Open Access | Commentary
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Pharmaceutical interventions to slow human aging. Are we ready for cocktails?
* Corresponding author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School
of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 21 June 2022 / Accepted: 24 June 2022 / Published: 30 June 2022
DOI: 10.31491/APT.2022.06.086
Abstract
Slowing human aging with pharmaceuticals is now recognized as a feasible strategy. However, the design of clinical trials is still focused on single drug approaches. The process of aging has multiple pathways, which no current drug has been shown to effectively target. Therefore, it is of interest to study combinations, or cocktails, of drugs. A recently published article reported that a drug cocktail of rapamycin, acarbose and phenylbutyrate slowed aging in middle-aged mice treated for three months. The impact of this report is discussed, with the implications for determining endpoints in humans for testing drug cocktails as well as testing other drug combinations.
Keywords
Healthy aging, drug cocktail, aging mice, rapamycin, acarbose, phenylbutyrate
A panel of experts held a workshop in 2013 in Erice, Italy
entitled “Interventions to slow aging in humans: are we
ready yet?” The panel selected a subset of the most promising strategies that could be tested in humans for effects
on enhancing healthy aging [1]. Among these were specific pathways associated with aging that were targeted by
one or more repurposed drugs, ie., drugs that had already
been approved for clinical use for other disease entities.
The pathways included mTOR, AMPK, histone deacetylase (epigenetics), and generic inflammation, as well as
others. Several drugs were mentioned, such as rapamycin
and metformin, that were considered safe for clinical trials
in older people. What was not discussed at the time was
the challenge of targeting multiple processes of aging with
just one drug.
To address this issue, we published an editorial entitled
“Testing drug combinations to slow aging” [2] promoting
the concept that in order to effectively slow aging, combinations of drugs were going to be needed. The rationale
behind using a combination of drugs is that multiple pathways are involved in aging, which will require drug cocktails to target these pathways in order to effectively slow aging (Figure 1). Currently, there is no single drug that
can target the multiple pathways involved, and whether at
some point in the future there will be is not known. Drugs,
such as rapamycin and acarbose, that have previously
been shown to extend lifespan in mice [3] are of interest
because they target different aging pathways.
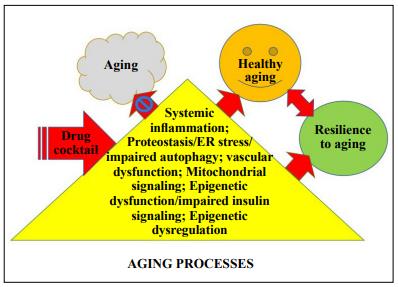
Figure 1. A drug cocktail that targets multiple aging processes will enhance resilience to aging.
Testing drugs in mouse lifespan studies has been the gold
standard but is costly and time consuming as well as having complicated translational relevance. A new approach
has been developed where middle-aged mice can be treated with a promising drug for several months and the antiaging effects of the drug can be assessed by changes in the severity of age-related lesions [4]. This approach can be
used for testing drug cocktails.
Jiang et al. [5] published a study in middle-aged mice
treated for three months with a combination of rapamycin,
acarbose, and phenylbutyrate and showed the cocktail to
be superior to any of the individual drugs in slowing aging as defined by decreased age-related lesion severity as
well as increased physiological performance. The drug
cocktail targets multiple pathways of aging. Rapamycin targets autophagy and vascular deficits, acarbose
indirectly targets insulin signaling and oxidative stress,
and phenylbutyrate targets protein folding and histone
deacetylase (epigenetic) activity. The study used C57BL/6
middle aged mice as they are a popular preclinical model
for aging research, and HET3 four-way cross mice as they
were utilized by the NIA Intervention Testing Program to
show the drugs used in the study extended lifespan. The
study showed that similar effects of slowing aging were
observed in this strain as in C57BL/6 mice, thus providing
validation for the short-term effects of the drug cocktail.
These results are very promising and demonstrate the effectiveness of a drug cocktail over a single drug in slowing aging. The cocktail serves as an excellent prototype,
but is it the ultimate pharmaceutical combination to enhance healthy aging? Most likely not, as each of the three
drugs did not contribute equally to the overall effects of
the cocktail, with rapamycin seemingly contributing the
most. Therefore, several of the drugs could be replaced by
other drugs, or additional drugs could be added, and tested
in the same mouse model system.
An equally important feature of the study was the reduction in study time by starting with middle-aged mice on a
three-month treatment regimen. This, of course, requires
having access to middle-aged mice, which is possible in
the United States through the National Institute on Aging Aged Rodent Colony. Are the positive results of the
short-term study in middle-aged mice translationally relevant? The implications are yes. A 3-month study in mice
would be roughly equivalent to a 6-year study in middleaged people. Moving from mouse studies to clinical trials brings up many questions, such as who gets the drug
cocktail and at what point should people start taking the
drug cocktail.
But more importantly, what would be the endpoints for
determining the effectiveness of the cocktail in middleaged people who necessarily will not have strong agerelated dysfunctions? Certainly, assessing the severity of
age-related lesions would not be as comprehensive since
autopsies for accessing multiple tissues would not be a
part of any short-term study. Biopsies, such as skin and
possibly a few other tissues, and peripheral white blood
cells would be accessible. The use of serum protein biomarkers for aging would be of high interest in these types
of studies, but unfortunately have not been fully developed and validated yet, therefore more research is needed
in this area.
So, are we ready for clinical trials to see if drug cocktails
can enhance resilience to aging in middle-aged people?
The study by Jiang et al. [5] provides promising evidence
that cocktails work in middle-aged mice and paves the
way for more comprehensive studies in mice and preliminary studies in humans.
Declarations
Author contributions
The authors contributed equally to writing this manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
Grants NIH R56 AG058543, Ladiges (PI); NIH R01 AG057381, Ladiges (PI).
Conflicts of interest
Warren Ladiges is a member of the Editorial Board of Aging Pathobiology and Therapeutics. The author declares that there are no conflicts.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell, 2015, 14(4): 497-510.[Crossref]
2. Ladiges W, Liggitt D. Testing drug combinations to slow aging. Pathobiol Aging Age Relat Dis, 2017, 8(1): 1407203. [Crossref]
3. Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell, 2014, 13(2): 273-282. [Crossref]
4. Ladiges W, Snyder JM, Wilkinson E, Imai DM, Snider T, Ge X, et al. A new pre-clinical paradigm for testing antiaging therapeutics. J Gerontol A Biol Sci Med Sci, 2017, 72(6): 760-762. [Crossref]
5. Jiang Z, Wang J, Imai D, Snider T, Klug J, Mangalindan R, et al. Short term treatment with a cocktail of rapamycin, acarbose and phenylbutyrate delays aging phenotypes in mice. Sci Rep, 2022, 12(1): 7300. [Crossref]