replace damaged or lost cells and tissues in vivo.
Thus far, MSCs are widely used for regenerative medicine
and tissue engineering and are currently the focus of over
thousands of clinical trials, showing significant therapeutic capacity in a broad range of diseases, such as pulmonary fibrosis [14], myocardial infarction [15], and diabetes
mellitus [16].
Senescence is a physiological process of organisms and
is associated with a decline in MSC activity, which slows
tissue repair and maintenance [17]. In vitro, proliferation arrest is the major characteristic of cell senescence.
With the accumulation of undegraded macromolecules,
senescent cells show morphological enlargement, flattening, and extensive vacuolization [18], accompanied by increased senescence-associated β-galactosidase (SA-β-gal)
activity, DNA damage, telomere shortening, and genomic
instability.
In tissues or organisms, senescent cells can transmit signals to surrounding tissues through senescence-associated
secretory phenotype (SASP), which consists of basic
fibroblast growth factor (FGF), cytokines (interleukin-6
(IL-6), IL-1β), chemokines (IL-8, and monocyte chemoattractant protein-1 (MCP-1)), extracellular proteases (matrix metalloproteinases (MMPs)), growth factors (transforming growth factor-beta (TGF-β), hepatocyte growth
factor (HGF)), and vascular endothelial growth factor
(VEGF) [19]. SASP can in a way help eliminate senescent
cells and/or tissue remodeling by promoting phagocytic
immune cells and promote the occurrence and development of tumors and age-related diseases by creating a proinflammatory microenvironment.
The aging of adult resident MSCs is directly proportional
to the old donor, and the functional properties of MSCs
deteriorate severely with the increase of donor age. Compared with MSCs from adult tissues, some MSCs from
neonatal tissues have a stronger proliferative capacity in
vitro, especially under hypoxic conditions [20]. The differentiation efficacy of adult MSCs into certain lineagespecific cells is also influenced by the donor age, while
their ex vivo proliferative potential depends on population
doubling (PD) and cell passage [21]. The senescence of
MSCs influences their replicative potential and properties
(e.g., morphology, function, and biomarker), which may
affect their therapeutic efficacy. The functional degradation and potentially harmful effects of senescence have
limited the application of MSCs in regenerative medicine
and tissue engineering. Therefore, it is important to understand the senescence features of MSCs and identify common methods for assessing the MSC state.
During the long-term culture of MSCs in vitro, their
proliferative capacity and colony-forming units (CFU)
decreased. The proliferation of MSCs slows down at 30-
40 PD, stops proliferation, and enters the senescence state
when PD reaches a certain level [22]. The number of
colonies indicates the clonogenic potential and proliferation ability, and the level of CFU decreased in senescent
cells. The CFU of MSCs decreased continuously with the
increase of passage and could hardly be detected after the
20th passage [23]. Therefore, detecting PD and CFU indicators of MSCs is a shared method for detecting senescence in vitro.
The size and morphology of MSCs changed significantly
during senescence. With long-term culture in vitro, the
early MSCs, similar to spindle-forming fibroblasts form,
became larger in size, flattened in shape, and increased in
cytoplasmic granules [24]. The in vitro imaging system
analysis showed that the cell volume of MSCs began to
expand at the 5th passage, and the area of the 9th passage
cells increased by 4.8 times compared with the 1st passage [25]. The cell size was strongly associated with the
increase of SA-β-gal expression and actin stress fibers [26].
Therefore, assessing the morphology and size of MSCs
is also a shared method for detecting senescence in vitro.
Especially based on the unique morphology of senescent
cells, the development of image recognition-related detection technology has excellent application prospects.
MSCs continuously lose their adipogenic and osteogenic
differentiation potential during prolonged culture [27]. It
has been reported that senescence can transform the osteogenic differentiation potential of MSCs into adipogenic
[28]. Rapamycin, an autophagy activator, can restore the
biological characteristics of senescent MSCs by increasing
proliferation and osteogenic and decreasing adipogenic
differentiation [29].
MSCs are involved in regulating the activation and phenotype of innate and adaptive immune cells, including
dendritic cells, macrophages, monocytes, natural killer
cells, and lymphocytes. When co-cultured with young
mice MSCs, mice macrophages retained their original
phagocytosis and M2 polarization and showed higher
migration rates [30]. With senescence, the protective immunomodulatory functions of MSCs may be altered, such
as their reduced ability to inhibit lymphocyte proliferation. With the increase of passages, the ability of MSCs
co-cultured with peripheral blood mononuclear cells to
inhibit the proliferation of CD4+
and CD8+
T cells were
continuously weakened [31]. In addition to proliferation,
senescent MSCs attenuated the inhibitory effects of phytohemagglutinin-stimulated T-cell cytokine and activationantigen production [32].
The secretory properties of MSCs also change with senescence. The expression of growth factors (TGF-β and
HGF), inflammatory cytokines (IL-1, IL-6, and IL-8),
and extracellular proteases (MMP1, MMP3, and MMP9)
increased in SASP secreted by senescent MSCs [33].
SASP-related factors were increased in the conditioned
medium of late passages compared with that of early passages. SASP-related factors drive the senescence of their
own or neighboring cells in a cell-autonomous manner or
paracrine manner, resulting in negative effects on cellular
functions (such as cell adhesion, differentiation, proliferation, and migration) [34].
Specific molecules associated with MSCs--CD markers show different expression patterns at early and late
stages. The expression of CD264 is up-regulated during
the intermediate stage of cell senescence and continues
to be up-regulated during cell senescence, which can be
used to evaluate therapeutic potential. When the CD264+
proportion is 75%, the regenerative potential of MSCs is
severely impaired [35]. On the other hand, the CD146+
proportion decreases with the increase in donor age and
generation [36]. The expression of CD90+
and CD106+
is also decreased in senescent MSCs [26]. Leptin receptor (CD295) can be used to mark apoptotic cells and its
expression increased with MSCs of advancing biological
aging [37].
Telomere shortening and DNA damage are the major
mechanisms of senescence. Telomere length is closely
related to the replicative potential of cells and tissues.
Telomerase prevents telomere shortening and induces
elongation by bringing repeated TTAGGG to chromosome ends [38]. However, telomerase almost does not
express itself throughout the life cycle of MSCs. Due to
the lack of telomerase activity, adult MSCs showed irreversibly shortened telomeres during continuous passages
[39]. Oxidative stress is the major cause of DNA damage.
Increased oxidative stress-related molecules can induce
senescence and growth arrest in MSCs, which are highly
sensitive to the accumulation of DNA damage [40]. Elevated intracellular reactive oxygen species (ROS) levels
can reduce MSCs proliferation and DNA synthesis [41].
The activity of the antioxidant enzyme (superoxide dismutase (SOD)) decreased in late-generation MSCs, while
the levels of nitrogen monoxide (NO), ROS, and gluconate oxidizing enzyme increased [42].
Phosphatidylinositol 3-kinase (PI3K)/v-akt murine thymoma viral oncogene homolog (Akt)/mechanistic target
of rapamycin (mTOR) pathways are activated by the
high concentration of ROS and are key regulators of the
oxidative stress response [43]. Nuclear factor erythrocyte 2-related factor 2 (NRF2) plays an important role
as a transcription and regulator factor in oxidative stress
response by regulating a variety of antioxidant response
element-dependent antioxidant genes [44]. NRF2 activity decreased with the senescence of MSCs. Activation of
NRF2 may be an effective method for preventing the deterioration of the MSC growth state under oxidative stress
and maintaining stemness [45].
In addition, mitochondrial membrane potential changes
in senescent cells, are accompanied by increased cellular
oxygen consumption and ROS production [46]. Mitochondrial dysfunction has been shown to contribute to
senescence. When mitochondrial function is impaired,
oxidative stress increases, leading to apoptosis [47]. Mitochondrial fusion increased and mitochondrial fission decreased in senescence MSCs. The efficiency and function
of autophagy gradually decline with age, and enhanced
autophagy may prolong the life span of organisms [48].
In vitro MSC senescence induced by the high glucose
concentration showed increased autophagy levels, while
down-regulation of autophagy alleviated the senescence,
suggesting autophagy is involved in MSC senescence [49].
MiRNAs in MSC senescence
MiRNAs are important contributors to epigenetic regulation, affecting the translation and stability of targeted mRNAs to regulate post-transcriptional gene expression [50]. Mounting evidence indicates that individual miRNAs participate in the regulation of target mRNAs and mediate numerous cellular processes by influencing different signaling networks [51], including senescence-related multiple signaling molecules and pathways (Figure 1 and Table 1).
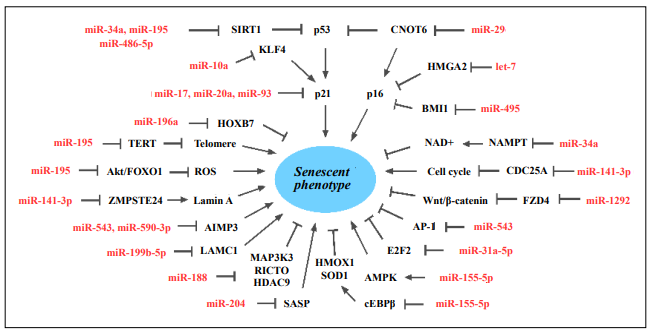
Figure 1. MiRNAs are involved in MSC senescence. AIMP3, Aminoacyl-tRNA synthetase-interacting multifunctional protein 3; AMPK, AMPactivated protein kinase; AP-1, activating protein 1; BMI1, B-cell-specific moloney murine leukemia virus insertion site 1; CDC25A, cell division cycle 25A; cEBPβ, CCAAT/enhancer binding protein β; CNOT6, CCR4-NOT transcription complex subunit 6; E2F2, early 2 factor 2; FOXO1, forkhead box O1; FZD4, frizzled-4; HDAC9, histone deacetylase 9; HMGA2, high mobility group A2; HMOX1, heme oxygenase-1; HOXB7, homeobox B7; KLF4, krüpple-like factor 4; LAMC1, laminin gamma 1; MAP3K3, mitogen-activated protein kinase kinase kinase 3; NAMPT, nicotinamide phosphoribosyl-transferase; RICTOR, RPTOR-independent companion of MTOR complex 2; SASP, senescence-associated secretory phenotype; SIRT1, sirtuin 1; SOD1, superoxide dismutase 1; TERT, telomerase reverse transcriptase; ZMPSTE24, zinc metallopeptidase STE24.
Table 1
MiRNAs are involved in MSC senescence.
| miRNA | miRNA Target | Mechanism | Reference |
|---|---|---|---|
| let-7 | HMGA2 | Regulate the p16INK4a/pRB pathway | [55] |
| miR-10a | KLF4 | Reduce p21 expression | [59] |
| miR-17 | SMURF1 | Regulate p53 pathway | [61, 62] |
| miR-20a/93 | p21 | Regulate p53 pathway | [56- 58] |
| miR-29 | CNOT6 | Activate the p16INK4a/pRB and p21/p53 pathways | [63] |
| miR-31a | E2F2 | DNA damage and heterochromatin | [64] |
| miR-34a | NAMPT | Regulate mitochondrial dysfunction and SIRT1/ FOXO3a activation | [65- 67] |
| miR-141 | BMI1, SDF1, SVCT2, DLX5, ZMPSTE24 | Regulate differentiation, migration, proliferation, and cell cycle | [68- 71] |
| miR-155 | Cab39, cEBPβ | Regulate AMPK pathway and ROS production | [72, 73] |
| miR-188 | RICTOR, MAP3K3, HDAC9 | Regulates differentiation | [74, 75] |
| miR-195 | SIRT1, TERT,Akt/FOXO1 | Shorten telomere length and ROS production | [76] |
| miR-196a | HOXB7 | Repress proliferation | [53] |
| miR-199b | LAMC1 | Regulate LAMC network | [52] |
| miR-204 | SIRT1 | SASP expression | [82, 83] |
| miR-335 | AP1 | Disrupts immunomodulatory properties and chondrogenic differentiation | [84, 85] |
| miR-486 | SIRT1 | Repress cell proliferation and differentiation | [86] |
| miR-495 | BMI1 | Increased p16, p21 and p53 expression,SA-β-gal activity, and suppress cell migration | [87, 88] |
| miR-543/590 | AIMP3 | Affect differentiation potential | [89, 90] |
| miR-1292 | ALP, RUNX2, FZD4 | Regulate Wnt/β-catenin pathway | [91, 92] |
Specific miRNA function and expression profiles may reflect unique developmental stage-specific, tissue-specific, or disease-specific patterns. Several miRNAs are expressed differently between young and senescent MSCs (Figure 2). The miScript miRNA assay was used to identify 43 miRNAs in senescent MSCs, of which 23 miRNAs were analyzed. Fourteen miRNAs (miR-10, miR-27b, miR-30b, miR-30d, miR-103a, miR-103a-2, miR-136, miR-140- 5p, miR-323-3p, miR-330-5p, miR-361-5p, miR-409-3p,miR-424, and miR-455-3p) were up-regulated in response to senescence, and five miRNAs (miR-16-2, miR-29b, miR-199b-5p, miR-454, and miR-618) were down-regulated [52]. MiRNA expressed on MSCs from old donors (39-78 years) and young (3-13 years old) donors were also shown different, and 7 miRNAs (miR-99a, miR-100, miR- 196, miR-337-5p, miR-376b, miR-431, and miR-543) were particularly identified, with miR-196 rarely detected in the old-donors [53]. By analyzing the replicative senescence-induced miRNAs expression changes of MSCs derived from young and old donors, twelve miRNAs were shown to be differentially expressed jointly in young and old donor MSCs. Among them, ten miRNAs (miR-150- 3p, miR-371a-5p, miR-762, miR-1207-5p, miR-1225-5p, miR-1915-3p, miR-2861, miR-3665, miR-4281, and miR- 4327) were found to be up-regulated and two miRNAs (miR-25-3p and miR-93-5p) were down-regulated [54].
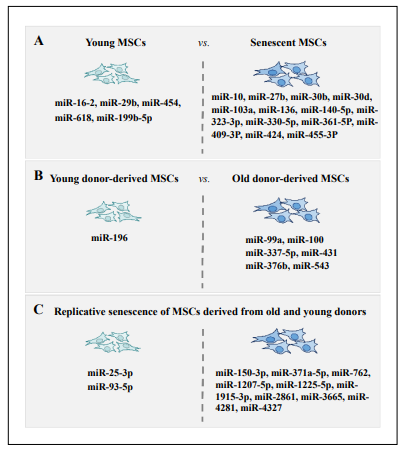
Figure 2. Characteristics of miRNAs profile of young and senescent MSCs.
Functionally, overexpression or downregulation of particular miRNAs has been proven to mediate senescence
by targeting candidate genes on the p16INK4a/pRB and p53/
p21 pathways, which primarily control cell senescence.
Histone deacetylase inhibitors induce senescence in
MSCs. The up-regulation of miRNAs in the let-7 family
can reduce high mobility group A2 (HMGA2) expression during cell senescence [55]. Decreased HMGA2 leads to the activation of the p16INK4a gene, which in turn
induces MSCs senescence [56] through the regulation of
the p16INK4a/pRB pathway by let-7. In senescent MSCs,
the expression of miR-20a and miR-93 (members of the
miR-17 family) decreased [57]. The decreased expression
of miR-20a was critical for the upregulation of p21, and
overexpression of miR-20a significantly attenuated senescence [58].
The expression of miR-10a in MSCs also affected by donor age. MiR-10a attenuated cell senescence by inhibiting
Krüpple-like factor 4 (KLF4) and increased the differentiation capacity of aged BM-MSCs [59]. KLF4, a zinc
finger transcription factor, is involved in the regulation of
important processes such as cell cycle, cell growth, and
apoptosis. Overexpression of KLF4 can induce cell senescence, which is mainly caused by inducing p21 expression
[60].
MiR-17 partially rescues the osteogenic differentiation
of senescent MSCs in vitro and in vivo. Smad ubiquitin
regulatory factor 1 (SMURF1), as a direct target gene, is
an important contributor to the cascade of p53/miR-17 in
osteogenesis [61]. The miR-17 family participates in senescence regulation by directly targeting p21 [62].
The expression of miR-29 showed an increasing trend
during the aging of human MSCs. MiR-29c-3p may regulate MSCs senescence depending on the p53 pathway.
Overexpression of miR-29c-3p resulted in enhanced SA-
β-gal staining and SASP expression, delayed osteogenic
differentiation, and reduced proliferation, whereas that of
silencing had the opposite results. MiR-29c-3p was shown
to target CCR4-NOT transcription complex subunit 6
(CNOT6) and activated the p16INK4a /pRB and p53/p21
pathways in MSCs [63].
The expression of miR-31a-5p was significantly elevated
in old rat BMSCs, which exhibited increased adipogenesis
and senescence phenotypes. MiR-31a-5p affects osteoblastic and osteoclastic differentiation and mediates the
age-related bone marrow microenvironment. MiR-31a-5p
induces DNA damage, cell senescence, and senescenceassociated heterochromatin foci by targeting E2F2, which
is involved in senescence-related changes of heterochromatin [64].
The expression of miR-34a increased in senescent MSCs
with continuous passage. MiR-34a promotes apoptosis by
regulating mitochondrial dysfunction and activating sirtuin 1(SIRT1)/forkhead box O3a (FOXO3a) and intrinsic
apoptosis pathways. In replicative and naturally senescent
MSCs, inhibition of miR-34a contributes to the alleviation
of senescence-related phenotypic features [65]. MiR-34a
is up-regulated by p53 and then down-regulates SIRT1
expression (a p53 inhibitor), thus forming a positive feedback loop [66]. Exception of p53/p21, overexpression of
miR-34a reduces cycle-dependent kinases and cyclins. In
addition, overexpression of miR-34a in young MSCs induces long-term proliferation, increased SA-β-gal activity,
and decreased osteogenic differentiation capacity. MiR-
34a significantly reduced SIRT1 activity, nicotinamide
adenine dinucleotide (NAD)+
content, and NAD+
/nicotinamide adenine dinucleotide (NADH) ratio by targeting
nicotinamide phosphoribosyl-transferase (NAMPT) [67].
In MSCs, miR-141 target genes include B-cell-specific
moloney murine leukemia virus insertion site 1 (BMI1),
stromal cell-derived factor 1 (SDF1), sodium-dependent
from vitamin C-2 (SVCT2), and distal-less homeobox
5 (DLX5), which are involved in the regulation of differentiation, migration, and proliferation. The expression
of miR-141-3p depends on histone acetylation at the promoter and increases in senescent MSCs [68, 69]. MiR-
141-3p directly inhibited zinc metallopeptidase STE24
(ZMPSTE24) (enzyme for processing pre-lamin A into
lamin A) [70]. In the subculture of aged MSCs, the cells
have abnormal nuclear morphology due to the increase
of pre-Lamin A. MiR-141-3p targeted cell division cycle
25A (CDC25A) leads to inhibiting MSC proliferation by
arresting cell cycle at the G1 phase [71].
The expression of miR-155-5p in MSCs from old donors
was significantly higher than that from young donors.
In young donor-derived MSCs, high expression of miR-
155-5p resulted in increased cell senescence. MiR-155-5p
increases mitochondrial fusion and inhibits mitochondrial
fission in MSCs through the AMP-activated protein kinase
(AMPK) pathway, thereby leading to cell senescence by
inhibiting Cab39 expression [72]. In addition, miR-155-
5p promotes ROS production. MiR-155-5p suppressed the expression of antioxidant genes (heme oxygenase-1
(HMOX1) and superoxide dismutase 1 (SOD1)) by repressing CCAAT/enhancer binding protein β (cEBPβ, a
common transcription factor regulating these genes) [73].
MiR-188 regulates the senescence-associated transition of
BMSCs from osteogenesis to adipogenesis and has additional significance in senescence. The expression of miR-
188 increased in BMSCs of elderly mice and humans. In
lineage-negative myeloid cells, overexpression of miR-
188 promotes senescence. MiR-188 targeted genes including RPTOR-independent companion of MTOR complex
2 (RICTOR), mitogen-activated protein kinase kinase
kinase 3 (MAP3K3), and histone deacetylase 9 (HDAC9)
[74, 75].
The expression of miR-195 increased in senescent and old
donor MSCs, and the miRNA directly targeted SIRT1 and
telomerase reverse transcriptase (TERT) [76]. SIRT1 is
a regulator of p53 deacetylation and exerts an inhibitory
role in aging [77]. TERT encodes telomerase, which prevents telomere shortening [78]. MiR-195 affects telomere
length changes by targeting TERT. Increased miR-195
expression shortens telomere length in MSCs from old
donors. Inhibition of miR-195 significantly reduced SA-
β-gal expression in senescent MSCs. MiR-195 also affects
the phosphorylation of Akt and FOXO1 [76]. FOXO is
a downstream target of the PI3K-Akt signaling pathway,
which regulates the ROS pathway during cell senescence
[79]. Among them, FOXO1 is a transcription factor involved in the expression of antioxidant enzymes (SOD
and catalase) and acts on SIRT1-mediated ROS increase
and maintenance during senescence [80, 81].
Expression of miR-196a increased with senescence. Compared with the children group, the expression level of
miR-196a increased and Ki-67 decreased in adult MSCs.
MiR-196a is negatively correlated with MSC proliferation
by directly targeting homeobox B7 (HOXB7). Overexpression of HOXB7 can reduce senescence and improve
cell growth, which is related to the increase of basic
fibroblast growth factor secretion. HoxB7 acts in cell differentiation, proliferation, and signal transduction, and is a
major factor driving the behavioral longevity of progenitor cells to optimize MSC performance [53].
Compared to young (average 21 years) and old (average 65 years) donor MSCs, miR-199b-5p is dysregulated
in senescent MSCs. MiR-199b-5p directly represses the
expression of laminin gamma 1 (LAMC1) to regulate the
LAMC network, thereby indirectly affecting the senescence of MSCs [52]. LAMC1 promotes tumor cell migration and proliferation through the Akt-NF-κB signaling
pathway.
The expression of miR-204 is upregulated in senescent
human umbilical vein endothelial cells (HUVECs) and
stress-induced senescent chondrocytes [82, 83]. In mice,
ectopic expression of miR-204 is sufficient to promote
osteoarthritis development, while knockdown improved
surgically-induced osteoarthritis and repressed SASP expression [83]. SIRT1 is considered to be a key regulator of
inflammation and aging. miRNAs post-transcriptionally
downregulated SIRT1 during the differentiation of mouse
embryonic stem cells, and maintain low levels of SIRT1
expression in differentiated tissues, where MiR-204 was
found to be involved in inhibiting SIRT1 protein expression [82].
The expression of miR-335 was increased in BMSCs
from old donors and senescent MSCs. Forced expression
of miR-335 in MSCs induces a senescent phenotype and
disrupts immunomodulatory properties and chondrogenic
differentiation ability by repressing activating protein 1
(AP-1), which regulates cell proliferation, differentiation,
and migration [84, 85].
MiR-486-5p plays a role in senescence by targeting the
SIRT1. In adipose-derived MSCs (AMSCs), miR-486-
5p is increased during aging and replicative senescence.
Overexpression of miR-486-5p represses cell proliferation
and adipogenic and osteogenic differentiation and induces
senescence phenotype. MiR-486-5p directly regulates
SIRT1 expression and deacetylase activity, and downregulation of SIRT1 can induce senescence [86].
In MSCs, miR-495 increased p16INK4a, p21, and p53 expression and SA-β-gal activity by targeting BMI1 [87].
BMI1 is an inhibitor of cell senescence and a regulator of
p16INK4a [88]. Conditioned medium collected from MSCs
overexpressing miR-495 suppressed the cell migration,
which is consistent with the paracrine effect of SASP to
trigger cell senescence into healthy adjacent cells [87].
Aminoacyl-tRNA synthetase-interacting multifunctional
protein 3 (AIMP3) affects the senescence and differentiation potential of MSCs, and its protein expression level
increases with senescence, while miR-543 and miR-590-
3p can significantly reduce the expression of AIMP3.
Overexpression of miR-543 or miR-590-3p alleviated
the late passage MSCs, whereas inhibition of miR-543 or
miR-590-3p aggravated senescence by increasing AIMP3
[89, 90].
MiR-1292 acts to accelerate senescence in adipose-derived MSCs and is negatively correlated with osteogenic
markers alkaline phosphatase (ALP) and runt-related transcription factor 2 (RUNX2) in bone. MiR-1292 mediates
its influence through the Wnt/β-catenin pathway by targeting frizzled-4 (FZD4) [91]. The Wnt/β-catenin signaling
pathway is an important contributor to the self-renewal
and differentiation of MSCs by promoting the intracellular
production of ROS [92].
EV, EV-miRNA in MSC senescence
Based on their differences in size and secretion pathway,
EVs are classified into three subtypes: exosomes, microvesicles, and apoptotic bodies [93]. Exosomes (less
than 120 nm) originate from the endoplasmic reservoir,
producing multivesicular bodies that fuse with the plasma
membrane to secrete their contents. Microvesicles (100
to 500 nm) are budding vesicles that may arise from the
plasma membrane under various conditions of stress.
Apoptotic bodies (500 nm to 5 μm) are released from the
plasma membrane of apoptotic cells [94].
EVs are composed of nucleic acids (mRNA, DNA, miRNAs, and long noncoding RNAs), lipids, and proteins
[95]. The contents reflect the origin of the cell and convey
specific molecules for specific cell types. EV-miRNA exchange between cells may be a key for intercellular communication and the miRNAs encapsulated into EVs are
strictly regulated by various microenvironmental conditions and stress stimuli. The miRNA content of EVs may
reflect the pathological state of released cells and serve as
promising biomarkers for multiple pathologies. EVs are
highly enriched for ALG-2 interacting protein X (ALIX),
CD63, CD81, and tumor susceptibility gene 101 (TSG-
101). Various techniques have been used to characterize
EVs, including atomic force microscopy, dynamic light
scattering, enzyme-linked immunosorbent assay, electrochemical biosensors, flow cytometry, fluorescenceactivated cell sorting, microfluidics, nanoparticle tracking
analysis, resistance pulse sensing, scanning electron microscopy, and transmission electron microscopy [96, 97].
Senescence-related EVs can transfer regulatory factors
such as miRNAs and proteins to promote the senescence
process in autocrine, endocrine, and paracrine ways. Senescent cells secrete high levels of EVs and regulate the
microenvironment. P53 regulates the transcription of other
endosomal genes associated with vesicle biosynthesis.
DNA damage-induced senescence induces an increase in
p53-dependent EV biogenesis. Senescent cell-derived EVs
are partially dependent on p53 and its downstream target
tumor suppressor-activated pathway 6 (TSAP6) [98].
Senescent cell-derived EVs enable neighboring cells to
respond particularly rapidly and efficiently to stress by
regulating the surrounding environment. On the hand,
these EVs may play a role in promoting SASP by transmitting pro-senescence signals, which facilitate the regenerative potential of surrounding cells and the elimination
of senescent cells and also enhance local inflammation
levels by recruiting immune cells and spreading senescence throughout tissues. A recent study has just shown
that senescence-associated exosomes influence the genetic
information and immunomodulatory potential of the microenvironment [99].
At present, a variety of inflammation-related miRNAs
have been identified in EVs, such as miR-19b, miR-20a,
miR-21, miR-126, miR-146a, and miR-155 [100]. The expression pattern of different miRNAs in MSC-EVs changes with senescence [101]. Compared with young rats, the
expression levels of miRNA-294 and miRNA-872-3p
in MSC-EVs decreased with age [102]. The expression
of miRNA-146a was elevated in late passage MSC-EVs
compared with the early passage [103]. Mouse senescent
MSC-EVs contain miRNA-183-5p, which promotes senescence in young MSCs [104].
Old bone marrow-derived EVs were absorbed by young
MSCs and repressed osteogenic differentiation. Overexpression of miR-183-5p reduced Hmox1 protein level and
cell proliferation and promoted senescence in MSCs [104].
MiR-34a increases with age in muscle-derived EVs and
induces senescence of BMSCs. That is, EVs may induce
MSC senescence through miR-34a-5p targeting SIRT1
[105].
MiR-17-3p and miR-199b-5p were decreased in senescent fibroblast-derived EVs. In particular, miR-199b-5p is
decreased in senescent MSCs and elderly donor-derived
MSCs [52]. MiR-17-3p is also decreased in senescent
MSCs and skin fibroblasts as a cellular model. MiR-23a-
5p has been proven to regulate the osteogenic differentiation of BMSCs, and its expression was increased in senescent fibroblast-derived EVs [106]. MiR-23a-5p promotes
osteogenic differentiation by targeting transmembrane
protein 64 (TMEM64), whereas inhibition of miR-23a-5p
expression promotes adipogenic differentiation in MSCs
[107].
MSC-EVs containing let-7a, miR-21, miR-191, and miR-
222 are known to regulate cell proliferation and cycle progression [108]. The expression of miR-21 was decreased
in EVs of senescent MSCs and adult MSCs, and this
miRNA was also decreased in MSCs from ovariectomized
mice and postmenopausal osteoporotic patients [109]. In
breast cancer cells, this miR-21 targets E2F2, a downstream effector of p21 and p16INK4a [110].
MiR-31 is a circulating miRNA that is differentially expressed with senescence and increased in the blood of
osteoporosis patients. The expression of miR-31 is also elevated in senescent endothelial cell MVs. These MVs repress the osteogenic differentiation of MSCs by targeting
FZD3 [111]. MiR-31a-5p was found in senescent MSCderived exosomes, which trigged osteogenesis of cocultured bone marrow cells [112]. Compared with young
mice, exosomes secreted from older mice-isolated muscle
cells are enriched with miR-34a. MiR-34a is related to
senescence and inflammation. Myoblast exosomes overexpressing miR-34a can reduce MSCs proliferation and
induce senescence by promoting SA-β-gal activity [105].
Induced pluripotent stem cell-derived MSC-EVs (iMSCEV) enriched with miR-105-5p could rejuvenate senescent
nucleus pulposus cells by activating the SIRT6 pathway
in vitro. miR-105-5p plays a pivotal role in the iMSC-EVmediated therapeutic effect by decreasing the level of the
cAMP-specific hydrolase phosphodiesterase 4D (PDE4D)
[113]. It has been reported that suppression of PDE4D expression can promote the migration, invasion, colony formation, and proliferation of colorectal cancer cells [114].
MiR-146a-5p is increased in senescent MSC-derived EVs.
This miRNA is known to regulate the NF-κB signaling
activation and SASP production of senescent cells [103].
In a mouse model of allergic airway inflammation, MSCEV suppresses the function of group 2 innate lymphoid
cells, reducing inflammatory infiltration and T helper 2
cytokines production by transporting miR-146a-5p [115].
MSC-EV effectively represses the inflammatory response
of cardiomyocytes by delivering miR-146a-5p to reduce
v-myb myeloblastosis viral oncogene homolog-like 1
(MYBL1) expression [116].
Exosomes enriched with miR-188-3p ameliorate senescence by regulating the mTOR complex. Incubation of
old MSCs with this exosome decreased senescence markers and mTOR pathway proteins, and up-regulate the pluripotency markers. Inhibition of miR-188-3p in MSCEVs significantly increased the expression of RICTOR,
decreased the expression and phosphorylation of Akt, and
downregulated the proportion of SA-β-gal staining cells
[117].
Interestingly, EVs from MSCs of young donors or early
passages have been shown to reverse the senescent phenotypes of late passages MSCs or that from pre-mature aged
patients. In our study, we found that adding early passage iMSC-EV to the senescent iMSC culture promoted
cell growth, downregulated the expression of age-related
genes, reduced mitochondrial density, and improved mitochondrial membrane potential (Figure 3). This, even still
at the preliminary stage, may suggest that the addition of
exogenous exosomes, ideally engineered with elevated
expression of specific miRNAs, to the MSC culture, may
be feasible for promoting MSC proliferation in culture or
scaling-up the manufacture of MSCs to a significant extent.
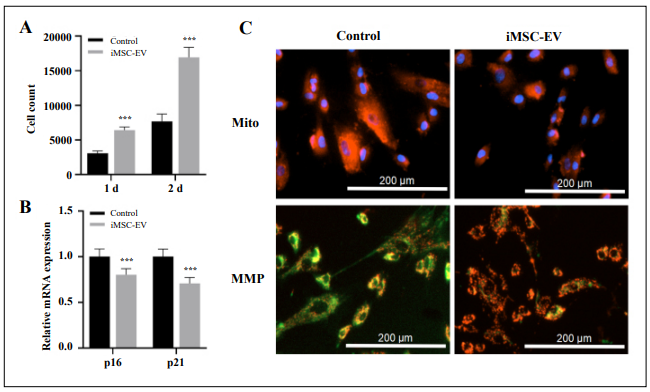
Figure 3. Effect of early passage iMSC-EV on senescence characteristics of late passage iMSCs.A. Changes in cell count of senescent iMSCs after early passage iMSC-EV incubation. B. Expression of cell cycle genes of senescent iMSCs. C. Mitochondrial density and mitochondrial membrane potential (MMP) of senescent iMSCs. n=3, *** p < 0.001.
Prospects of MSC-EV therapy
In initial studies in most animals, MSCs have shown
encouraging positive results in various types of cell
therapy, but the concerns of human MSC therapy remain
unneglectable, including immune rejection and various
cancer promotion. Due to the clinical outcomes of MSCbased therapy remaining nonoptimal, so far, a large proportion of the majority of registered clinical trials applying MSC therapy for human diseases have indeed fallen
short of expectations.
Emerging evidence suggests that MSC-EV therapy has
equal or better efficacy than MSCs in many diseases, and
the risk of MSC-based therapy is significantly reduced.
The advantages of cell-free therapies based on MSC-EVs
are considerable. The incapability of MSC-EVs to selfreplicate greatly reduces the risk of expansion and tumor
and increases safety. The small size also provides faster
tissue penetration [118]. The potential to stimulate the immune system is limited, reducing the risk during allogeneic transplantation. EVs are easier to handle in transportation and storage, which makes EV therapeutic potential optimal.
The effects of MSC-EVs, including anti-senescence, antiinflammatory, and wound healing, play a positive role in
various diseases. In pilocarpine-induced status epilepticus
mice treated with MSC-EVs, EVs reach the hippocampus
within 6 h and exert anti-inflammatory and neuroprotective effects, which are coupled with normal hippocampal neurogenesis and cognitive and memory functions [119].
Melatonin-pretreated MSC-EVs affect the ratio of macrophage M2 polarization to M1 polarization by regulating
the activation of phosphatase and tensin homolog (PTEN)/
Akt signaling pathway, thus suppressing inflammatory
response and promoting diabetic wound healing [120]. In
the rat model of skin burn treated with human MSC-EVs,
EVs accelerate the re-epithelialization of the wound, promote the nuclear transfer of β-catenin, and enhance skin
cell migration and proliferation, thus facilitating wound healing [121].
Although preclinical data have demonstrated the scalability of EV isolation methods and the safety of therapy, the
clinical use of MSC-EVs is still limited. Currently, there
is a lack of well-defined and standardized optimal culture
conditions of parental cells and optimal protocols for
EV isolation and storage, optimal therapeutic doses and
dosing schedules, as well as reliable potency and safety
profiles. Currently, studies have investigated the effectiveness of MSC-EVs in the clinical setting, and most clinical
trials are still recruiting and active (Table 2). The delivery
routes oral, intranasal administration, intravenous and intraperitoneal injection.
Table 2
List of clinical trials using MSC-EVs.
| No. | Condition or disease | Treatment | Trial Phase | Trial ID |
|---|---|---|---|---|
| 1 | Cerebrovascular Disorders | Allogenic MSC-EVs enriched by miR-124 | Phase I Phase II (Recruiting) | NCT03384433 |
| 2 | Metastatic Pancreas Cancer With KrasG12D Mutation | MSC-EVs with KRAS G12D siRNA | Phase I (Recruiting) | NCT03608631 |
| 3 | Chronic Graft Versus Host Diseases | Artificial tears for 14 days of UMSC-EVs 10ug/drop | Phase I Phase II (Recruiting) | NCT04213248 |
| 4 | Alzheimer Disease | Twice a week for 12 weeks nasal drip of MSC-EVs (5, 10, 20μg) | Phase I Phase II (Recruiting) | NCT04388982 |
| 5 | Hospital-acquired pneumonia | 7 times aerosol inhalation of MPC-EVs (8×108 or 16×108 particles) | Phase I Phase II (Recruiting) | NCT04544215 |
| 6 | Acute Respiratory Distress Syndrome | Basic treatment and 7 times aerosol inhalation of MSC-EVs (2×108, 8×108, or 16×108 particles) | Phase I Phase II (Recruiting) | NCT04602104 |
| 7 | Moderate SARS-CoV2 Infection | Intravenous injection of MSC-EVs | Phase II Phase III (Recruiting) | NCT05216562 |
| 8 | Degenerative Meniscal Injury | Intra-articular administration of synovial fluid-derived MSC-EVs | Phase II (Recruiting) | NCT05261360 |
| 9 | Perianal Fistula | Placenta-MSC-EVs | Phase I Phase II (Recruiting) | NCT05402748 |
| 10 | Retinitis Pigmentosa | Subtenon injection of Wharton jelly-derived MSC-EVs | Phase II Phase III (Recruiting) | NCT05413148 |
| 11 | Chronic Ulcer | MSC conditioned media | Phase I (Completed) | NCT04134676 |
| 12 | Novel Coronavirus Pneumonia | 5 times aerosol inhalation of MSC-EVs (2×108 nanovesicles/3 ml) | Phase I (Completed) | NCT04276987 |
| 13 | Healthy | Once aerosol inhalation of MSC-EVs (2×108, 4×108, 8×108, 12×108, or 16×108 nanovesicles/3 ml) | Phase I (Completed) | NCT04313647 |
| 14 | SARS-CoV-2 Associated Pneumonia | Twice a day for 10 days inhalation of MSC-EVs (0.5-2×108 nanovesicles/3 ml) | Phase I Phase II (Completed) | NCT04491240 |
| 15 | C O V I D - 1 9 A s s o c i a t e d A c u t e Respiratory Distress Syndrome | Intravenous administration of BM-MSC-EVs | Phase II (Completed) | NCT04493242 |
Note: * Information obtained from https://clinicaltrials.gov/ on 30 November 2022.
The heterogeneity of MSC-EVs is probably one of the key
factors affecting their therapeutic properties. EV variability lies in the contents of RNA and proteins, particularly
non-coding RNAs with properties such as inflammation
resolution, potency, and tissue regeneration. The developLiangge He, et al.
All Rights Reserved
ment of technology for detecting EV contents is helpful
to promote the study of maintaining EV characteristics.
Currently, contents are analyzed using chemical, physical,
biological, and nanotechnological methods, usually involving the use of multiple antibodies, nucleic acid fitting,
or molecular markers as recognition components, coupled
with various chemical labels (e.g., redox probes and optical dyes), nanoparticle tags or DNA oligonucleotide [122].
For example, Raman spectroscopy is used to distinguish
the overall chemical bond characteristics of EVs based on
the spectral patterns generated by vibration and rotation.
EV particles are captured on a specially modified plane or
spherical interface and fluorescent dye labeling is added
to detect and quantify the membrane proteins and internal
miRNAs [122-124]. The analysis and identification of
specific contents can be achieved by using surface-sensitive label-free physical analysis methods (e.g., electrical
impedance spectroscopy, quartz crystal microbalance, and surface plasmon resonance) or external chemical tags to
monitor the binding of EV contents to receptors on the
array [125-127]. Although the diversity of EV detection
methods has been achieved, the standardization of identification and analysis is still very important. The above detection methods are more or less affected by the difference
in the quality of reagents provided by different suppliers.
The control of high-quality biologics and the evaluation
of binding parameters helps to improve the reproducibility
of detection.
Promoting or inhibiting expression levels of specific miRNAs in EVs can improve therapeutic efficiency for specific diseases or specific repair tissues. The culture conditions and external stimuli of stem cells can alter their EV
yield and content composition. While the EV components
cannot be fully controlled in gene-manipulated cells, currently, breakthroughs have been made in the use of EVs
as a carrier for the better delivery of specified molecules,
including passive loading (e.g., incubation stimulation) or
active loading (e.g., extrusion, electroporation, hypotonic
dialysis, sonication, saponin permeabilization, and transfection) [128]. The miRNA enrichment techniques can be
achieved by constructing overexpressed cell lines or direct
loading miRNAs into EVs by physical or chemical methods. Due to the complex EV loading mechanisms involving the endosomal sorting complex required for transport
(ESCRT)/Rab protein family, multivesicular bodies, intracellular tubules, and actin networks, the generated EVs
loaded with specific miRNA molecules by transfection
of parental stem cells are unreliable and unpredictable.
In addition to cell transfection, direct delivery of desired
miRNAs into EVs is an efficient and feasible method for
enriching miRNAs, which can enhance the interaction of
miRNAs with the surface of EVs by using calcium chloride (CaCl2) buffered medium and promote the incubation
of selected miRNAs into EVs [129]. The heat shock method can alter the fluidity of EV membranes, and promote
miRNA entry into EVs [130]. Electroporation is another
technology to promote miRNA entry, but electroporation
may trigger EV aggregation and change its morphological
characteristics, thus affecting the effect. The existing limitations still need to be improved.
Conclusion
Cell senescence is a dynamic process evolving with time,
and its specific regulation remains unknown. Analyzing
the senescence properties of MSCs is very important for
developing methods to assess MSC senescence, as well as
for understanding how senescence affects the quality and
efficacy of MSCs. A comprehensive analysis of miRNAs
provides a more detailed and in-depth insight into how
senescence influences MSCs. Advances in understanding
the role of miRNAs in aging may provide new ways to alleviate MSC senescence. Undoubtedly, continued in-depth
studies of miRNAs within MSC senescence will shed
light on their mechanisms of action during senescence and
may reveal clues for the potential roles in the extracellular
environment.
Senescence may influence the production rate and cargo
type of MSCs and their EVs. Systematic analysis and
comparison of miRNAs related to MSC senescence and
those contained in MSC-EVs will help to discover universal senescence markers to identify senescent cells.
Translating preclinical results into the clinic faces different challenges related to EV dynamics and biology. Effective MSC-EV therapy may depend on the physiological
function and state of the parental cells, as senescent may
deprive cells of reverse/reduce disease efficacy. A correct
understanding of the detailed mechanisms involved in
miRNAs and EV-miRNAs during senescence may contribute to the regulation of MSC efficacy, as well as the
development of MSC-EVs to improve tissue regeneration
and aging-related diseases.
Declarations
Authors’ contributions
Conceptualization, Liangge He and Guangqian Zhou; Investigation, Arshad Ahmed Padhiar, and Zhen Liu; Writing-Original Draft Preparation, Liangge He and Mingzhu Li; Writing-Review & Editing, Liangge He and Guangqian Zhou; Supervision, Guangqian Zhou; Project Administration, Guangqian Zhou; Funding Acquisition, Guangqian Zhou. All authors were involved in approving the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work is supported, in part, by the National Natural Science Foundation of China (2072480 and 32100603), and Shenzhen Commission of Development Reform (Funding for Shenzhen Engineering Laboratory for Orthopedic Diseases and Regenerative Technologies).
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval and informed consent statement
Not applicable.
Consent for publication
Not applicable.
References
1. Khademi-Shirvan M, Ghorbaninejad M, Hosseini S, & Baghaban Eslaminejad M. The Importance of Stem Cell Senescence in Regenerative Medicine. Adv Exp Med Biol 2020, 1288: 87-102. [Crossref]
2. Maqsood M, Kang M, Wu X, Chen J, Teng L, & Qiu L. Adult mesenchymal stem cells and their exosomes: Sources, characteristics, and application in regenerative medicine. Life Sci, 2020, 256: 118002. [Crossref]
3. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol, 2007, 213(2): 341-347. [Crossref]
4. Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, & Alimonti A. Cellular Senescence: Aging, Cancer, and Injury. Physiol Rev, 2019, 99(2): 1047-1078. [Crossref]
5. Lujambio A. To clear, or not to clear (senescent cells)? That is the question. Bioessays, 2016, 38 Suppl 1: S56- 64. [Crossref]
6. He L, & Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet, 2004, 5(7): 522- 531. [Crossref]
7. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 2004, 116(2): 281-297. [Crossref]
8. Suh N. MicroRNA controls of cellular senescence. BMB Rep, 2018, 51(10): 493-499. [Crossref]
9. Potter ML, Hill WD, Isales CM, Hamrick MW, & Fulzele S. MicroRNAs are critical regulators of senescence and aging in mesenchymal stem cells. Bone, 2021, 142: 115679. [Crossref]
10. van Niel G, D’Angelo G, & Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol, 2018, 19(4): 213-228. [Crossref]
11. Mathieu M, Martin-Jaular L, Lavieu G, & Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol, 2019, 21(1): 9-17. [Crossref]
12. Liu ZJ, Zhuge Y, & Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem, 2009, 106(6): 984-991. [Crossref]
13. Kangari P, Talaei-Khozani T, Razeghian-Jahromi I, & Razmkhah M. Mesenchymal stem cells: amazing remedies for bone and cartilage defects. Stem Cell Res Ther, 2020, 11(1): 492. [Crossref]
14. Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J, et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology, 2014, 19(7): 1013-1018. [Crossref]
15. Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med, 2006, 355(12): 1199-1209. [Crossref]
16. Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, Alrabadi A, et al. Safety and Potential Therapeutic Effect of Two Intracavernous Autologous Bone Marrow Derived Mesenchymal Stem Cells injections in Diabetic Patients with Erectile Dysfunction: An Open Label Phase I Clinical Trial. Urol Int, 2018, 101(3): 358-365. [Crossref]
17. Zhou X, Hong Y, Zhang H, & Li X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front Cell Dev Biol, 2020, 8: 364. [Crossref]
18. Muñoz-Espín D, & Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol, 2014, 15(7): 482-496. [Crossref]
19. Tchkonia T, Zhu Y, van Deursen J, Campisi J, & Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest, 2013, 123(3): 966-972. [Crossref]
20. Hass R, Kasper C, Böhm S, & Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissuederived MSC. Cell Commun Signal, 2011, 9: 12. [Crossref]
21. Baksh D, Yao R, & Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells, 2007, 25(6): 1384-1392. [Crossref]
22. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, & Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells, 2004, 22(5): 675-682. [Crossref]
23. Schellenberg A, Stiehl T, Horn P, Joussen S, Pallua N, Ho AD, et al. Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy, 2012, 14(4): 401-411. [Crossref]
24. Wang S, Wang Z, Su H, Chen F, Ma M, Yu W, et al. Effects of long-term culture on the biological characteristics and RNA profiles of human bone-marrow-derived mesenchymal stem cells. Mol Ther Nucleic Acids, 2021, 26: 557- 574. [Crossref]
25. Oja S, Komulainen P, Penttilä A, Nystedt J, & Korhonen M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res Ther, 2018, 9(1): 6. [Crossref]
26. Bertolo A, Baur M, Guerrero J, Pötzel T, & Stoyanov J. Autofluorescence is a Reliable in vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells. Sci Rep, 2019, 9(1): 2074. [Crossref]
27. Denu RA. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxid Med Cell Longev, 2017, 2017: 5841716. [Crossref]
28. Yang YK, Ogando CR, Wang See C, Chang TY, & Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther, 2018, 9(1): 131. [Crossref]
29. Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH,et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell, 2018, 17(1) [Crossref]
30. Yin Y, Wu RX, He XT, Xu XY, Wang J, & Chen FM. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res Ther, 2017, 8(1): 153. [Crossref]
31. de Witte SFH, Lambert EE, Merino A, Strini T, Douben H, O’Flynn L, et al. Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy, 2017, 19(7): 798-807. [Crossref]
32. Li XY, Ding J, Zheng ZH, Li XY, Wu ZB, & Zhu P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol Med Rep, 2012, 6(5): 1183-1189. [Crossref]
33. Rodier F, & Campisi J. Four faces of cellular senescence. J Cell Biol, 2011, 192(4): 547-556. [Crossref]
34. Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E, et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell, 2019, 18(3): e12933. [Crossref]
35. Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, & O’Connor KC. Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther, 2017, 8(1): 201. [Crossref]
36. Maijenburg MW, Kleijer M, Vermeul K, Mul EP, van Alphen FP, van der Schoot CE, et al. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica, 2012, 97(2): 179-183. [Crossref]
37. Laschober GT, Brunauer R, Jamnig A, Fehrer C, Greiderer B, & Lepperdinger G. Leptin receptor/CD295 is upregulated on primary human mesenchymal stem cells of advancing biological age and distinctly marks the subpopulation of dying cells. Exp Gerontol, 2009, 44(1-2): 57-62. [Crossref]
38. Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, et al. Telomerase maintains telomere structure in normal human cells. Cell, 2003, 114(2): 241-253. [Crossref]
39. Guillot PV, Gotherstrom C, Chan J, Kurata H, & Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells, 2007, 25(3): 646-654. [Crossref]
40. Banimohamad-Shotorbani B, Kahroba H, Sadeghzadeh H, Wilson DM, 3rd, Maadi H, Samadi N, et al. DNA damage repair response in mesenchymal stromal cells: From cellular senescence and aging to apoptosis and differentiation ability. Ageing Res Rev, 2020, 62: 101125. [Crossref]
41. Chen X, Wang L, Hou J, Li J, Chen L, Xia J, et al. Study on the Dynamic Biological Characteristics of Human Bone Marrow Mesenchymal Stem Cell Senescence. Stem Cells Int, 2019, 2019: 9271595. [Crossref]
42. Jeong SG, & Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun, 2015, 460(4): 971-976. [Crossref]
43. Samakova A, Gazova A, Sabova N, Valaskova S, Jurikova M, & Kyselovic J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol Res, 2019, 68(Suppl 2): S131-s138. [Crossref]
44. Yoon DS, Choi Y, & Lee JW. Cellular localization of NRF2 determines the self-renewal and osteogenic differentiation potential of human MSCs via the P53-SIRT1 axis. Cell Death Dis, 2016, 7(2): e2093. [Crossref]
45. Yuan Z, Zhang J, Huang Y, Zhang Y, Liu W, Wang G, et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem Biophys Res Commun, 2017, 491(1): 228-235. [Crossref]
46. Summer R, Shaghaghi H, Schriner D, Roque W, Sales D, Cuevas-Mora K, et al. Activation of the mTORC1/PGC- 1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am J Physiol Lung Cell Mol Physiol, 2019, 316(6): L1049-l1060. [Crossref]
47. Ghosh-Choudhary SK, Liu J, & Finkel T. The role of mitochondria in cellular senescence. Faseb j, 2021, 35(12): e21991. [Crossref]
48. Revuelta M, & Matheu A. Autophagy in stem cell aging. Aging Cell, 2017, 16(5): 912-915. [Crossref]
49. Stolzing A, Coleman N, & Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res, 2006, 9(1): 31-35. [Crossref]
50. Jonas S, & Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet, 2015, 16(7): 421-433. [Crossref]
51. Krol J, Loedige I, & Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet, 2010, 11(9): 597-610. [Crossref]
52. Yoo JK, Kim CH, Jung HY, Lee DR, & Kim JK. Discovery and characterization of miRNA during cellular senescence in bone marrow-derived human mesenchymal stem cells. Exp Gerontol, 2014, 58: 139-145. [Crossref]
53. Candini O, Spano C, Murgia A, Grisendi G, Veronesi E, Piccinno MS, et al. Mesenchymal progenitors aging highlights a miR-196 switch targeting HOXB7 as master regulator of proliferation and osteogenesis. Stem Cells, 2015, 33(3): 939-950. [Crossref]
54. Kilpinen L, Parmar A, Greco D, Korhonen M, Lehenkari P, Saavalainen P, et al. Expansion induced microRNA changes in bone marrow mesenchymal stromal cells reveals interplay between immune regulation and cell cycle. Aging (Albany NY), 2016, 8(11): 2799-2813. [Crossref]
55. Lee S, Jung JW, Park SB, Roh K, Lee SY, Kim JH, et al. Histone deacetylase regulates high mobility group A2- targeting microRNAs in human cord blood-derived multipotent stem cell aging. Cell Mol Life Sci, 2011, 68(2): 325-336. [Crossref]
56. Lin SP, Chiu FY, Wang Y, Yen ML, Kao SY, & Hung SC. RB maintains quiescence and prevents premature senescence through upregulation of DNMT1 in mesenchymal stromal cells. Stem Cell Reports, 2014, 3(6): 975-986. [Crossref]
57. Guo J, Zhao Y, Fei C, Zhao S, Zheng Q, Su J, et al. Dicer1 downregulation by multiple myeloma cells promotes the senescence and tumor-supporting capacity and decreases the differentiation potential of mesenchymal stem cells. Cell Death Dis, 2018, 9(5): 512. [Crossref]
58. Sokolova V, Fiorino A, Zoni E, Crippa E, Reid JF, Gariboldi M, et al. The Effects of miR-20a on p21: Two Mechanisms Blocking Growth Arrest in TGF-β-Responsive Colon Carcinoma. J Cell Physiol, 2015, 230(12): 3105-3114. [Crossref]
59. Dong J, Zhang Z, Huang H, Mo P, Cheng C, Liu J, et al. miR- 10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res Ther, 2018, 9(1): 151. [Crossref]
60. Xu Q, Liu M, Zhang J, Xue L, Zhang G, Hu C, et al. Overexpression of KLF4 promotes cell senescence through microRNA-203-survivin-p21 pathway. Oncotarget, 2016, 7(37): 60290-60302. [Crossref]
61. Liu W, Qi M, Konermann A, Zhang L, Jin F, & Jin Y. The p53/miR-17/Smurf1 pathway mediates skeletal deformities in an age-related model via inhibiting the function of mesenchymal stem cells. Aging (Albany NY), 2015, 7(3): 205-218. [Crossref]
62. Wang Z, Liu M, Zhu H, Zhang W, He S, Hu C, et al. Suppression of p21 by c-Myc through members of miR-17 family at the post-transcriptional level. Int J Oncol, 2010, 37(5): 1315-1321.
63. Shang J, Yao Y, Fan X, Shangguan L, Li J, Liu H, et al. miR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways. Biochim Biophys Acta, 2016, 1863(4): 520-532. [Crossref]
64. Xu R, Shen X, Si Y, Fu Y, Zhu W, Xiao T, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell, 2018, 17(4): e12794. [Crossref]
65. Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z, et al. Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem Cell Res Ther, 2015, 6: 195. [Crossref]
66. Yamakuchi M, & Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle, 2009, 8(5): 712-715. [Crossref]
67. Pi C, Ma C, Wang H, Sun H, Yu X, Gao X, et al. MiR-34a suppression targets Nampt to ameliorate bone marrow mesenchymal stem cell senescence by regulating NAD(+)-Sirt1 pathway. Stem Cell Res Ther, 2021, 12(1): 271. [Crossref]
68. Dimri M, Carroll JD, Cho JH, & Dimri GP. microRNA-141 regulates BMI1 expression and induces senescence in human diploid fibroblasts. Cell Cycle, 2013, 12(22): 3537-3546. [Crossref]
69. Fariyike B, Singleton Q, Hunter M, Hill WD, Isales CM, Hamrick MW, et al. Role of MicroRNA-141 in the Aging Musculoskeletal System: A Current Overview. Mech Ageing Dev, 2019, 178: 9-15. [Crossref]
70. Yu KR, Lee S, Jung JW, Hong IS, Kim HS, Seo Y, et al. MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J Cell Sci, 2013, 126(Pt 23): 5422-5431. [Crossref]
71. Qiu W, & Kassem M. miR-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta, 2014, 1843(9): 2114-2121. [Crossref]
72. Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, et al. miR- 155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell, 2020, 19(4): e13128. [Crossref]
73. Onodera Y, Teramura T, Takehara T, Obora K, Mori T, & Fukuda K. miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging Cell, 2017, 16(6): 1369-1380. [Crossref]
74. Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest, 2015, 125(4): 1509-1522. [Crossref]
75. Zheng Y, Liu H, & Kong Y. miR-188 promotes senescence of lineage-negative bone marrow cells by targeting MAP3K3 expression. FEBS Lett, 2017, 591(15): 2290- 2298. Retraction of: Zheng Y, Liu H, & Kong Y. FEBS Lett. 2022, 596(16): 2086. [Crossref]
76. Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, & Ashraf M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells, 2016, 34(1): 148- 159. [Crossref]
77. Ong ALC, & Ramasamy TS. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev, 2018, 43: 64-80. [Crossref]
78. Dogan F, & Forsyth NR. Telomerase Regulation: A Role for Epigenetics. Cancers (Basel), 2021, 13(6): 1213. [Crossref]
79. Burgering BM, & Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol, 2003, 73(6): 689-701. [Crossref]
80. Xing YQ, Li A, Yang Y, Li XX, Zhang LN, & Guo HC. The regulation of FOXO1 and its role in disease progression. Life Sci, 2018, 193: 124-131. [Crossref]
81. Hao Z, Xu G, Yuan M, Tan R, Xia Y, Liu Y, et al. Leucine Supplementation in Middle-Aged Male Mice Improved Aging-Induced Vascular Remodeling and Dysfunction via Activating the Sirt1-Foxo1 Axis. Nutrients, 2022, 14(18): 3856. [Crossref]
82. Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY), 2010, 2(7): 415- 431. [Crossref]
83. Kang D, Shin J, Cho Y, Kim HS, Gu YR, Kim H, et al. Stressactivated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci Transl Med, 2019, 11(486): eaar6659. [Crossref]
84. Tomé M, López-Romero P, Albo C, Sepúlveda JC, Fernández-Gutiérrez B, Dopazo A, et al. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ, 2011, 18(6): 985-995. [Crossref]
85. Tomé M, Sepúlveda JC, Delgado M, Andrades JA, Campisi J, González MA, et al. miR-335 correlates with senescence/ aging in human mesenchymal stem cells and inhibits their therapeutic actions through inhibition of AP-1 activity. Stem Cells, 2014, 32(8): 2229-2244. [Crossref]
86. Kim YJ, Hwang SH, Lee SY, Shin KK, Cho HH, Bae YC, et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev, 2012, 21(10): 1749-1760. [Crossref]
87. Li X, Song Y, Liu D, Zhao J, Xu J, Ren J, et al. MiR-495 Promotes Senescence of Mesenchymal Stem Cells by Targeting Bmi-1. Cell Physiol Biochem, 2017, 42(2): 780-796. [Crossref]
88. Park IK, Morrison SJ, & Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest, 2004, 113(2): 175- 179. [Crossref]
89. Lee S, Yu KR, Ryu YS, Oh YS, Hong IS, Kim HS, et al. miR- 543 and miR-590-3p regulate human mesenchymal stem cell aging via direct targeting of AIMP3/p18. Age (Dordr), 2014, 36(6): 9724. [Crossref]
90. Oh YS, Kim DG, Kim G, Choi EC, Kennedy BK, Suh Y, et al. Downregulation of lamin A by tumor suppressor AIMP3/ p18 leads to a progeroid phenotype in mice. Aging Cell, 2010, 9(5): 810-822. [Crossref]
91. Fan J, An X, Yang Y, Xu H, Fan L, Deng L, et al. MiR-1292 Targets FZD4 to Regulate Senescence and Osteogenic Differentiation of Stem Cells in TE/SJ/Mesenchymal Tissue System via the Wnt/β-catenin Pathway. Aging Dis, 2018, 9(6): 1103-1121. [Crossref]
92. Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, et al. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem, 2013, 374(1-2): 13-20. [Crossref]
93. Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev, 2020, 159: 332-343. [Crossref]
94. van der Pol E, Böing AN, Harrison P, Sturk A, & Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev, 2012, 64(3): 676- 705. [Crossref]
95. Tkach M, & Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell, 2016, 164(6): 1226-1232. [Crossref]
96. Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, et al. Methodological Guidelines to Study Extracellular Vesicles. Circ Res, 2017, 120(10): 1632-1648. [Crossref]
97. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, & Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev, 2018, 118(4): 1917-1950. [Crossref]
98. Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ, 2008, 15(11): 1723-1733. [Crossref]
99. Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res, 2008, 68(19): 7864-7871. [Crossref]
100.Ahmadi M, & Rezaie J. Ageing and mesenchymal stem cells derived exosomes: Molecular insight and challenges. Cell Biochem Funct, 2021, 39(1): 60-66. [Crossref]
101.Fafián-Labora J, Lesende-Rodriguez I, Fernández-Pernas P, Sangiao-Alvarellos S, Monserrat L, Arntz OJ, et al. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci Rep, 2017, 7: 43923. [Crossref]
102.Wang Y, Fu B, Sun X, Li D, Huang Q, Zhao W, et al. Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-β1- mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res Ther, 2015, 6: 185. [Crossref]
103.Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, et al. Microvesicles as Potential Biomarkers for the Identification of Senescence in Human Mesenchymal Stem Cells. Theranostics, 2017, 7(10): 2673-2689. [Crossref]
104.Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, et al. MicroRNA-183-5p Increases with Age in BoneDerived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng Part A, 2017, 23(21-22): 1231- 1240. [Crossref]
105.Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y, et al. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging (Albany NY), 2019, 11(6): 1791-1803. [Crossref]
106.Terlecki-Zaniewicz L, Lämmermann I, Latreille J, Bobbili MR, Pils V, Schosserer M, et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany NY), 2018, 10(5): 1103-1132. [Crossref]
107.Godfrey TC, Wildman BJ, Beloti MM, Kemper AG, Ferraz EP, Roy B, et al. The microRNA-23a cluster regulates the developmental HoxA cluster function during osteoblast differentiation. J Biol Chem, 2018, 293(45): 17646- 17660. [Crossref]
108.Yoo JK, Kim J, Choi SJ, Noh HM, Kwon YD, Yoo H, et al. Discovery and characterization of novel microRNAs during endothelial differentiation of human embryonic stem cells. Stem Cells Dev, 2012, 21(11): 2049-2057. [Crossref]
109.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiencyinduced osteoporosis. J Bone Miner Res, 2013, 28(3): 559-573. [Crossref]
110.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res, 2009, 37(14): 4850-4861. [Crossref]
111.Cho JH, Dimri M, & Dimri GP. MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence. J Biol Chem, 2015, 290(16): 10555-10567. [Crossref]
112.Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K, et al. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials, 2021, 272: 120718. [Crossref]
113.Sun Y, Zhang W, & Li X. Induced pluripotent stem cellderived mesenchymal stem cells deliver exogenous miR- 105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res Ther, 2021, 12(1): 286. [Crossref]
114.Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H, et al. miR- 203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res, 2018, 8(12): 2387-2401.
115.Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles, 2020, 9(1): 1723260. [Crossref]
116.Liu C, Xue J, Xu B, Zhang A, Qin L, Liu J, et al. Exosomes Derived from miR-146a-5p-Enriched Mesenchymal Stem Cells Protect the Cardiomyocytes and Myocardial Tissues in the Polymicrobial Sepsis through Regulating MYBL1. Stem Cells Int, 2021, 2021: 1530445. [Crossref]
117.Fafián-Labora J, Morente-López M, Sánchez-Dopico MJ, Arntz OJ, van de Loo FAJ, De Toro J, et al. Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Res Ther, 2020, 11(1): 13. [Crossref]
118.Jafarinia M, Alsahebfosoul F, Salehi H, Eskandari N, & Ganjalikhani-Hakemi M. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy. Immunol Invest, 2020, 49(7): 758-780. [Crossref]
119.Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, et al. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A, 2017, 114(17): E3536-e3545. [Crossref]
120.Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, et al. Melatoninstimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther, 2020, 11(1): 259. [Crossref]
121.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells, 2015, 33(7): 2158-2168. [Crossref]
122.Jiang C, Fu Y, Liu G, Shu B, Davis J, & Tofaris GK. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nanomicro Lett, 2021, 14(1): 3. [Crossref]
123.Ćulum NM, Cooper TT, Lajoie GA, Dayarathna T, Pasternak SH, Liu J, et al. Characterization of ovarian cancerderived extracellular vesicles by surface-enhanced Raman spectroscopy. Analyst, 2021, 146(23): 7194-7206. [Crossref]
124.Ćulum NM, Cooper TT, Bell GI, Hess DA, & Lagugné- Labarthet F. Characterization of extracellular vesicles derived from mesenchymal stromal cells by surface-enhanced Raman spectroscopy. Anal Bioanal Chem, 2021, 413(20): 5013-5024. [Crossref]
125.Gool EL, Stojanovic I, Schasfoort RBM, Sturk A, van Leeuwen TG, Nieuwland R, et al. Surface Plasmon Resonance is an Analytically Sensitive Method for Antigen Profiling of Extracellular Vesicles. Clin Chem, 2017, 63(10): 1633- 1641. [Crossref]
126.Suthar J, Parsons ES, Hoogenboom BW, Williams GR, & Guldin S. Acoustic Immunosensing of Exosomes Using a Quartz Crystal Microbalance with Dissipation Monitoring. Anal Chem, 2020, 92(5): 4082-4093. [Crossref]
127.Kilic T, Cho YK, Jeong N, Shin IS, Carter BS, Balaj L, et al. Multielectrode Spectroscopy Enables Rapid and Sensitive Molecular Profiling of Extracellular Vesicles. ACS Cent Sci, 2022, 8(1): 110-117. [Crossref]
128.Barile L, & Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther, 2017, 174: 63-78. [Crossref]
129.Zhang D, Lee H, Zhu Z, Minhas JK, & Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol, 2017, 312(1): L110-l121. [Crossref]
130.Mansilla MC, Cybulski LE, Albanesi D, & de Mendoza D. Control of membrane lipid fluidity by molecular thermosensors. J Bacteriol, 2004, 186(20): 6681-6688. [Crossref]