Open Access | Research Article
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Long-term treatment with Elamipretide enhances healthy aging phenotypes in mice
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School
of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 28 June 2022 / Revised: 28 July 2022 / Accepted: 29 August 2022 / Published: 30 September 2022
DOI: 10.31491/APT.2022.09.089
Abstract
Background: Disruption of metabolic and bioenergetic homeostasis related to mitochondrial dysfunction is a
key driver of aging biology. Therefore, targeting mitochondrial function would be a rational approach to slowing aging. Elamipretide (Elam, a.k.a. SS-31) is a peptide known to target mitochondria and suppress mammalian signs of aging. The present study was designed to examine the phenotypic effects of long-term Elam treatment on aging in C57BL/6 mice starting at 18 months of age.
Methods: Mice were fed regular chow (RC diet) or a diet high in fat and sugar (HF diet) and treated with 3 mg/
kg of Elam or saline subcutaneously 5 days per week for 10 months. Physiological performance assessments
were conducted at 28 months of age.
Results: Elam improved the physical performance of males but not females, while in females Elam improved
cognitive performance and enhanced the maintenance of body weight and fat mass. It also improved diastolic
function in both males and females, but to a greater extent in males. The HF diet over 10 months had a negative effect on health span, as it increased body fat and decreased muscle strength and heart function, especially
in females.
Conclusions: Elam enhanced healthy aging and cardiac function in both male and female mice, although the
specific effects on function differed between sexes. In females, the treatment led to better cognitive performance and maintenance of body composition, while in males, performance on a rotating rod was preserved.
These overall observations have translational implications for considering additional studies using Elam in
therapeutic or preventive approaches for aging and age-related diseases.
Keywords
Aging, mitochondria, elamipretide, C57BL/6 mice, high fat and sugar diet
Introduction
Enhancing mitochondrial function can delay or reverse
some of the untoward effects of aging by targeting metabolic and bioenergetic processes [1, 2, 3]. Several reports
have focused on mitochondrial-targeted catalase, a reactive oxygen species (ROS) scavenger that was found to
be protective of a number of aging phenotypes [4, 5, 6, 7].These studies led to a more translational pharmacotherapeutic approach involving the peptide elamipretide
(Elam), previously known as SS-31 or MTP-131 [8]. Elam has been found to reverse mitochondrial dysfunction
associated with aging. It has now been shown that Elam
can reverse the preexisting loss of function in multiple
organ systems of aging mice, including skeletal muscle,
heart, and brain [7, 9, 10], organs with the greatest energy
demands. Progress on understanding how to deliver these
benefits in clinical settings could substantially enhance the
human health span, as loss of cardiac and skeletal muscle
function, and cognitive impairment are great contributors
to frailty in the elderly, resulting in numerous lifesty leconsequences, including increased susceptibility to inactivity, social isolation, and falls.
Healthspan is the length of life during which one is generally healthy and free from serious disease. Importantly,
the end of the health span limits the ability to perform
regular activities of daily living and signals a progression
to frailty and inability to maintain a high quality of life. It
is becoming increasingly evident that lifespan and health
span are not necessarily correlated [11, 12, 13], creating
substantial social and economic consequences. Therefore,
the overall goal is to achieve an extended health span
along with an extended lifespan. In mice, health span is
determined by the onset of pathology and loss of physiologic performance. Increasing age and unhealthy diets
are strongly associated with susceptibility to metabolic
conditions such as obesity, diabetes, dyslipidemia, and
liver disease, as well as comorbid conditions such as heart
disease, cancer, infertility, neurodegeneration, and deficits
in cognitive and executive functioning. With the growing
elderly population and the increasingly early age of exposure to unhealthy diets that increase susceptibility to lateronset metabolic disease, there is an urgent and unmet need
to understand the long-term consequences of pathologic
changes during high-risk diet exposure, as well as preventive and treatment-related approaches.
The efficacy of Elam has been previously described in
studies of mice, rats, pigs, sheep, guinea pigs, and rabbits
[8]. Several phases I studies have also found Elam to be
safe and well tolerated in healthy human subjects when
administered as an intravenous infusion [14, 15, 16].
However, previous studies have been relatively short-term
in nature, with some studies in mice lasting as long as two
months using subcutaneous infusion pumps. We report
here on the extended health span effects of subcutaneous
Elam in mice treated 5 days per week, over a 10-month
period starting at 18 months of age.
Methods
Animals and Elamipretide treatment
C57BL/6 male and female mice were obtained from the National Institute on Aging aged rodent colony at 18 months of age. Mice were group housed in a specific pathogen-free facility at the University of Washington under a 12-hour light and 12-hour dark cycle with a room temperature of 25 ± 4℃ and reverse osmosis water in an automatic watering system. Body weight was measured weekly. Lean mass and fat mass were measured monthly by quantitative magnetic resonance imaging (EchoMRI). Blood glucose (CONTOUR® NEXT EZ meter) was tested from tail vein blood monthly. Food consumption in the first week of each month was recorded. All studies were approved by the University of Washington IACUC, protocol 2174-23. Elam was obtained from Stealth BioTherapeutics (Needham, MA). Mice were given 3 mg/kg Elam, or an equal volume of saline, subcutaneously 5 days per week, starting at 18 months of age and ending at 28 months of age (i.e., 10 months duration).
Diet
Mice were acclimated to their new environment for two weeks to avoid the stress that could potentially confound the results, then randomly assigned to a high fat, high sucrose (HF), or regular chow (RC) diet. The HF diet was obtained from Bioserv (S1850 Mouse Diet, Paste, Gamma Irradiated) and contained lard, sucrose, casein, maltodextrin, and a vitamin-mineral mixture with 20% protein, 36% carbohydrate, 36% fat, and 0% fiber. The RC diet (Picolab Rodent Diet 20, 5053, irradiated) consisted of corn, soybean, wheat, fish meal, and vitamin-mineral mixture with 20% protein, 65% carbohydrate, 11% fat, and 8% fiber. The caloric differences in the two diets are provided in Table 1. Each respective diet was placed in a widemouthed, flat-bottom porcelain container on the cage floor at a volume of 200 gm per cage and replenished weekly for 10 months. Caloric intake was determined monthly by multiplying the average daily amount of food consumed per mouse in each cage over 3 days by the Kcal per g (gram) of the respective diet received.
Table 1
Dietary caloric values. The fat macronutrient added more
caloric value to the high fat (HF) diet compared to the regular chow (RC)
diet.
| Macronutrient component | HF diet Kcal/g | RC diet Kcal/g |
|---|---|---|
| Carbohydrate | 1.48 (all from sucrose) | 1.91 |
| Protein | 0.82 | 0.76 |
| Fat | 3.24 | 0.41 |
| Total | 5.54 | 3.07 |
Physical and behavioral assessments
Mice were put under 2% isoflurane anesthesia and tested
for cardiac function before starting the study (baseline), at
the midpoint (5 months after starting the study), and then
again at the end of the 10-month feeding period when
mice were 28 months of age. However, echocardiography
was not able to be done in female mice at the last time
point (after 10 months when mice were 28 months of age)
because of laboratory access (COVID-19 restrictions).
The Siemens Acuson CV-70 system was used with standard imaging planes, including M-mode conventional and
Tissue Doppler imaging [17].
All other assessments were conducted at the end of the
10-month treatment period, when mice were 28 months
old, with at least 2 days between each test, including grip
strength, rotarod, and spatial navigation learning task. For
grip strength, mice were stretched on a force meter rod [18]
using their forelimb and pulled by the tail until the grasp
was broken. Mice were tested 5 consecutive times, and
the maximum force was recorded. For the rotarod test [19],
mice were placed on a rotating rod with an increasing rate
of acceleration, and their latencies to fall were recorded.
Mice were tested one time for acclimatization and then
three times on the second day. A spatial navigation task
designated as the Box maze was used to assess changes in learning impairment [20]. Mice were placed in a square
box with seven blocked exits and one escape hole leading
to a dark non-stressful cage. In each trial, mice were given
120 seconds to escape. Mice were tested in four consecutive trials, and their escape times were recorded.
In vivo muscle function
Maximal torque and fatigue of ankle plantar flexors were performed as described [21] using an Aurora Scientific 305C servomotor (Aurora, Ontario, Canada). Briefly, each mouse was anesthetized with isoflurane (4% for induction and ~2% for maintenance) and laid on its side on a temperature-controlled platform maintained at 37°C. The right knee was clamped in place and the foot was secured to a footplate with the ankle positioned at 90°. The tibial nerve was stimulated with a Grass Instruments S88X stimulator (Astro-Med, Inc., West Warwick, Rhode Island, USA) at an optimal voltage (1.5 V) using percutaneous electrodes. Maximal tetanic torque was assessed by a force-frequency curve, where the muscle was stimulated every other minute at frequencies from 10 Hz to 200 Hz. Maximal tetanic torque was assessed at baseline and study endpoint. Muscle fatigue was assessed at the endpoint. After two minutes of rest following force frequency, fatigue was induced by repeated contractions (100 Hz) every 5 seconds for 120 contractions. Fatigue was assessed by the ratio of torque of the last contraction to the initial contraction (% initial). Analyses of muscle contractions were performed using DMA software (Aurora, ON) to quantify torque.
Statistical analysis
Significance analysis was done by students via T-test and one- and two-way ANOVA. Mean values ± standard error of the mean (SEM) were used. Statistical difference was identified as p ≤ 0.05.
Results
Elamipretide treatment altered body composition and caloric intake in a sex- and diet-dependent manner
Female mice fed the RC diet and treated with Elam maintained body weight throughout the 10-month study, while
those in the saline group lost weight (Figure 1A). There
was no difference in body weight observed in males. All
groups (males and females, Elam- and saline-treated) fed
HF diet showed an increase in body weight after three
months (Figure 1B). After six months on the HF diet,
Elam-treated female mice maintained the same body
weight they attained at three months, whereas body weight
in saline-treated female mice continued to increase (Figure 1B). At the end of 10 months, body weight was decreased
to near baseline levels in all cohorts. Changes in body fat
mirrored changes in body weight, except for earlier in the
study period (Figure 1D). Elam prevented the decrease in
body fat in female mice fed the RC diet and the increase
in body fat in females fed the HF diet (Figure 1C, 1D).
Elam treatment had no effect on body fat in males fed either diet.
Changes in caloric intake were also different in the two
sexes. Male mice treated with Elam and fed RC diet maintained a constant caloric intake over the 10-month study,
while treatment with saline resulted in increased caloric
intake through 6 months, which then decreased back toward baseline levels at 10 months (Figure 1E). Caloric
intake for females fed the RC diet gradually increased
over 10 months with no treatment effect. Interestingly,
both males and females fed the HF diet had a significant
decrease in caloric intake over the first three months of the
study regardless of treatment, and this continued in males,
but females showed an increase over the remaining study
period (Figure 1F).
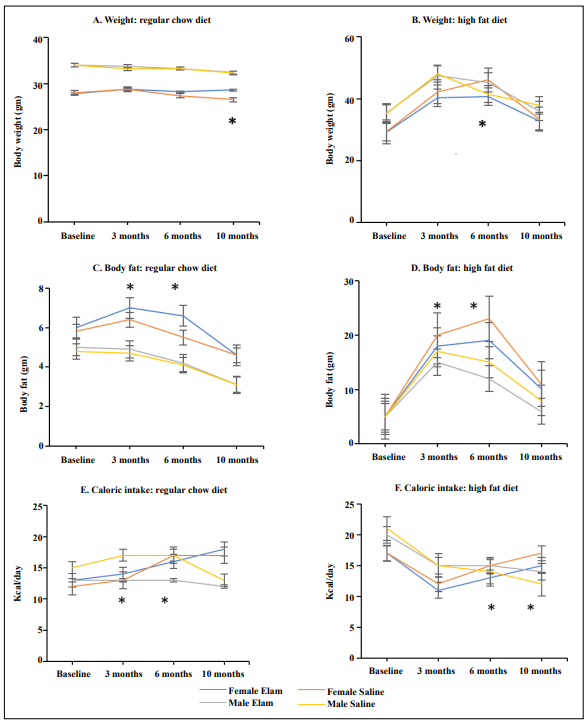
Figure 1. Mice treated with Elamipretide for 10 months showed selected alterations in body conformation and caloric intake. (A). Body weight in mice fed regular chow diet; (B). Body weight in mice fed high-fat diet; (C). Body fat in mice fed regular chow diet; (D). Body fat in mice fed high-fat diet; (E). Caloric intake in mice fed regular chow diet; (F). Caloric intake in mice fed a high-fat diet. N = 9-14/cohort; *p ≤ 0.05.
Elamipretide enhanced physical performance in males but not females
Physical performance was assessed by grip strength and
rotarod tests, as well as plantarflexion torque and fatigue.
Elam treatment had no effect on grip strength in either
males or females fed RC diet (Figure 2A). HF diet decreased grip strength in both males and females; however,
Elam treatment resulted in significant improvements in
grip strength in males, which approached test levels seen
in males fed RC diet. Male mice treated with Elam and
fed an RC diet were able to stay on the rotating rod longer
than saline-treated animals, but no effect was seen in females (Figure 2B). The differences seen in these two tests
were not related to changes in lean muscle mass, as this
was not altered in any of the cohorts over the study period
as determined by QMRI (data not shown). It is of interest
to note that Elam treatment in males had no effect on body
fat, so the increase in grip strength and rotarod agility may
be associated with other metabolic or nonmetabolic factors.
The loss in maximal torque of plantar flexors was tested
by stimulating the tibial nerve using electrodes in anesthetized animals. Maximal torque of plantar flexors was
not affected by diet or Elam treatment. However, females
had a greater decline in maximal torque throughout the
10-month intervention period compared to males. Fatigue
of plantar flexors was assessed by the torque of the final
contraction compared to the initial contraction and was
also not affected by diet or Elam treatment.
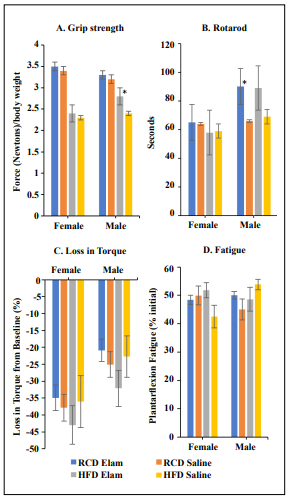
Figure 2. Male mice but not female mice treated with Elamipretide for 10 months showed enhanced physical performance. (A). Grip strength test results, N=8-17/cohort; (B). Rotarod test results, N=8-16/cohort; (C). Loss in maximal plantarflexion torque, N = 4-14/cohort; Female HF and Elam HF N=4/cohort; (D). Plantarflexion fatigue, N=3-14/cohort; Female Elam HF N=4 and HF N=3. *p ≤ 0.05.
Elamipretide prevented cognitive decline in females but not males
The Box maze is a spatial navigation task that is used to assess the ability to learn new tasks with increasing age by measuring the time it takes to find an escape hole in a novel environment [20]. Female mice treated with Elam and fed RC diet were able to find the escape hole more quickly in trials 3 and 4 compared to mice treated with saline (Figure 3A), indicating improved cognitive ability. Female mice fed HF diet and treated with Elam also found the escape hole more quickly than saline-treated mice but only at trial 4, suggesting that Elam may be less effective in improving age-related cognitive decline under conditions of metabolic stress. Elam treatment in males fed either diet had no effect on cognitive function (Figure 3B).
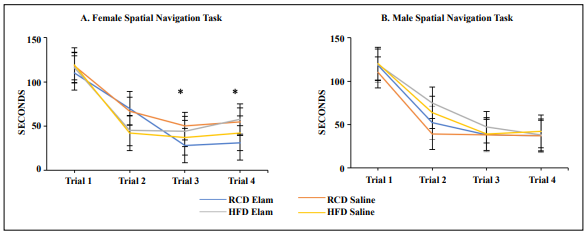
Figure 3. Female mice but not male mice treated with Elamipretide for 10 months showed improved cognition. (A). Female spatial navigation task results, N=8-13/cohort; (B). Male spatial navigation task results, N=11-17/cohort. *p ≤ 0.05.
Elamipretide enhanced selective parameters of cardiac function
Echocardiography was used to assess three aspects of cardiac health: diastolic function, myocardial performance
index (MPI), and left ventricular mass (LVM). Diastolic
function is measured by Ea/Aa, the ratio between the early
(E) and late (A) diastolic filling velocities, with increased
values indicating improved diastolic performance [22]. MPI is an indication of the proportion of the cardiac cycle
in which no volume change occurs. An increase in this
parameter indicates reduced performance. LVM measures
changes in muscle mass of the left ventricle, the major
compartment for pumping oxygenated blood to systemic
organs and the brain. Increased LVM would suggest cardiac hypertrophy, either due to increased functional demand
or pathological conditions specifically within the heart or
in combination with other organs.
Male mice fed RC diet and treated with Elam showed
enhanced diastolic function, i.e., increased Ea/Aa, at the
5-month midpoint (23 months old) and 10-month endpoint
of the study (28 months old) compared to males treated
with saline, where Ea/Aa values were unchanged over
time (Figure 4A), consistent with our previous studies [9].
In male mice fed HF diet, diastolic function decreased
at the end of the study with saline treatment, while this
negative effect was prevented in males treated with Elam.
For females, echocardiography was performed only at
baseline and the 5-month midpoint because of COVID19
restrictions in place at the study endpoint. Diastolic function declined with age at the midpoint in female mice fed
either diet and this decline was prevented in both diet cohorts treated with Elam but not saline (Figure 4B).
For the other two cardiac parameters, MPI increased significantly at the 10-month endpoint in male mice fed HF
diet and treated with saline (Figure 4C). This increase
was prevented by Elam treatment. No changes were seen
over time in males fed RC diet treated with either Elam
or saline. In females fed HF diet and treated with Elam,
MPI at the 5-month midpoint showed a decrease, i.e.,
improvement (Figure 4D). No changes in MPI were seen
in females fed RC diet and treated with Elam. For LVM
measurements, no effect was seen with Elam treatment
in males or females on either diet (Figure 4E and 4F).
However, the HF diet did increase LVM at the 5-month
midpoint in females and the 10-month endpoint in males.
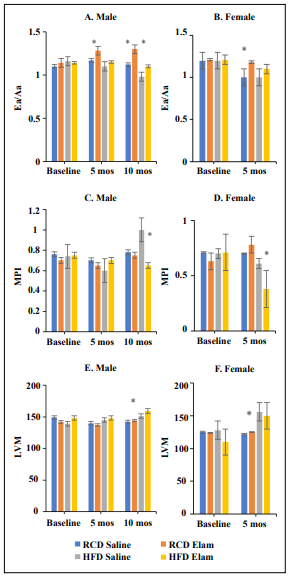
Figure 4. Parameters of cardiac function were assessed by echocardiography. (A) and (B). Diastolic function measured as Ea/ Aa values in males and females, respectively; (C) and (D). Myocardial performance index (MPI) values in males and females, respectively; (E) and (F). Left ventricular mass (LVM) values in males and females, respectively. N = 7-13/cohort; *p ≤ 0.05.
Discussion
C57BL/6 mice treated with Elam for 10 months starting at
18 months of age and ending at 28 months of age showed
selective delays in aging depending on sex, diet, and individual organs as determined by physiological performance
tests. Elam-treated females were more responsive to maintaining body weight and fat mass homeostasis as well as
improved cognitive performance than Elam-treated males,
which showed increased physical performance. Mice that
were metabolically stressed with a diet high in fat and
sugar and treated with Elam, in general, showed similar
but non-significant responses.
Elam is known to target and improve mitochondrial function, which, in turn, has been shown to improve resilience
to aging in organ systems such as cardiac and skeletal
muscle [9, 21]. This is supported in our study since Elam
enhanced diastolic function in the heart in both sexes. In
other studies, Elam has been shown to enhance heart function by improving left ventricle (LV) function, improving
the rate of ATP synthesis, and reducing ROS formation
[23, 24]. Elam interacts with cardiolipin and proteins on
the inner mitochondrial membrane to enhance membrane
structure and ATP production while decreasing mitochondrial oxidative stress in dysfunctional mitochondria [25, 26, 27]. One such interaction, with the adenine nucleotide
transporter, has been demonstrated to reduce proton leak
in aged cardiomyocytes [28]. This then improves energy
production, which is linked to heart function. HF diets can
cause an increase in triacylglycerol (TG) in cardiac myocytes where TG regulates fatty acid oxidation that feeds
mitochondrial ATP production [29]. An excess of TG
causes a dysregulation of this process, which can cause
energy starvation and is linked to heart failure, potentially
through elevated oxidant production and redox stress.
Since Elam targets the mitochondrial inner membrane and
the ATP production process, there would be an expectation
that it should alleviate the negative effects of an HF diet
on cardiac function [30]. The improved diastolic function
and positive effect on MPI values support this hypothesis.
It is important to note that the positive results may be affected by selection bias, with the least healthy mice dying
during the 10-month study. This is especially important
to consider for the female mice where we were not able
to collect 10-month cardiac data. In contrast, the data for
cardiac function showed no significant effect of Elam
treatment on LVM measure of hypertrophy, although the
HF led to elevated hypertrophy in the female mice.
Elam also had a positive effect on physical performance,
increasing age-related muscle strength and agility, as
shown by grip strength and rotarod data. This is supported
by several studies as Elam is known to counter age-related
muscle fatigue and reverse the mitochondrial deficits of
ATP production in skeletal muscle [3]. Interestingly, our
data only supports this for male mice, with no difference
in physical performance between Elam and control-treated
female mice; even though Elam has been shown in past
studies to improve exercise tolerance in females as well as
males [9, 21]. Since Elam treatment helped stabilize body
weight and fat gain in females but not males, it would be
expected that stabilization of fat would lead to a positive
effect on muscle performance in females, but no such effect was observed. Instead, Elam treatment only had a positive effect on the grip strength of males fed the HF diet.
Despite previous reports demonstrating increased muscle
fatigue resistance in aged mice with Elam treatment, in
this study there was no significant effect on fatigue or
force production in electrically stimulated muscle [7]. An important difference is that in the present study Elam
was administered by daily SC injections 5 days/week,
while the previous work demonstrating improved fatigue resistance used continuous delivery for 8 weeks with a
subcutaneous osmotic pump. The difference between the
positive effects observed on complex whole-body function
compared to skeletal muscle-specific assays supports an
interpretation where reducing mitochondrial dysfunction
throughout the body may cause subtle effects on multiple
physiological systems (e.g. cardiovascular, neuromuscular) that contribute to better performance while the effects
on an individual muscle or organ may not be as apparent.
Diets high in fat and sugar are known to increase the risk
for metabolic conditions such as obesity and insulin resistance. A high-fat diet can lead to an increase in body fat
and adipose tissue [31, 32] This increase in body fat correlates with a loss of strength. Our data shows that aging
mice fed a long-term diet high in saturated fat and sugar
had increased body weight and body fat compared to mice
fed regular chow. This could help explain the decreased
muscle strength further supporting observations that obesity negatively effects muscle tissue and therefore overall
strength. However, our study showed that the HF diet had
no effect on how mice performed in the rotarod assay,
though other studies have shown that high amounts of adipose tissue have a negative effect on mobility and agility
[31].
Elam decreased the susceptibility to cognitive decline
in females; however, no difference was seen in males.
Elam has been shown in previous studies on male mice
to cross the blood-brain barrier but only had a positive effect when a stressor was introduced [33]. In aging female
mice, Elam prevented the cognitive impairment caused
by short-term sleep disruption, but males were not tested
[10]. Having a better understanding of the differences in
how Elam effects the aging brain in male and female mice
would help to develop studies on how to treat cognitive
impairment and dementia in more focused sex-specific approaches.
The overall observations of this study have translational
implications for considering additional studies using Elam
in therapeutic or preventive approaches for aging and agerelated diseases. Sex and organ specificity of Elam in the
mouse may be different in humans under long-term treatment conditions, but there should be an awareness that
these effects might occur in one form or another and so
should be considered in any clinical study design. Gender
differences observed in this study may be related to underlying differences in metabolic pathways targeted by Elam
in female mice compared to male mice but additional
studies are warranted to address this issue. Drugs in combination (cocktails) that target multiple processes of aging
have now been shown to be more effective in slowing aging than any individual drug in the cocktail [34], so there
is always the possibility of combining Elam with other
anti-aging drugs to target a variety of aging pathways.
Declarations
Authors’ contributions
All authors participated in the study. WL and KN wrote the manuscript and all other authors helped with the final draft for submission.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by NIH grants: P01 AG01751 (Rabinovitch, PI); R01 AG057381 (Ladiges, PI).
Conflicts of interest
Warren Ladiges is a member of the Editorial Board of Aging Pathobiology and Therapeutics. The author declares that there are no conflicts.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
1. Liu SZ, Marcinek DJ. Skeletal muscle bioenergetics in aging and heart failure. Heart Fail Rev, 2017, 22(2): 167- 178. [Crossref]
2. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell, 2018, 17(2). [Crossref]
3. Roshanravan B, Liu SZ, Ali AS, Shankland EG, Goss C, Amory JK, et al. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS One, 2021, 16(7): e0253849. [Crossref]
4. Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science, 2005, 308(5730): 1909-1911. [Crossref]
5. Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci, 2008, 63(8): 813-822. [Crossref]
6. Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab, 2010, 12(6): 668-674. [Crossref]
7. Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, et al. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell, 2013, 12(5): 763- 771. [Crossref]
8. Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol, 2014, 171(8): 2029-2050. [Crossref]
9. Chiao YA, Zhang H, Sweetwyne M, Whitson J, Ting YS, Basisty N, et al. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. Elife, 2020, 9. [Crossref]
10. Wu J, Dou Y, & Ladiges WC. Adverse Neurological Effects of Short-Term Sleep Deprivation in Aging Mice Are Prevented by SS31 Peptide. Clocks Sleep, 2020, 2(3): 325- 333. [Crossref]
11. Sierra F, Kohanski R. Geroscience and the trans-NIH Geroscience Interest Group, GSIG. Geroscience, 2017, 39(1): 1-5. [Crossref]
12. Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest, 2013, 123(8): 3272-3291. [Crossref]
13. Ladiges W, Ikeno Y, Niedernhofer L, McIndoe RA, Ciol MA, Ritchey J, et al. The Geropathology Research Network: An Interdisciplinary Approach for Integrating Pathology Into Research on Aging. J Gerontol A Biol Sci Med Sci, 2016, 71(4): 431-434. [Crossref]
14. Karaa A, Haas R, Goldstein A, Vockley J, & Cohen BH. A randomized crossover trial of elamipretide in adults with primary mitochondrial myopathy. J Cachexia Sarcopenia Muscle, 2020, 11(4): 909-918. [Crossref]
15. Butler J, Khan MS, Anker SD, Fonarow GC, Kim RJ, Nodari S, et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J Card Fail, 2020, 26(5): 429-437. [Crossref]
16. Reid Thompson W, Hornby B, Manuel R, Bradley E, Laux J, Carr J, et al. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genet Med, 2021, 23(3): 471-478. [Crossref]
17. Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation, 2009, 119(21): 2789-2797. [Crossref]
18. Ge X, Cho A, Ciol MA, Pettan-Brewer C, Snyder J, Rabinovitch P, et al. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol Aging Age Relat Dis, 2016, 6: 32981. [Crossref]
19. Ge X, Ciol MA, Pettan-Brewer C, Goh J, Rabinovitch P, & Ladiges W. Self-motivated and stress-response performance assays in mice are age-dependent. Exp Gerontol, 2017, 91: 1-4. [Crossref]
20. Darvas M, Mukherjee K, Lee A, & Ladiges W. A Novel One-Day Learning Procedure for Mice. Curr Protoc Mouse Biol, 2020, 10(1): e68. [Crossref]
21. Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Merrihew GE, Egertson JD, et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med, 2019, 134: 268-281. [Crossref]
22. Mottram PM, & Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart, 2005, 91(5): 681-695. [Crossref]
23. Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, & Zhang K. Chronic Therapy With Elamipretide (MTP- 131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ Heart Fail, 2016, 9(2): e002206. [Crossref]
24. Chatfield KC, Sparagna GC, Chau S, Phillips EK, Ambardekar AV, Aftab M, et al. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl Sci, 2019, 4(2): 147-157. [Crossref]
25. Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, et al. The mitochondrial-targeted compound SS-31 reenergizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol, 2013, 24(8): 1250-1261. [Crossref]
26. Chavez JD, Tang X, Campbell MD, Reyes G, Kramer PA, Stuppard R, et al. Mitochondrial protein interaction landscape of SS-31. Proc Natl Acad Sci U S A, 2020, 117(26): 15363-15373. [Crossref]
27. Mitchell W, Ng EA, Tamucci JD, Boyd KJ, Sathappa M, Coscia A, et al. The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action. J Biol Chem, 2020, 295(21): 7452-7469. [Crossref]
28. Zhang H, Alder NN, Wang W, Szeto H, Marcinek DJ, & Rabinovitch PS. Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. Elife, 2020, 9. [Crossref]
29. Kienesberger PC, Pulinilkunnil T, Nagendran J, & Dyck JR. Myocardial triacylglycerol metabolism. J Mol Cell Cardiol, 2013, 55: 101-110. [Crossref]
30. Steggall A, Mordi IR, & Lang CC. Targeting Metabolic Modulation and Mitochondrial Dysfunction in the Treatment of Heart Failure. Diseases, 2017, 5(2). [Crossref]
31. Addison O, Marcus RL, Lastayo PC, & Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol, 2014, 2014: 309570. [Crossref]
32. Messa GAM, Piasecki M, Hurst J, Hill C, Tallis J, & Degens H. The impact of a high-fat diet in mice is dependent on duration and age, and differs between muscles. J Exp Biol, 2020, 223(Pt 6). [Crossref]
33. Zhao W, Xu Z, Cao J, Fu Q, Wu Y, Zhang X, et al. Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation, 2019, 16(1): 230. [Crossref]
34. Jiang Z, Wang J, Imai D, Snider T, Klug J, Mangalindan R, et al. Short term treatment with a cocktail of rapamycin, acarbose and phenylbutyrate delays aging phenotypes in mice. Sci Rep, 2022, 12(1): 7300. [Crossref]