Open Access | Review
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Age effect on mesenchymal stem cell properties: a concise review
# These authors contributed equally to this work.
* Corresponding author: Tsz Kin Ng, PhD
Mailing address: Joint Shantou International Eye Center of Shantou University and the Chinese University of Hong Kong, North
Dongxia Road, Shantou, Guangdong 515041, China.
Email: micntk@hotmail.com
* Corresponding author: Herman S. Cheung, PhD
Mailing address: Department of Biomedical Engineering, College
of Engineering, University of Miami, Coral Gables, FL 33124,
USA.
Email: hcheungster@gmail.com
This article belongs to the Special Issue: Age-linked Stem Cell-Mediated dysfunction
Received: 02 November 2022 / Revised: 01 December 2022 / Accepted: 14 December 2022 / Published: 29 December 2022
DOI: 10.31491/APT.2022.12.101
Abstract
Mesenchymal stem cells belong to one of the multipotent stem cell types isolated from almost all tissues in the human body. They function to maintain tissue homeostasis with their highly proliferative property, and they also possess immunomodulatory properties. The properties of mesenchymal stem cells can be influenced by multiple factors, among which donor ages have been indicated negatively correlated with the proliferation, migration, and differentiation of mesenchymal stem cells. Telomerase activity, telomere length, and cell senescence have been studied to understand the mechanisms of the age effect on mesenchymal stem cell properties. Rejuvenation treatments are the critical research direction to attenuate the deterioration of mesenchymal stem cell properties by the age effect. This review article summarized the updated research on the impact and mechanisms of aging and age-related factors on different mesenchymal stem cell properties. In addition, the treatments to rejuvenate the aged mesenchymal stem cells will also be discussed. This review article aims to enlighten scientific researchers in better preparing and nursing the autologous mesenchymal stem cells from the elderly for future applications in tissue engineering and regenerative medicine.
Keywords
Mesenchymal stem cells, aging, proliferation, migration, differentiation
Introduction
Stem cells belong to the undifferentiated cells with the
ability to self-renewal and differentiation into mature cells.
They are essential for tissue growth, development, and
homeostasis. Adult stem cells refer to the stem cells found
in the developed tissues, and they function to maintain
adult tissue specificity by homeostatic cell replacement
and tissue regeneration [1]. They are presumed to be inactive within the adult tissues, but they can be stimulated to
divide into a stem cell clone and a transiently amplifying
cell. The latter will undergo limited divisions before terminally differentiating into mature functional tissue cells.
Because of lineage-restricted differentiation for adult stem
cells, different types of adult stem cells are equipped with
specific functions in different tissues and organs. Apart
from the most-studied blood-forming hematopoietic stem
cells (HSCs) [2], mesenchymal stem cells (MSCs), also
known as marrow stromal cells, belong to another multipotent adult stem cell population with potentials differentiating into the mesodermal lineages, including adipocytes, chondrocytes, and osteocytes [3]. Although MSCs
were first identified in bone marrow, they reside within the
connective tissues of many organs, including adipose tissue, umbilical cord, and teeth [4]. MSCs isolated from the
fetal tissues, including the umbilical cord, umbilical cord
blood, Wharton’s jelly, placenta, and amniotic membrane,
are considered the fetal MSCs, and the alternative is considered adult MSCs. Human MSCs can be sorted with the
positive selection by CD29, CD44, CD73, CD90, CD105,
CD146, and STRO-1 as well as the negative selection by CD31, CD34, CD45, CD49f, and CD133 [5]. Apart from
the expression of specific cell surface markers, MSCs
are also defined to be growing in adherence to the plastic
surface while maintained in standard culture conditions
and are capable to be in vitro induced into mesenchymal
lineages with the appropriate medium as recommended by
the International Society of Cellular Therapy [6].
MSCs have been applied as a therapeutic agent for the
treatment of various diseases, such as cardiovascular [7]
and neurodegenerative diseases [8, 9]. Autologous MSCs
are developed from the patients themselves without immune rejection, whereas allogeneic MSCs are established
from the selected donors allowing expansion on a large
scale and cryopreservation to provide a readily available
source of stem cells. Current allogeneic volunteer MSC
donors mostly are of a young age [10]. Yet, there are
substantial pieces of evidence demonstrating the aging
process adversely affects the properties of MSCs [11]. The
impacts of age and age-related factors on the MSC properties are of great importance for autologous or allogeneic
MSC transplantation, especially among the elderly. In this
review article, we will summarize the donor age effect on
MSC properties, together with the underlying molecular
mechanisms. In addition, the treatments attenuating or delaying the age effect on MSCs will also be discussed.
Age effect on mesenchymal stem cell yield from tissues
A study on MSCs derived from anterior cruciate ligaments demonstrated that the mean proportion of isolated MSCs was slightly but significantly higher in older donors (67.96 ± 5.22 years) than the younger donors (29.67 ± 10.92 years) [12]. In contrast, a study on MSCs in adiposederived stromal vascular fraction reported a negative correlation of MSC count with donor age [13]. Yet, human adipose-derived MSCs harvested from the same subjects with a time window of 7 to 12 years apart (initial age of the 3 donors: 17, 21, and 72 years old) show no significant difference was found in cell yield, stromal-vascular fraction subpopulation, proliferation, and tri-lineage differentiation [14]. These studies indicated that the effect of donor ages on the cell yield of MSCs harvested from donor tissues is still controversial.
Age effect on mesenchymal stem cell surface marker expression
A study reported that the subpopulations of bone marrowderived MSCs harvested from younger donors are composed of more CD71+ , CD146+ , and CD274+ MSCs than that from older donors, and the fluorescence per cell of CD71, CD90, CD106, CD140b, CD146, CD166, and CD274 is negatively correlated with the donor age [15]. Similarly, human bone marrow-derived MSCs express CD13, CD44, CD90, CD105, and Stro-1 regardless of age, but those from the donors over 40 years old showed significantly lower expression of CD90, CD105, and Stro- 1 [16]. Moreover, lower SSEA-4 expression was found in the elderly bone marrow-derived MSCs as compared to the young MSCs [17]. Consistently, our previous study also reported that lower expression of SSEA4 was found in human MSCs derived from periodontal ligaments with a donor age of > 40 years old as compared to those with a donor age of ≤ 20 years old [18]. In contrast, no significant difference in cell surface marker expression was reported in bone marrow-derived MSCs from the pediatric and adult donors [19]. Human adipose-derived MSCs from all age groups also show comparable expression of CD3, CD14, CD19, CD34, CD44, CD45, CD73, CD90, and CD105 [20]. The effect of donor ages on MSC marker expression could be exhibited in donors with older ages.
Age effect on mesenchymal stem cell proliferation
MSC proliferation is related to the availability and abundance of stem cells present to exert a regenerative effect. The number of colony-forming unit-fibroblasts (CFU-F) colonies with alkaline phosphatase (ALP) activity in bone marrow-derived MSCs of younger donors (3–36 years old) is significantly higher than that of the older donors (41 –70 years old) [21]. Consistently, there is a significant decline in the CFU-F number in bone marrow-derived MSCs from older donors (21–40 years old) as compared to younger donors (0–20 years old) [16]. Similar results were also observed in adipose-derived stem cells that a 30% decline in CFU numbers and with 38% increase in population doubling time from the donors with age > 50 years old as compared to those with age < 20 years old [22]. Moreover, the cumulative population doubling of bone marrow-derived MSCs from pediatric donors is twice that of young adult donors [19], and the doubling time is 1.7- fold longer in bone marrow-derived MSCs from the older subjects as compared to the younger subjects [23]. In addition, our previous study also found that human MSCs derived from periodontal ligaments with a donor aged ≤ 20 years old show significantly higher proliferation than that of a donor aged 21–40 years old and > 40 years old [18]. On the contrary, no significant differences in CFU numbers of bone marrow-derived MSCs among different donor ages were also reported [24- 26]. Interestingly, umbilical cord-derived MSCs from older mothers also show lower proliferative and colony-forming capacity as compared to those from younger mothers [27]. Yet, other studies demonstrated that human fetal membrane-derived MSCs from older mothers show a higher proliferation rate than those from younger mothers [28]. Collectively, there are prominent pieces of evidence that the proliferation of MSCs would be reduced in donors of older ages.
Age effect on mesenchymal stem cell migration
The movement of stem cells and their capacity to migrate to injury sites are the determining factors of stem cell regenerative potentials. The migration ability of human adipose-derived MSCs is significantly decreased in the elderly donors as compared to the child donors with a significant reduction in CXCR4 and CXCR7 expression in the elderly group [29]. Moreover, the migratory activity of human periodontal ligament-derived MSCs with a donor age of 56–75 years old is significantly decreased as compared to those with a donor age of 16–30 years old [30]. Consistently, our previous study also demonstrated that human periodontal ligament-derived MSCs with donor age > 40 years old show significantly lower migration as compared to that with donor age ≤ 20 years old and 20– 40 years old, accompanied by lower expression of PTK2 in the periodontal ligament-derived MSCs with donor age > 40 years old [18]. Notably, bone marrow from the aged mice can induce a slower migration ability of murine MSC cell line C3H10T1/2 as compared to that from the young mice [31]. Collectively, aging, together with the aged tissue microenvironment, could reduce the migration ability of MSCs.
Age effect on mesenchymal stem cell differentiation
MSCs, equipped with multipotent differentiation potential,
can give rise to mesenchyme tissue cells, including adipocytes, osteoblasts, chondrocytes, myocytes, and cardiomyocytes. As compared to the younger adipose-derived
MSCs, the aged MSCs show decreased chondrogenic and
osteogenic potential, but are in favor of shifting towards
adipogenic differentiation with increasing age [32]. Yet,
another study reported that the osteogenic and chondrogenic potentials of adipose-derived MSCs decline with the
donor age, but the adipogenic potential of adipose-derived
MSCs is independent of the donor age [20]. Advancing
age has been demonstrated to have a significant negative
effect on the adipogenic and osteogenic differentiation
potentials of human adipose-derived MSCs [29], while no
differences in the differentiation efficiency in adipogenesis
and osteogenesis between young (≤ 35 years old) and old
(≥ 55 years old) adipose-derived MSCs have also been
reported [33]. In contrast, the adipogenic and osteogenic
potentials of bone marrow-derived MSCs decrease with
increasing age while the chondrogenic potential did not
change [34]. Besides, the osteogenic differentiation of
bone marrow-derived MSCs is more affected by age than
the adipose-derived MSCs [35]. No significant differences in the osteogenic differentiation capacity of bone
marrow-derived MSCs between young and aged donors
have also been reported [15, 26]. Under a moderate level
of inflammatory stimuli, osteogenic differentiation of
bone marrow-derived MSCs from elderly donors could be
greatly diminished, and adipogenic differentiation remains
unchanged, while the bone marrow-derived MSCs from
young and intermediately aged donors show better osteogenic differentiation but reduced adipogenic differentiation [36]. For human periodontal ligament-derived MSCs,
the osteogenic and adipogenic differentiation capacities of
human periodontal ligament-derived MSCs are reduced
when age increases [30]. Consistently, our previous study
demonstrated that the osteogenic, chondrogenic, and adipogenic differentiation abilities of human periodontal
ligament-derived MSCs with donor age > 40 years old
are all reduced as compared to those with donor age ≤ 20
years old [18]. Collectively, the age effect on the differentiation of different mesodermal lineages of MSCs could be
dependent on the originated cell sources and the microenvironments.
Apart from mesodermal lineage differentiation, we have
previously demonstrated that human periodontal ligamentderived MSCs and adipose-derived MSCs can be induced
into neural and retinal lineages [37- 39]. It has been reported that the neuroectodermal differentiation potential
of human bone marrow-derived MSCs from old donors
(> 45 years old) is completely lost, with no cells showing
mature neuroectodermal phenotypes and fewer cells expressing early neuroectodermal marker proteins as compared to that of the young donors (18–35 years old) [40].
Yet, additional studies are needed to validate the age effect
on the neural differentiation of MSCs.
Age effect on immunomodulation of mesenchymal stem cells
The allogeneic transplantation of MSCs can be achieved because of the immunomodulatory properties of MSCs. It has been reported that adult adipose-derived MSCs (< 65 years old) inhibit the activated CD4+ T-lymphocytes more effectively than elderly adipose-derived MSCs (≥ 65 years old) with increasing mean CD4+ T-lymphocyte proliferation by 0.5 % for any 1-year increase in age [41]. However, it was also shown that gingival tissue-derived MSCs display effective immunoregulation in a mouse model of lipopolysaccharide-induced acute lung injury irrespective of donor age [42]. Similarly, human dental pulp-derived MSCs have been shown effectively regulate the CD4+ T cells; yet, their effects on Th1 and Th2 cells are not affected by the donor ages [43]. In mouse, the aged MSCs presented with a lower immunomodulatory property to induce T cell apoptosis in the co-culture system as compared to the young MSCs [44]. For our previous study, we demonstrated that human periodontal ligament-derived MSCs with donors ages 20–40 and > 40 years old show higher IL6 and CXCL8 expression [18]. Elevated expressions of IL6 and CXCL8 are also reported in adult MSCs as compared with pediatric MSCs [45]. These could indicate that the microenvironment around the aged MSCs could be inflammatory, reflected by the accumulation of inflammatory T and B lymphocytes [44].
Age effect on the neuroprotective effect of mesenchymal stem cells
We have previously demonstrated that human periodontal ligament-derived MSCs can protect retinal ganglion cells from optic nerve injury by secreting the brain-derived neurotrophic factor and interacting with the host cells in the retina [46]. It has been reported that bone marrow-derived MSCs from both young (16–18 years) and old (67–75 years) donors in a co-culture system significantly enhance total neurite length of dorsal root ganglia neurons, and only the MSCs from young donors, but not the old donors, can further be potentiated by the treatment of growth factors [47]. Moreover, under the culture with a conditioned medium of bone marrow-derived MSCs, the rescue ability of MSCs on the reduced survival of rat cortical neurons by trophic factor withdrawal decrease with increasing MSC donor age [48]. In addition, it has been suggested that the composition of the secreted bio-active materials of MSCs derived from human tooth germ is influenced by the passage number of the cells [49]. These indicate that increasing MSC age could weaken its ability to neurotrophic factor secretion and compositions, which leads to the reduced neuroprotective effect of the aged MSCs.
Molecular mechanisms of age effect on mesenchymal stem cells
Telomere length
The length of the telomere is an indicator of the mitotic capacity of a cell. Telomere shortening is considered a hallmark of stem cell aging [50]. It has been reported that the infant adipose-derived MSCs exhibited longer telomere lengths than the elderly MSCs [51]. Consistently, our previous study demonstrated that human periodontal ligament-derived MSCs with a donor age > 40 years old have shorter telomere lengths than those with a donor age ≤ 20 years old [18]. However, the same telomere length, regardless of the donor’s age, has also been demonstrated in human adipose-derived MSCs [52]. Similarly, no difference in telomere length was found in bone marrowderived MSCs from younger (8 months–6 years old) and older (38–58 years old) donors [53]. The telomere lengths in native bone marrow-derived MSC are also not related to the ages of the donors [54]. In placenta-derived MSCs, the telomere lengths could be related to cell division rather than the aging of the mothers [55]. Collectively, the role of telomere length in the age effect on MSC properties is still controversial.
Telomerase activity
Telomerase (telomere terminal transferase) is a reverse transcriptase responsible for maintaining the telomere length via de novo telomere synthesis [56]. Telomerase activity is related to the proliferation capability of MSCs [57]. Low levels of telomerase activity were reported in bone marrow-derived MSCs in a study [54], and another study reported no telomerase activity is detected in bone marrow-derived MSCs from different ages of human donors [15]. Yet, the analysis of the microarray datasets GSE97311 and GSE68374 revealed that some of the downregulated genes in the aged adult bone marrow-derived MSCs are involved in the telomerase activity as compared to the fetal MSCs [58]. In addition, telomerase expression was reported to be lower in bone marrow-derived MSCs from the adult rats as compared to that from the young rats [59]. The role of telomerase activity in the age effect on MSC properties requires further investigations.
Cell senescence
Cellular senescence is a special form of durable cell cycle arrest, leading to the gradual decline in the ability of cell proliferation, differentiation, and physiological function over time. Senescent cells are characterized by durable growth arrest, expressions of anti-proliferative molecules, such as p16INK4a, and activation of damage-sensing signaling pathways, including p38 and NF-κB [60]. A significant increase in quiescence of the G2 and S phase was reported in adipose-derived MSCs from the aged donors with increased expression of CHEK1 and p16INK4a genes with age [22]. The donor age of adipose-derived MSCs is associated with an increase in the expression of senescenceassociated β-galactosidase staining with p16 and p21 gene expression higher in adipose-derived MSCs from the aged donors (> 50 years) than the young donor (< 40 years) [20]. The increase in senescence-associated β-galactosidasepositive cells in the elderly human adipose-derived mesenchymal stem cells is accompanied by increased mitochondrial-specific reactive oxygen species production and the p21 expression [29]. Similarly, the percentage of senescence-associated β-galactosidase-positive cells is tremendously increased in bone marrow-derived MSCs from the aged donors (> 60 years old) as compared to the young donors (< 30 years old) [61]. Moreover, the numbers of p21-positive and p53-positive cells were also found to be significantly higher in bone marrow-derived MSCs from the aged donors (> 40 years old) as compared to the young donors (7–18 years old) [16]. Critically, NAP1L2 is a regulator for cell senescence of bone marrow-derived MSCs through the activation of the NF-κB pathway [62], whereas follistatin is a marker for human bone marrowderived MSC aging [63]. For the gingival tissue-derived MSCs, an increase in p53 and sirtuin-1 expression was shown in MSCs from the elderly donors (59–80 years old) as compared to the young donors (13–31 years old) [42]. Yet, no evidence of cellular senescence was reported in bone marrow-derived MSCs from pediatric and adult donors [19]. Collectively, the pieces of evidence of the involvement of cell senescence in the age effect of MSC properties are substantial (Figure 1).
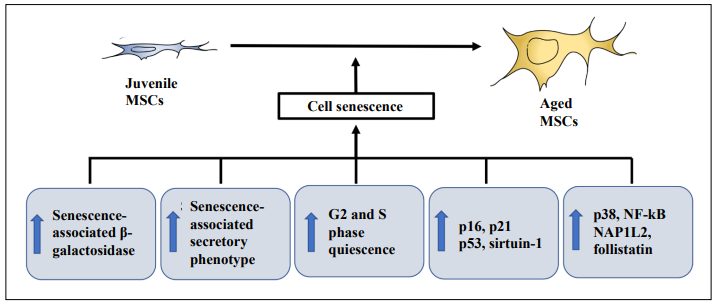
Figure 1. Cell senescence in aged mesenchymal stem cells. Increase in senescence-associated β-galactosidase staining, quiescence of G2 and S phases, expressions of p16, p21, p38, p53, and sirtuin-1, activation of nuclear factor-κB (NF-κB), nucleosome assembly protein 1 like 2 (NAP1L2), follistatin, and senescence-associated secretory phenotype (SASP) have been shown contributing to cell senescence in the aged mesenchymal stem cells (MSCs).
A hallmark of aging is chronic, low-grade, “sterile” inflammation [64]. Cellular senescence is associated with the production of pro-inflammatory chemokines, cytokines, and extracellular matrix remodeling proteases, which comprise the senescence-associated secretory phenotype (SASP) [65]. Accumulation of senescent fat progenitor cells has been found in adipose tissue with aging, and the senescent cells acquire SASP and provoke inflammation in adipose tissue with JAK pathway activation in adipose tissue with aging [66]. Exposure to TNF-α could induce the upregulation of SASP components in adiposederived MSCs, including interleukin (IL)-6, IL-8, and monocyte chemoattractant protein 1 (MCP-1) [67]. It has been reported that transplanting relatively small numbers of senescent cells into young mice is sufficient to spread cellular senescence to host tissues and cause persistent physical dysfunction [68], indicating the endocrine effects of the senescent cells. Consistently, transplanting adiposederived MSCs from old donors, but not from young donors, induces physical dysfunction in older recipient mice owing to a naturally occurring senescent cell-like population in adipose-derived MSCs primarily from old donors [69]. Therefore, the senescent MSCs could limit the application of exogenous autologous delivery of MSCs from aged donors and impose a potential risk to the shortening of the health- and lifespan of the recipients. Rejuvenation of the senescent MSCs could be helpful to improve autologous MSC transplantation in elderly individuals.
Rejuvenation of the aged mesenchymal stem cells
Rejuvenation refers to the restoration of youthful vigor. Multiple strategies have been studied to rejuvenate the aged mesenchymal stem cells (Figure 2) to improve their properties for treatments.
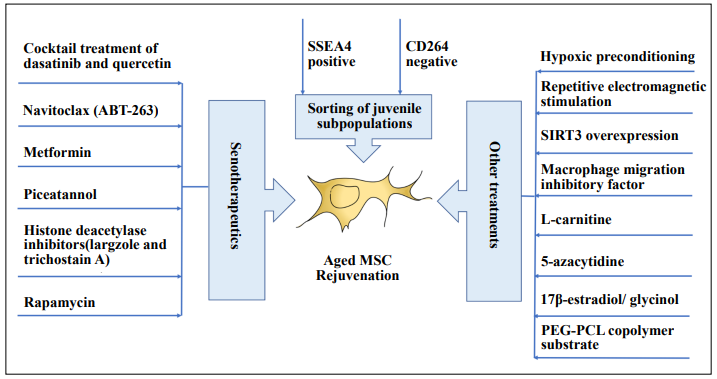
Figure 2. Rejuvenation of the aged mesenchymal stem cells. Sorting of juvenile subpopulations among the aged mesenchymal stem cells (MSCs), senotherapeutics, hypoxic preconditioning, repetitive electromagnetic stimulation, sirtuin-3 (SIRT3) and macrophage migration inhibitory factor overexpression, treatment with L-carnitine, 17β-estradiol, glycinol, and 5-azacytidine, and culturing on the poly(ethylene glycol)-poly(ε-caprolactone) (PEG-PCL) copolymer substrate have been studied as the rejuvenation strategies on the aged MSCs.
Sorting of juvenile subpopulations
MSCs are heterogeneous in the population [1]. We have previously isolated the pluripotent neural crest subpopulation from human periodontal ligament-derived MSCs [70], suggesting that there could be “juvenile” cells residing in the aged MSCs as a rare subpopulation. Consistent with our study, 8% of the SSEA-4-positive subpopulation was identified in human bone marrow-derived MSCs from elderly donors and exhibits a “youthful” phenotype that is similar to that of young MSCs with the number of cells increased by 17,000 folds [17]. Moreover, it has been shown that the sorted CD264+ human bone marrowderived MSCs have elevated β-galactosidase activity, decreased differentiation potential, and are inefficient in colony formation relative to the CD264- MSCs [71], indicating that CD264- is a selection method for the “juvenile” MSCs. Yet, CD271 might not be the marker for the isolation of the “juvenile” cells from the aged MSCs [72].
Senotherapeutics
Senotherapeutics refers to a strategy targeting cellular senescence to delay the aging process. Senotherapeutics are composed of analytics (selectively inducing senescent cell death) and xenomorphic (indirectly suppressing senescence by inhibiting SASP to delay the progression of senescence and tissue dysfunction) [73]. Treatment with dasatinib significantly increases the number of apoptotic PEadipose-derived MSCs from women with preeclampsia as compared to those from normotensive pregnancies by decreasing the gene expression of p16 and SASP components [67]. Cocktail treatment of dasatinib and quercetin can decrease the number of naturally occurring senescent All Rights Reserved cells and their secretion of frailty-related pro-inflammatory cytokines in explants of human adipose tissue [68], and improve the osteogenic capacity of bone marrow-derived MSCs from the aged mice [74]. Navitoclax (ABT-263) has been demonstrated with a moderate senolytic effect on senescent human bone marrow-derived MSCs by reducing the senescence-associated β-galactosidase expression [75], whereas metformin reduces the replicative senescence and cell death associated with the prolonged cultivation of human adipose-derived MSCs [76]. Moreover, piceatannol has been shown to reduce the number of senescent human bone marrow-derived MSCs after genotoxic stress and in senescent replicative cultures by promoting the recovery of cell proliferation and the stemness of MSCs [77]. Similarly, largazole and trichostatin A, the histone deacetylase inhibitors, can improve human umbilical cord-derived MSCs proliferation and delay its aging [78]. In addition, rapamycin has also been reported to reverse the senescent phenotype and improve the immunoregulation of human bone marrow-derived MSCs from systemic lupus erythematosus patients by inhibiting the mTOR signaling pathway [79]. Collectively, senotherapeutics should be a promising and emerging treatment strategy to remove senescent MSCs from aged donors.
Other treatments
Hypoxic preconditioning induced by 2, 4-dinitrophenol can improve the regeneration potential of aging bone marrow-derived MSCs into pancreatic β-cells [80]. Similarly, hypoxic preconditioning can improve the in vivo angiogenic capacities of human adipose-derived MSCs from older donors [81]. Moreover, preconditioning the bone marrow-derived MSCs with repetitive electromagnetic stimulation can enhance CFU-F and cell proliferation in bone marrow-derived MSCs, more effectively from the older donors than the young donors, via transient nitric oxide production and extracellular signal-regulated kinase 1/2 activation [82]. For gene modulation, SIRT3 overexpression can protect human bone marrow-derived MSCs from older donors against oxidative damage by activating catalase and manganese-dependent SOD through FOXO3a and improved their cell myocardial repair effect [83]. The improvement of myocardial repair by the aged MSCs can also be achieved by modulating the macrophage migration inhibitory factor that overexpressing macrophage migration inhibitory factor in human bone marrow-derived MSCs from older donors can reduce cellular senescence, activate autophagy, induce angiogenesis, prevent cardiomyocyte apoptosis, and improve the heart function and cell survival after myocardial infarction [84]. In addition, treatment with L-carnitine has been demonstrated to increase the gene expression of human telomerase reverse transcriptase and telomere length in human adipose tissue-derived MSCs isolated from healthy aged volunteers [85]. For the osteogenic differentiation, we have previously demonstrated that treatment of 5 μmol/L curcumin can enhance the osteogenic differentiation of human bone marrow-derived MSCs via matrix metalloproteinase-13 expression and activity [86]. Treatments with 17β-estradiol and glycinol have also been demonstrated to rescue the age-related reduction in osteogenic differentiation of bone marrow-derived MSCs isolated from older donors through estrogen receptor signaling [87], whereas treatment of 5-azacytidine induces the proliferation and improves the osteogenic differentiation potential of adipose-derived MSCs from older donors with DNA demethylation and increased TET2 and TET3 gene expression [88]. Furthermore, it has been reported that culture of the bone marrow-derived MSCs from aged human donors on a poly(ethylene glycol)-poly(ε-caprolactone) copolymer substrate can decrease levels of detected intracellular ROS levels in the aged MSCs and promoting the osteogenic differentiation [89].
Challenges and prospects
Aging is a life-long process of living toward old age,
which is characterized by the progressive loss of physiological functions that could lead to diseases and death.
The effect of aging on MSCs is complex and complicated,
involving genetic martial deterioration, non-coding RNAs,
exosomes, protein imbalance, mitochondrial dysfunction,
reactive oxygen species as well as the mTOR, and insulin/
IGF-1-like signaling pathways [90]. However, as a lifelong process, MSCs are not just influenced by aging. In
the real world, other environmental exposures and behaviors can also influence the properties of MSCs [91,92].
The influences of these personalized factors also need to
be considered in the analysis of the donor effect. Refine
phenotyping and grouping with larger sample sizes could
help to resolve the effects of specific factors on MSC
properties. Single-cell and spatial transcriptomics could
also help to delineate the specific aging cells among the
heterogeneous subpopulations of MSCs [93].
The induced pluripotent (iPS) stem cells [94] is demonstrated as an example of rejuvenation. There is still a
lack of consensus on the standard/clinically recognized
rejuvenation strategies for aged MSCs although numerous
anti-aging strategies have been proposed [95]. Yet, MSCs
possess diversified properties for different treatment approaches [9], and different rejuvenation approaches might
be needed for different MSC properties. Further studies
are needed to optimize the condition and quality of MSCs
in the treatment regime for each MSC property. Despite the uncertainties regarding the application of aged MSCs,
MSC therapy would still be a promising and important
strategy for the treatment of different diseases.
Declarations
Authors’ contributions
T.K.N. and H.S.C. conception and design. Y.H. and T.K.N. financial support. C.B.C., X.B., and Y.H. collection and/or assembly of data. C.B.C., X.B., Y.H., and T.K.N. data analysis and interpretation. C.B.C., X.B., Y.H., T.K.N., and H.S.C. manuscript writing.
Financial support and sponsorship
This work was supported in part by the Special Fund of Guangdong Province for Science and Technology (project code: 210714156882753 to Y.H.), and the Joint Regional Basic Science and Applied Basic Science Research Fund of Guangdong Province (project code: 2019A1515110685 to T.K.N.), China.
Conflicts of interest
The authors declare no potential conflicts of interest.
References
1. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell, 2004, 116(5): 639-648.[Crossref]
2. Dunn CD. The differentiation of haemopoietic stem cells. Ser Haematol, 1971, 4(4): 1-71.
3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science, 1999, 284(5411): 143- 147. [Crossref]
4. Young HE, Mancini ML, Wright RP, Smith JC, Black AC Jr, Reagan CR, et al. Mesenchymal stem cells reside within the connective tissues of many organs. Dev Dyn, 1995, 202(2): 137-144. [Crossref]
5. Lv FJ, Tuan RS, Cheung KM, & Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells, 2014, 32(6): 1408-1419. [Crossref]
6. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006, 8(4): 315-317. [Crossref]
7. Bolli R, Mitrani RD, Hare JM, Pepine CJ, Perin EC, Willerson JT, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail, 2021, 23(4): 661-674. [Crossref]
8. Ng TK, Fortino VR, Pelaez D, & Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells, 2014, 6(2): 111-119. [Crossref]
9. Tan S, Yao Y, Yang Q, Yuan XL, Cen LP, & Ng TK. Diversified Treatment Options of Adult Stem Cells for Optic Neuropathies. Cell Transplant, 2022, 31: 9636897221123512. [Crossref]
10. Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, & Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med, 2016, 14(1): 145. [Crossref]
11. Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, & Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell, 2011, 10(1): 66-79. [Crossref]
12. Lee DH, Ng J, Kim SB, Sonn CH, Lee KM, & Han SB. Effect of donor age on the proportion of mesenchymal stem cells derived from anterior cruciate ligaments. PLoS One, 2015, 10(3): e0117224. [Crossref]
13. Karadağ Sarı EÇ, Ovalı E. Factors Affecting the Population of Mesenchymal Stem Cells in Adipose-Derived Stromal Vascular Fraction. Balkan Med J, 2022, In press. [Crossref]
14. Kokai LE, Traktuev DO, Zhang L, Merfeld-Clauss S, DiBernardo G, Lu H, et al. Adipose Stem Cell Function Maintained with Age: An Intra-Subject Study of Long-Term Cryopreserved Cells. Aesthet Surg J, 2017, 37(4): 454- 463. [Crossref]
15. Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, & Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med, 2013, 11: 146. [Crossref]
16. Stolzing A, Jones E, McGonagle D, & Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev, 2008, 129(3):163-173. [Crossref]
17. Block TJ, Marinkovic M, Tran ON, Gonzalez AO, Marshall A, Dean DD, et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther, 2017, 8(1): 239. [Crossref]
18. Ng TK, Chen CB, Xu C, Xu Y, Yao X, Huang L, et al. Attenuated regenerative properties in human periodontal ligament-derived stem cells of older donor ages with shorter telomere length and lower SSEA4 expression. Cell Tissue Res, 2020, 381(1): 71-81. [Crossref]
19. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem, 2006, 97(4): 744-754. [Crossref]
20. Choudhery MS, Badowski M, Muise A, Pierce J, & Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med, 2014, 12: 8. [Crossref]
21. D’Ippolito G, Schiller PC, Ricordi C, Roos BA, & Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res, 1999, 14(7): 1115-1122. [Crossref]
22. Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res, 2012, 8(2): 215-225. [Crossref]
23. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell, 2008, 7(3): 335-343. [Crossref]
24. Stenderup K, Justesen J, Eriksen EF, Rattan SI, & Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res, 2001, 16(6): 1120-1129. [Crossref]
25. Dexheimer V, Mueller S, Braatz F, & Richter W. Reduced reactivation from dormancy but maintained lineage choice of human mesenchymal stem cells with donor age. PLoS One, 2011, 6(8): e22980. [Crossref]
26. Prall WC, Saller MM, Scheumaier A, Tucholski T, Taha S, Böcker W, et al. Proliferative and osteogenic differentiation capacity of mesenchymal stromal cells: Influence of harvesting site and donor age. Injury, 2018, 49(8): 1504- 1512. [Crossref]
27. Huang S, Feng C, Wu Y, Yang S, Ma K, Wu X, et al. Dissimilar characteristics of umbilical cord mesenchymal stem cells from donors of different ages. Cell Tissue Bank, 2013, 14(4): 707-713. [Crossref]
28. Alrefaei GI, Alkarim SA, & Abduljabbar HS. Impact of Mothers’ Age on Telomere Length and Human Telomerase Reverse Transcriptase Expression in Human Fetal Membrane-Derived Mesenchymal Stem Cells. Stem Cells Dev, 2019, 28(24): 1632-1645. [Crossref]
29. Liu M, Lei H, Dong P, Fu X, Yang Z, Yang Y, et al. AdiposeDerived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell Transplant, 2017, 26(9): 1505-1519. [Crossref]
30. Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, & Chen FM. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials, 2012, 33(29): 6974-6986. [Crossref]
31. Yang YM, Li P, Cui DC, Dang RJ, Zhang L, Wen N, et al. Effect of aged bone marrow microenvironment on mesenchymal stem cell migration. Age (Dordr), 2015, 37(2): 16. [Crossref]
32. Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, & Henry BM. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Stem Cells Int, 2016, 2016: 2152435. [Crossref]
33. Horinouchi CD, Barisón MJ, Robert AW, Kuligovski C, Aguiar AM, & Dallagiovanna B. Influence of donor age on the differentiation and division capacity of human adipose-derived stem cells. World J Stem Cells, 2020, 12(12): 1640-1651. [Crossref]
34. Zaim M, Karaman S, Cetin G, & Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol, 2012, 91(8): 1175-1186. [Crossref]
35. Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med, 2012, 16(3): 582-593. [Crossref]
36. Laschober GT, Brunauer R, Jamnig A, Singh S, Hafen U, Fehrer C, et al. Age-specific changes of mesenchymal stem cells are paralleled by upregulation of CD106 expression as a response to an inflammatory environment. Rejuvenation Res, 2011, 14(2): 119-131. [Crossref]
37. Ng TK, Yung JS, Choy KW, Cao D, Leung CK, Cheung HS, et al. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci Rep, 2015, 5: 16429. [Crossref]
38. Ng TK, Yang Q, Fortino VR, Lai NY, Carballosa CM, Greenberg JM, et al. MicroRNA-132 directs human periodontal ligament-derived neural crest stem cell neural differentiation. J Tissue Eng Regen Med, 2019, 13(1): 12-24. [Crossref]
39. Huang Y, Ng TK, Chen CB, Huang B, Liang J, Pang CP, et al. Notch Signaling Activation Enhances Human AdiposeDerived Stem Cell Retinal Differentiation. Stem Cells Int, 2018, 2018: 9201374. [Crossref]
40. Hermann A, List C, Habisch HJ, Vukicevic V, EhrhartBornstein M, Brenner R, et al. Age-dependent neuroectodermal differentiation capacity of human mesenchymal stromal cells: limitations for autologous cell replacement strategies. Cytotherapy, 2010, 12(1): 17-30. [Crossref]
41. Kizilay Mancini O, Shum-Tim D, Stochaj U, Correa JA, & Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res Ther, 2015, 6(1): 140. [Crossref]
42. Dave JR, Chandekar SS, Behera S, Desai KU, Salve PM, Sapkal NB, et al. Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age. Sci Adv, 2022, 8(25): eabm6504. [Crossref]
43. Özgül Özdemir RB, Özdemir AT, Kırmaz C, Eker Sarıboyacı A, Karaöz E, Erman G, et al. Age-related changes in the immunomodulatory effects of human dental pulp derived mesenchymal stem cells on the CD4+ T cell subsets. Cytokine, 2021, 138:155367. [Crossref]
44. Aung KT, Akiyama K, Kunitomo M, Mun AY, Tosa I, Nguyen HTT, et al. Aging-Affected MSC Functions and Severity of Periodontal Tissue Destruction in a Ligature-Induced Mouse Periodontitis Model. Int J Mol Sci, 2020, 21(21): 8103. [Crossref]
45. O’Hagan-Wong K, Nadeau S, Carrier-Leclerc A, Apablaza F, Hamdy R, Shum-Tim D, et al. Increased IL-6 secretion by aged human mesenchymal stromal cells disrupts hematopoietic stem and progenitor cells’ homeostasis. Oncotarget, 2016, 7(12): 13285-13296. [Crossref]
46. Cen LP, Ng TK, Liang JJ, Zhuang X, Yao X, Yam GH, et al. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells, 2018, 36(6): 844-855. [Crossref]
47. Brohlin M, Kingham PJ, Novikova LN, Novikov LN, & Wiberg M. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS One, 2012, 7(9), e45052. [Crossref]
48. Sarkar P, Redondo J, Kemp K, Ginty M, Wilkins A, Scolding NJ, et al. Reduced neuroprotective potential of the mesenchymal stromal cell secretome with ex vivo expansion, age and progressive multiple sclerosis. Cytotherapy, 2018, 20(1): 21-28. [Crossref]
49. Yalvaç ME, Yarat A, Mercan D, Rizvanov AA, Palotás A, & Şahin F. Characterization of the secretome of human tooth germ stem cells (hTGSCs) reveals neuro-protection by fine-tuning micro-environment. Brain Behav Immun, 2013, 32: 122-130. [Crossref]
50. Fathi E, Charoudeh HN, Sanaat Z, & Farahzadi R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig, 2019, 6: 7. [Crossref]
51. Wu W, Niklason L, & Steinbacher DM. The effect of age on human adipose-derived stem cells. Plast Reconstr Surg, 2013, 131(1): 27-37. [Crossref]
52. Ding DC, Chou HL, Hung WT, Liu HW, & Chu TY. Human adipose-derived stem cells cultured in keratinocyte serum free medium: Donor’s age does not affect the proliferation and differentiation capacities. J Biomed Sci, 2013, 20(1): 59. [Crossref]
53. Lund TC, Kobs A, Blazar BR, & Tolar J. Mesenchymal stromal cells from donors varying widely in age are of equal cellular fitness after in vitro expansion under hypoxic conditions. Cytotherapy, 2010, 12(8): 971-981. [Crossref]
54. Parsch D, Fellenberg J, Brümmendorf TH, Eschlbeck AM, & Richter W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med (Berl), 2004, 82(1): 49-55. [Crossref]
55. Guerrero EN, Vega S, Fu C, De León R, Beltran D, & Solis MA. Increased proliferation and differentiation capacity of placenta-derived mesenchymal stem cells from women of median maternal age correlates with telomere shortening. Aging (Albany NY), 2021, 13(22): 24542- 24559. [Crossref]
56. Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell, 1987, 51(6): 887-898. [Crossref]
57. Piper SL, Wang M, Yamamoto A, Malek F, Luu A, Kuo AC, et al. Inducible immortality in hTERT-human mesenchymal stem cells. J Orthop Res, 2012, 30(12): 1879-1885. [Crossref]
58. Liu X, Yin M, Liu X, Da J, Zhang K, Zhang X, et al. Analysis of Hub Genes Involved in Distinction Between Aged and Fetal Bone Marrow Mesenchymal Stem Cells by Robust Rank Aggregation and Multiple Functional Annotation Methods. Front Genet, 2020, 11: 573877. [Crossref]
59. Hell RC, Ocarino NM, Boeloni JN, Silva JF, Goes AM, Santos RL, et al. Physical activity improves age-related decline in the osteogenic potential of rats’ bone marrowderived mesenchymal stem cells. Acta Physiol (Oxf), 2012, 205(2): 292-301. [Crossref]
60. He S, Sharpless NE. Senescence in Health and Disease. Cell, 2017, 169(6): 1000-1011. [Crossref]
61. Mohd Ali N, Boo L, Yeap SK, Ky H, Satharasinghe DA, Liew WC, et al. Probable impact of age and hypoxia on proliferation and microRNA expression profile of bone marrow-derived human mesenchymal stem cells. PeerJ, 2016, 4: e1536. [Crossref]
62. Hu M, Xing L, Zhang L, Liu F, Wang S, Xie Y, et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell, 2022, 21(2): e13551. [Crossref]
63. Alves H, van Ginkel J, Groen N, Hulsman M, Mentink A, Reinders M, et al. A mesenchymal stromal cell gene signature for donor age. PLoS One, 2012, 7(8): e42908. [Crossref]
64. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell, 2013, 153(6): 1194- 1217. [Crossref]
65. Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol, 2008, 6(12): 2853-2868. [Crossref]
66. Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A, 2015, 112(46): E6301-6310. [Crossref]
67. Suvakov S, Cubro H, White WM, Butler Tobah YS, Weissgerber TL, Jordan KL, et al. Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ, 2019, 10(1): 49. [Crossref]
68. Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med, 2018, 24(8): 1246-1256. [Crossref]
69. Wang B, Liu Z, Chen VP, Wang L, Inman CL, Zhou Y, et al. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell, 2020, 19(3): e13106. [Crossref]
70. Pelaez D, Huang CY, & Cheung HS. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev, 2013, 22(21): 2906-2914. [Crossref]
71. Madsen SD, Jones SH, Tucker HA, Giler MK, Muller DC, Discher CT, et al. Survival of aging CD264+ and CD264- populations of human bone marrow mesenchymal stem cells is independent of colony-forming efficiency. Biotechnol Bioeng, 2020, 117(1): 223-237. [Crossref]
72. Jones E, English A, Churchman SM, Kouroupis D, Boxall SA, Kinsey S, et al. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum, 2010, 62(7): 1944-1954. [Crossref]
73. Wong PF, Dharmani M, & Ramasamy TS. Senotherapeutics for mesenchymal stem cell senescence and rejuvenation. Drug Discov Today, 2022, 28(1): 103424. [Crossref]
74. Zhou Y, Xin X, Wang L, Wang B, Chen L, Liu O, et al. Senolytics improve bone forming potential of bone marrow mesenchymal stem cells from aged mice. NPJ Regen Med, 2021, 6(1): 34. [Crossref]
75. Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, & Wagner W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther, 2018, 9(1): 108. [Crossref]
76. Acar MB, Ayaz-Güner Ş, Gunaydin Z, Karakukcu M, Peluso G, Di Bernardo G, et al. Proteomic and Biological Analysis of the Effects of Metformin Senomorphics on the Mesenchymal Stromal Cells. Front Bioeng Biotechnol, 2021, 9: 730813. [Crossref]
77. Alessio N, Squillaro T, Lettiero I, Galano G, De Rosa R, Peluso G, et al. Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? Int J Mol Sci, 2021, 22(21): 11619. [Crossref]
78. Wang Y, Chen T, Yan H, Qi H, Deng C, Ye T, et al. Role of histone deacetylase inhibitors in the aging of human umbilical cord mesenchymal stem cells. J Cell Biochem, 2013, 114(10): 2231-2239. [Crossref]
79. Gu Z, Tan W, Ji J, Feng G, Meng Y, Da Z, et al. Rapamycin reverses the senescent phenotype and improves immunoregulation of mesenchymal stem cells from MRL/ lpr mice and systemic lupus erythematosus patients through inhibition of the mTOR signaling pathway. Aging (Albany NY), 2016, 8(5): 1102-1114. [Crossref]
80. Waseem M, Khan I, Iqbal H, Eijaz S, Usman S, Ahmed N, et al. Hypoxic Preconditioning Improves the Therapeutic Potential of Aging Bone Marrow Mesenchymal Stem Cells in Streptozotocin-Induced Type-1 Diabetic Mice. Cell Reprogram, 2016, 18(5): 344-355. [Crossref]
81. De Barros S, Dehez S, Arnaud E, Barreau C, Cazavet A, Perez G, et al. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol Ther, 2013, 21(2): 399- 408. [Crossref]
82. Nam S, Kim S, Yoon K, Hong HS, & Son Y. Preconditioning with repetitive electromagnetic stimulation enhances activity of bone marrow mesenchymal stem cells from elderly patients through Erk1/2 via nitric oxide. Int J Mol Med, 2020, 45(2): 678-686. [Crossref]
83. Zhang DY, Gao T, Xu RJ, Sun L, Zhang CF, Bai L, et al. SIRT3 Transfection of Aged Human Bone Marrow-Derived Mesenchymal Stem Cells Improves Cell Therapy-Mediated Myocardial Repair. Rejuvenation Res, 2020, 23(6): 453- 464. [Crossref]
84. Zhang Y, Zhu W, He H, Fan B, Deng R, Hong Y, et al. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging (Albany NY), 2019, 11(24): 12641-12660. [Crossref]
85. Farahzadi R, Mesbah-Namin SA, Zarghami N, & Fathi E. L-carnitine Effectively Induces hTERT Gene Expression of Human Adipose Tissue-derived Mesenchymal Stem Cells Obtained from the Aged Subjects. Int J Stem Cells, 2016, 9(1): 107-114. [Crossref]
86. Yang Q, Leong SA, Chan KP, Yuan XL, & Ng TK. Complex effect of continuous curcumin exposure on human bone marrow-derived mesenchymal stem cell regenerative properties through matrix metalloproteinase regulation. Basic Clin Pharmacol Toxicol, 2021, 128(1): 141-153. [Crossref]
87. Strong AL, Jones RB Jr, Glowacki J, Boue SM, Burow ME, & Bunnell BA. Glycinol enhances osteogenic differentiation and attenuates the effects of age on mesenchymal stem cells. Regen Med, 2017, 12(5): 513-524. [Crossref]
88. Yan X, Ehnert S, Culmes M, Bachmann A, Seeliger C, Schyschka L, et al. 5-azacytidine improves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PLoS One, 2014, 9(6): e90846. [Crossref]
89. Balikov DA, Crowder SW, Lee JB, Lee Y, Ko UH, Kang ML, et al. Aging Donor-Derived Human Mesenchymal Stem Cells Exhibit Reduced Reactive Oxygen Species Loads and Increased Differentiation Potential Following Serial Expansion on a PEG-PCL Copolymer Substrate. Int J Mol Sci, 2018, 19(2): 359. [Crossref]
90. Al-Azab M, Safi M, Idiiatullina E, Al-Shaebi F, & Zaky MY. Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting. Cell Mol Biol Lett, 2022, 27(1): 69. [Crossref]
91. Ng TK, Carballosa CM, Pelaez D, Wong HK, Choy KW, Pang CP, et al. Nicotine alters MicroRNA expression and hinders human adult stem cell regenerative potential. Stem Cells Dev, 2013, 22(5): 781-790. [Crossref]
92. Ng TK, Huang L, Cao D, Yip YW, Tsang WM, Yam GH, et al. Cigarette smoking hinders human periodontal ligamentderived stem cell proliferation, migration and differentiation potentials. Sci Rep, 2015, 5: 7828. [Crossref]
93. Shen X, Zhao Y, Wang Z, & Shi Q. Recent advances in high-throughput single-cell transcriptomics and spatial transcriptomics. Lab Chip, 2022, In press. [Crossref]
94. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007, 131(5): 861-872. [Crossref]
95. Weng Z, Wang Y, Ouchi T, Liu H, Qiao X, Wu C, et al. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl Med, 2022, 11(4): 356-371. [Crossref]