Open Access | Commentary
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
The Eph receptor A4-mediated demyelination in depression
* Corresponding author: Quanguang Zhang, PhD
Mailing address: Department of Neurology, LSU Health Sciences Center
Biomedical Research Institute (BRI), F7-22 (O), F7-35 (L)
1501 Kings Hwy, Shreveport, LA 71103, USA.
Email: qzh001@lsuhs.edu
Received: 21 October 2022 / Accepted: 24 October 2022 / Published: 29 December 2022
DOI: 10.31491/APT.2022.12.097
Abstract
Accumulating evidence reveals that major depressive disorder, one of the most common mental illnesses, is characterized by abnormal myelination. However, the relationship between demyelination and depression-related behaviors and the molecular mechanism underlying demyelination and synaptic deficits in depression is largely unknown. In a recent study, Li and his colleagues found that the ephrin A4 receptor (EphA4), a member of the Eph family of receptor tyrosine kinases, was essential to mediate demyelination and regulate synaptogenesis in depression. Using the chronic, unpredictable mild stress (CUMS) exposure or lipopolysaccharide (LPS) administration-induced animal model of depression, the authors found that depression could induce demyelination, and the increased EphA4 levels mediate demyelination and depression-like behaviors. In this commentary, we reviewed this critical finding and discussed future directions on this topic.
Keywords
Depression, Eph receptor A4, demyelination
Major depressive disorder (MDD), also known as clinical depression or depression, is one of the most common mental illnesses. It is characterized by pervasive and persistent low mood, disturbed memory, concentration, sleep, low motivation, and a loss of interest in enjoyable activities [1, 2]. Evidence from genetic, neuroimaging, non-human animals and post-mortem has suggested abnormal myelination in the MDD [3]. However, the relationship between demyelination and depression-related behaviors remains unclear. The molecular mechanism underlying demyelination and synaptic deficits in depression is largely unknown.
In a recent study, Li and his colleagues found that the ephrin A4 receptor (EphA4), a member of the Eph family of receptor tyrosine kinases, was essential to mediate demyelination and regulate synaptogenesis in depression [1]. The authors hypothesized that depression would include demyelination, and the increased EphA4 levels mediate demyelination and depression-like behaviors.
Using the chronic, unpredictable mild stress (CUMS) exposure or lipopolysaccharide (LPS) administration-induced animal model of depression, the authors first analyzed the association between depression and demyelination. They conducted the three most commonly used behavioral tests for depression and found depressive-like behavior in the CUMS and LPS-induced depression models [1]. Moreover, the myelin basic protein (MBP) staining and the luxor fast blue (LFB) staining demonstrated a significantly decreased myelination in the depressive animal models, which was also confirmed by Western blotting. Furthermore, the author also stained the contactin-associated protein (Caspr) and analyzed the nodal length between the Caspr-positive regions. Interestingly, the nodal length was increased in the CUMS mice, suggesting demyelination in the corpus callosum of these depressive animals, which was further confirmed by electron microscopy with thinner axonal myelin sheath and border nodal length. These findings confirmed demyelination in the mouse model of depression.
To further evaluate the role of demyelination in depression-related behavior, the authors next asked whether myelination could inhibit depressive-like behavior and restore normal behavior. To answer this question, clemastine, a widely accepted remyelination therapy, was applied to induce pharmacological remyelination [4]. MBP staining and electron microscopic analysis confirmed the remyelinating effect of clemastine in both the CUMS and LPS models. Intriguingly, remyelination induced by clemastine normalized depressive-like behaviors and restored synaptic deficits, as evidenced by increased numbers of synapses and thicker postsynaptic density in CUMS mice with clemastine treatment.
To elucidate the mechanism underlying the association between demyelination and depression, the authors performed RNA-seq using hippocampal tissue from CUMS mice. The expression of EphA4 was significantly upregulated in CUMS mice and selected for further analysis due to its essential role in regulating synapse development, synaptic plasticity, neuronal growth, and its increased levels in axonal lesions in the demyelinating brain disorder [1, 5]. Intriguingly, the authors found that the ubiquitin-mediated degradation pathway contributes to the changes in EphA4 levels. The decreased ubiquitinated EphA4 and the upregulated immunoprecipitated EphA4 suggested that decreased ubiquitination was involved in the upregulation of EphA4.
To further evaluate the role of EphA4 in CUMS-induced changes in myelination and depression-related phenotypes, EphA4 was knockdown by using an Adeno-associated viral (AAV) vector encoding EphA4 shRNA. As expected, the EphA4 knockdown inhibited depressive-like behaviors and significantly attenuated demyelination and synaptic deficits caused by CUMS. Furthermore, the authors stained EphA4 with various specific cell makers, including Vglut1 (excitatory neuronal marker), GAD65/67 (inhibitory neuronal marker), MBP (expressed in oligodendrocytes), Iba1 (microglial marker), or GFAP (astrocyte markers), to determine the specific cell type with EphA4 responding to the CUMS. The author confirmed that EphA4 was mainly expressed in the excitatory neurons under normal conditions, and the expression of EphA4 was significantly upregulated in response to CUMS. Moreover, consistent with the results of the EphA4 shRNA knockdown mentioned previously, specific knockdown of EphA4 in the excitatory neurons can also prevent behavioral deficits and alleviated changes in myelin sheaths and synapses induced by CUMS.
In the end, the authors examined the changes in myelination, synapse, and EphA4 levels in post-mortem brain tissue from patients with major depressive disorder (MDD) or control individuals. Consistent with the findings from CUMS mice, MDD patients displayed markedly demyelination, synaptic deficits, and significantly increased EphA4 expressions, suggesting the potential to translate their findings in the animal study to humans.
In summary, Li et al. demonstrated that the increased EphA4 expression is essential to induce depressive-like behaviors and demyelination in response to physical or inflammatory stress [1]. EphA4 knockdown could significantly attenuate demyelination and behavioral changes in the animal models of depression (Figure 1). Their study provides additional information about the association between demyelination and depression and extends our understanding of the mechanisms underlying demyelination and synaptic deficits in depression. Their study supports the potential use of the EphA4 inhibitor as a treatment approach for depression and provides evidence on promoting remyelination as a general treatment strategy for depression. Although Li et al. have made excellent progress in understanding EphA4-mediated demyelination in depression, several intriguing topics arise and deserve further investigation (Figure 1). First, oligodendrocyte precursor cells (OPCs) are necessary for remyelination and may be a potential therapeutic target for demyelinating diseases [6]. It would be helpful to examine the expression of EphA4 in OPCs to determine whether EphA4 is enriched in OPCs following physical or inflammatory stress. In addition, OPCs senescence contributes to diminished remyelination in the brain disorder. It would be interesting to investigate whether OPCs senescence exists in physical or inflammatory stress-induced depression and whether the changes of EphA4 in the excitatory neurons affect OPCs senescence. Second, synaptic metaplasticity and depression are displayed in a sex-specific manner [7, 8]. Therefore, it would be helpful to consolidate the role of EphA4 in depression if there is evidence demonstrating that the change of EphA4 in depressed female animals is similar to that in males. Third, their study also identified Epha 7, another member of the Eph family of RTKs, upregulated in the hippocampus CUMS mice. Investigating the role of Epha7 in demyelination following physical or inflammatory stress may further our understanding of the association between demyelination and depressive-like behavior. Finally, because anxious-depressive-like behavior is an early endophenotype and predictor of Alzheimer’s disease, the role of Epha7 in the initiation and development of Alzheimer’s disease deserves further investigation. Addressing these questions will help us identify potential therapeutic targets for depression and demyelination-related brain diseases.
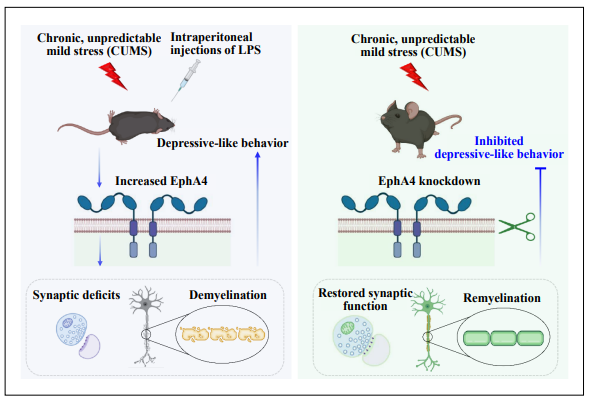
Figure 1. The Eph receptor A4-mediated demyelination in depression. The physical or inflammatory stress induces the increased expression of EphA4 in the excitatory neurons. The increased EphA4 levels induce demyelination and synaptic deficits in mouse models of depression (left panel). EphA4 knockdown in excitatory neurons can attenuate demyelination and rescue the synaptic deficits induced by chronic unpredictable mild stress (right panel).
Declarations
Authors’ contributions
LY reviewed the literature and drafted the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by a grant from the National Institute of Aging, National Institutes of Health (1RF1AG058603).
Conflicts of interest
The authors declare no competing financial interests.
References
1. Li Y, Su P, Chen Y, Nie J, Yuan TF, Wong AH, et al. The Eph receptor A4 plays a role in demyelination and depression-related behavior. J Clin Invest, 2022, 132(8). [Crossref]
2. Wu C, Yang L, Li Y, Dong Y, Yang B, Tucker LD, et al. Effects of Exercise Training on Anxious-Depressive-like Behavior in Alzheimer Rat. Med Sci Sports Exerc, 2020, 52(7): 1456-1469. [Crossref]
3. Sacchet MD, & Gotlib IH. Myelination of the brain in Major Depressive Disorder: An in vivo quantitative magnetic resonance imaging study. Sci Rep, 2017, 7(1): 2200. 2011, 364(23): 2227-2234. [Crossref]
4. Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet, 2017, 390(10111): 2481-2489. [Crossref]
5. Sobel RA. Ephrin A receptors and ligands in lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol, 2005, 15(1): 35-45. [Crossref]
6. Skaper SD. Oligodendrocyte precursor cells as a therapeutic target for demyelinating diseases. Prog Brain Res, 2019, 245: 119-144. [Crossref]
7. Yang L, Tucker L, & Zhang Q. Vasopressin Signaling Buffers Synaptic Metaplasticity in a Sex-specific Manner. Neurosci Bull, 2021, 37(9): 1377-1380. [Crossref]
8. Salk RH, Hyde JS, & Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull, 2017, 143(8): 783-822. [Crossref]