Open Access | Model Profile
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
A mouse model of naturally occurring age-related cognitive impairment
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School
of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 25 June 2022 / Revised: 08 August 2022 / Accepted: 29 August 2022 / Published: 30 September 2022
DOI: 10.31491/APT.2022.09.090
Abstract
Age-related cognitive impairment (ARCI) is a neurological condition that affects millions of older people, but little is known about the increased risk of developing more severe neurodegeneration and dementia. Preclinical research is needed to understand the mechanisms of the impairment and the neuropathology associated with it. We have characterized a model of naturally occurring ARCI in the C57BL/6J mouse strain that shows an age-dependent development of cognitive impairment. As in people, some mice have little cognitive impairment while others have more severe cognitive impairment. Therefore, mice can be categorized as resistant or susceptible and the two groups can be studied for behavioral and neuropathology differences. Preliminary observations show no difference in strength and agility test scores between ARCI resistant and susceptible mice of either sex suggesting the cognitive impairment in ARCI susceptible mice is not accompanied by impairment in daily living activities, similar to ARCI in humans. The hippocampal area of the brain from ARCI susceptible mice shows evidence of an increase in the inflammatory cytokine MCP-1 compared to ARCI resistant mice, suggesting inflammation may be associated with ARCI. These preliminary observations suggest that ARCI in C57BL/6J mice could be a high-impact model to study how resilience to brain aging may predict resilience to dementia associated with Alzheimer’s disease and other age-related neurological conditions.
Keywords
Age-related cognitive impairment, C57BL/6 mouse, brain aging, cognitive resilience
Cognitive decline with increasing age is a common aspect
of growing old [1]. It is a generic umbrella for all aspects
of cognitive dysfunction ranging from memory lapses associated with typical aging to prodromal stages of neurodegenerative diseases such as Alzheimer’s disease (AD).
Dementia is the term used for more severe cognitive impairment where patients experience memory loss as well
as difficulties in maintaining independence in normal living activities [2]. Mild cognitive impairment is often used
to describe a prodromal stage between cognitive impairment related to aging and dementia [3]. Age-related cognitive impairment (ARCI), which falls under the umbrella of
cognitive decline, is an extremely common condition that
affects millions of older people, but little is known about
how these people may be at increased risk for developing
more severe neurodegeneration and dementia.
We have characterized a mouse model of naturally occurring ARCI that will provide the opportunity to investigate
cellular, molecular and transcriptomic aspects of cognitive
impairment without the presence of disease factors such
as AD. Mice do not get AD naturally, so the mouse brain
is absent of amyloid plaques or tau fibrillary tangles. The
purpose of this short note is to describe the model. All live
mouse procedures were approved by the University of
Washington Animal Care and Use Committee.
C57BL/6 mice are used extensively in aging research.
To determine if cognitive impairment was age-dependent
in this strain, male mice were obtained from the NIAAged Rodent Colony (Charles River) at ages 4, 12, 20,
and 28 months. They were acclimated to the new housing
environment for three weeks and subsequently tested for
cognition using the radial water tread maze [4]. Figure 1
clearly shows that 28-month-old mice performed poorly,
whereas 4-month-old mice showed superior performance and were not cognitively impaired. The 12- and 20-month-old mice were in the middle with 20-monthold mice trending toward more cognitive impairment than
12-month-old mice.
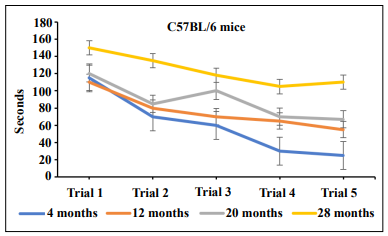
Figure 1. Cognitive impairment in C57BL/6 male mice develops in an age-dependent manner as shown by the radial water tread maze.
We next obtained 20-month-old C57BL6 mice, 20 males,
and 20 females, from the NIA Aged Rodent Colony to
assess the variability of ARCI using a spatial navigation
learning task [5].
This is a one-day assessment procedure that measures escape times from an isolated box-like bin into an open hole
among 7 closed holes. After finding the median slope value across the number of mice in each cohort, a threshold
value was set so that mice that escaped more quickly after
4 trials could be distinguished from mice that were slow
to escape. This was done by plotting the raw data of each
mouse (Figure 2) on a logarithmic scale and determining the R2
value and slope using a trendline thus allowing
placement into an ARCI susceptible group and an ARCI
resistant group. The R2
value was used to further separate
fast learners.
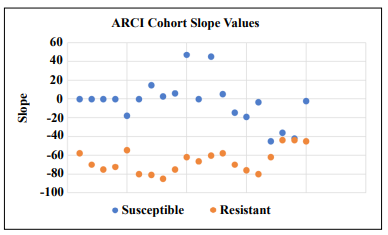
Figure 2. Regression analysis of escape times by 20-month-old C57BL/6 mice in a spatial navigation learning task. It can be seen that mice at this age can be stratified into a slow escape group and a fast escape group providing the rationale to designate these groups as susceptible and resistant, respectively, to ARCI.
To determine if ARCI susceptible mice at this age had any age-related physical challenges, agility and strength tests were performed. As can be seen in Figure 3, there were no differences in performance in strength or agility between the two groups of males or females suggesting ARCI susceptible mice were fully capable of performing basic living tasks requiring mobility for eating, drinking, and grooming. Interestingly, susceptible females performed better than susceptible males in the grip strength test but not the rotarod test, while resistant males performed better than resistant females in the rotarod test but not the grip strength test.
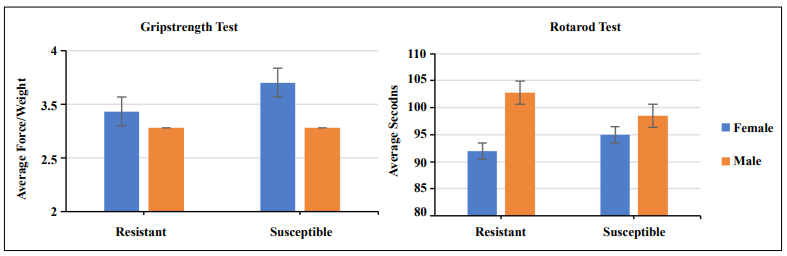
Figure 3. Grip strength and rotarod tests showed no significant difference in performance between ARCI resistant and ARCI susceptible mice in either males or females. Biostatistical analysis was done by the student's T-test.
It was then of interest to see if there were any molecular
differences in the brains of ARCI-resistant and ARCI
susceptible mice. MCP-1 is a prototype inflammatory
cytokine associated with aging [6]. We used immunohistochemistry and Qu-Path digital imaging [7] to show that
staining intensity for MCP-1 was less in the hippocampus
of ARCI-resistant mice compared to ARCI susceptible
mice (Figure 4) suggesting that inflammation may be associated with cognitive impairment in these mice.
In conclusion, we have presented preliminary observations in aging C57BL/6 mice of both sexes that describe
a naturally occurring model of ARCI. This is a potentially
high-impact translational model to study brain aging
and how mice resistant to ARCI might be more resistant
to developing AD, or other neurodegenerative diseases.
There is also the opportunity to explore, in exquisite detail
not possible in people, mechanistic aspects involved in
determining resistance or susceptibility, thus providing
insight into possible therapeutic targets for preventing or slowing the development of ARCI and decreasing the risk
of developing more severe neurodegenerative diseases.
We already have preliminary evidence that inflammation,
a process of aging, may be associated with impairment of
cognition in this model, and that mice that are resistant
to ARCI have less inflammation based on less MCP-1
expression. Therefore, the C57BL/6 ARCI mouse model
would be ideal to study strategies for therapeutically targeting inflammation. ARCI-resistant mice could also be
used to determine resistance factors to AD by challenge
with an AAV vector system linked with Aβ 42 and mutant
tau in a model we have previously described [8].
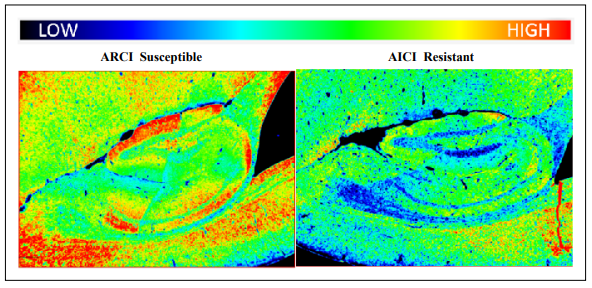
Figure 4. Staining intensities for MCP-1 were determined by immunohistochemistry and Qu-Path digital imaging. The heat map shows more intense staining in the brain of an ARCI susceptible mouse compared to the brain of an ARCI-resistant mouse.
Declarations
Authors’ contributions
All authors made contributions to generation of data and/or writing the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
Supported by NIH grants R01 AG067193, Ladiges and Darvas (co-PI’s) and R01 AG057381, Ladiges (PI).
Conflicts of interest
Warren Ladiges is a member of the Editorial Board of Aging Pathobiology and Therapeutics. The author declares that there are no conflicts.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
References
1. Jeste DV, Savla GN, Thompson WK, Vahia IV, Glorioso DK, Martin AS, et al. Association between older age and more successful aging: critical role of resilience and depression. Am J Psychiatry, 2013, 170(2): 188-196. [Crossref]
2. Kolanowski A, Boltz M, Galik E, Gitlin LN, Kales HC, Resnick B, et al. Determinants of behavioral and psychological symptoms of dementia: A scoping review of the evidence. Nurs Outlook, 2017, 65(5): 515-529. [Crossref]
3. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med, 2011, 364(23): 2227-2234. [Crossref]
4. Pettan-Brewer C, Touch DV, Wiley JC, Hopkins HC, Rabinovitch PS, & Ladiges WC. A novel radial water tread maze tracks age-related cognitive decline in mice. Pathobiol Aging Age Relat Dis, 2013, 3. [Crossref]
5. Darvas M, Mukherjee K, Lee A, & Ladiges W. A Novel One-Day Learning Procedure for Mice. Curr Protoc Mouse Biol, 2020, 10(1): e68. [Crossref]
6. Yousefzadeh MJ, Schafer MJ, Noren Hooten N, Atkinson EJ, Evans MK, Baker DJ, et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell, 2018, 17(2). [Crossref]
7. Lee A, Jiang Z, Zhu L, & Ladiges W. QuPath. A new digital imaging tool for geropathology. Aging Pathobiol Ther, 2020, 2(2): 114-116. [Crossref]
8. Darvas M, Keene D, & Ladiges W. A geroscience mouse model for Alzheimer’s disease. Pathobiol Aging Age Relat Dis, 2019, 9(1): 1616994. [Crossref]