Open Access | Research Article
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
A reappraisal of measured voriconazole concentration based on plasma albumin concentration during therapeutic drug monitoring
# These authors contributed equally to this work.
* Corresponding author: Xuben Yu
Mailing address: Department of Pharmacy, the First Affiliated
Hospital of Wenzhou Medical University, Wenzhou 325015,
China.
Email: yuxuben@126.com
* Corresponding author: Haina Zhang
Mailing address: Department of Pharmacy, the First Affiliated
Hospital of Wenzhou Medical University, Wenzhou 325015,
China.
Email: 443945147@qq.com
Received: 27 May 2022 / Revised: 07 June 2022 / Accepted: 15 June 2022 / Published: 30 June 2022
DOI: 10.31491/APT.2022.06.085
Abstract
Background: The unbound fraction of voriconazole can be elevated due to a decreased plasma albumin concentration. Given its nonlinear pharmacokinetic profile, this elevation can cause adverse effects even when the
total voriconazole concentration is within the therapeutic window. This study investigated the factors affecting the plasma protein binding (PPB) of voriconazole and developed a method for the reappraisal of measured
voriconazole concentration based on plasma albumin concentration.
Methods: An observational retrospective study was performed on adult patients receiving voriconazole and
therapeutic drug monitoring (TDM) from January 2019 to December 2020 at the First Affiliated Hospital of
Wenzhou Medical University. The unbound voriconazole in plasma samples was separated using high-throughput equilibrium dialysis. Total voriconazole and unbound voriconazole concentrations were determined using
liquid chromatography-tandem mass spectrometry. A Pearson correlation analysis was performed to analyze
the correlations between voriconazole PPB and plasma albumin concentration, liver function, and concomitant
medication.
Results: A total of 193 cases with 470 voriconazole plasma samples were included. The median plasma concentration of voriconazole was 2.78 [1.56, 4.40] mg/L, median concentration of unbound voriconazole was 1.34
[0.61, 2.18] mg/L, and median binding rate of voriconazole PPB was 51.45% [45.53%, 57.89%]. The Pearson
correlation analysis showed that voriconazole PPB was positively correlated with plasma albumin concentration (R = 0.664, P < 0.001). The current TDM window of voriconazole is defined as a total trough concentration
within 1 to 4.5 mg/L, assuming voriconazole PPB of 50%. However, fluctuations in plasma albumin levels were
found to have affected the unbound fraction of voriconazole, resulting in different responses or toxicity despite
the measured voriconazole concentration being within the therapeutic window. Therefore, we developed a formula to amend the measured concentration of voriconazole to reflect the influence of a fluctuation in plasma
albumin levels.
Conclusion: Plasma albumin levels can affect voriconazole PPB and thus change the unbound fraction of voriconazole. An adjustment to the measured total voriconazole concentration based on plasma albumin concentration is needed during TDM.
Keywords
Plasma albumin, voriconazole, therapeutic drug monitoring
Introduction
Plasma protein binding (PPB) has been investigated as a
crucial factor affecting the pharmacokinetics of antimicrobial agents [1-4]. As plasma protein level decreases,
the binding rate between a drug and protein decreases,
leading to an increase in the unbound fraction. Since only
unbound drugs have pharmacological activity, a change
in PPB will cause a fluctuation in the concentration of an
unbound form, which subsequently influences the efficacy
and safety of a drug. However, an increase in an unbound
form can be reversed by rapid distribution and elimination
via the liver or kidney, and this phenomenon is expected
to be of clinical significance only for highly protein-bound
drugs [2].
Because of broad-spectrum antibiotic use and immunosuppression, elderly individuals are prone to fungal infections. Voriconazole is a triazole antifungal agent with
broad-spectrum action against invasive aspergillosis and
Candida albicans infections. It is widely applied in clinical use as a first-line option for the treatment of invasive
fungal infections. Regarding its pharmacokinetics, voriconazole is rapidly absorbed after oral administration,
reaching peak plasma concentration within 1 to 2 hours,
and its oral bioavailability is over 90%. The PPB rate of
voriconazole is approximately 50% in healthy individuals
[5]. In addition, it is mainly metabolized in the liver but
shows a nonlinear pharmacokinetic profile [6]. Thus, in
patients with low plasma protein levels, an increase in the
unbound form of voriconazole cannot be rapidly eliminated.
The high inter-patient variability in pharmacokinetics and
narrow therapeutic window trigger the need for therapeutic drug monitoring (TDM) with voriconazole. Voriconazole concentrations may be higher in elderly patients
than in younger patients due to their relatively insufficient
liver function. The current guidelines for the TDM of
voriconazole suggest a trough concentration (of both the
bounded and unbound form) maintained within 1–4.5
mg/L [7], regardless of the effect of PPB fluctuations.
Given the complexity in determining the concentration of
unbound drugs, the current therapeutic window for voriconazole has been recommended based on the total form
of voriconazole while assuming fixed PPB of 50%. However, in patients with low plasma protein levels, especially
in patients with hypoalbuminemia, the risk of developing
voriconazole-related adverse reactions is increased even
when the trough concentration of total voriconazole is
within the therapeutic range [7], as the unbound form is
much higher in these patients.
The main objective of this study was to investigate the
factors that impact voriconazole PPB and propose an
emendation for the measured concentration of voriconazole based on plasma albumin concentration, thus obtaining a more suitable measure for patients with lower plasma albumin levels in the TDM process for voriconazole.
Materials and methods
Patients
Adult patients receiving voriconazole and its related TDM from January 2019 to December 2020 at the First Affiliated Hospital of Wenzhou Medical University were included. The inclusion criteria were as follows: a) having received voriconazole for at least 3 days; b) at least one plasma test for the concentration of voriconazole having been collected. The exclusion criteria were as follows: a) a total voriconazole concentration < 0.5 mg/L; b) an unbound voriconazole concentration < 0.1 mg/L.
Clinical data collection
Clinical data were collected from medical records, including basic demographic characteristics (age, sex, weight), voriconazole medication information, laboratory measurements of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), and albumin, and co-medications, especially drugs with high PPB values (> 70%), such as aspirin, phenytoin sodium, sodium valproate, amitriptyline, warfarin, and methotrexate.
Unbound voriconazole separation
The unbound voriconazole of the plasma samples was separated using rapid equilibrium dialysis (Thermo Fisher Scientific, USA) [8]. In brief, the plasma sample was added to a 96-well plate with a semipermeable membrane through which only the unbound voriconazole could permeate. The plate was covered with sealing tape and incubated at room temperature in a vortex mixer at 800 rpm for 4 hours to reach equilibrium.
Determination of voriconazole concentration
Validated liquid chromatography-tandem mass spectrometry was used to determine the voriconazole concentrations in both the plasma and buffer compartment [9]. The plasma samples and buffer compartment were prepared using a protein precipitation method. The concentration of voriconazole was quantified using a 4 × 3.0 mm Zorbax SB-C18 column with a Xevo TQ-S mass spectrometer (Waters, USA). The column temperature was 50°C, and the flow rate was set to 0.6 ml/min. The mobile phase consisted of water (A) and methanol-0.1% formic acid (B). The column was eluted with a gradient elution program and first ramped with a constant slope from a 30% mobile phase B to 100% at 0.4 minutes and then returned to a 30% mobile phase B at 0.8 minutes. The injection volume was 10 μL. The electrospray ion source interface parameters were as follows: positive-ion detection, drying gas (N2) flow rate set to 550 L/h, and drying gas temperature set to 400°C. Compounds were detected using multiple reaction-monitoring mode (voriconazole m/z 350.1–281.1). The calibration range for voriconazole was 0.1 to 10 μg/ mL. The method validations, including calibration curve, selectivity, accuracy, precision, matrix effect, recovery, and stability, met the requirements of U.S. Food and Drug Administration (FDA) principles.
Calculation of voriconazole PPB
The voriconazole PPB rate was calculated using the following formula:

Where Cserum is the total voriconazole plasma concentration, and Cbufferis the voriconazole concentration in the buffer compartment, which indicates the unbound voriconazole concentration.
Statistical analysis
The statistical analyses were performed using SPSS version 21.0 (IBM Corp, USA.). All the study variables were summarized by the descriptive statistics. Accordingly, the normally distributed variables were expressed as mean ± standard deviation (SD), while the non-normally distributed variables were expressed as median and interquartile range (IQR). A Pearson correlation analysis was used to analyze the correlation between the variables and voriconazole PPB. A P-value of < 0.05 was considered statistically significant.
Results
The demographic information is shown in Table 1. In total, 193 adult patients with a mean ± SD age of 61.83 ± 12.80 years were included in the study. A total of 470 plasma samples were included with a median [IQR] voriconazole concentration of 2.78 [1.56, 4.40] mg/L. In addition, the median unbound voriconazole concentration was 1.34 [0.61, 2.18] mg/L. The median protein binding rate of voriconazole was 51.45% [45.53%, 57.89%].
Table 1
Clinical characteristics of the patients.
| Characteristic | Valuea |
|---|---|
| Patients (n) | 193 |
| Age (years) | 61.83 ± 12.80 |
| Sex Male (n) Female (n) |
116 (60.10%) 77 (39.90%) |
| Body height (cm) | 163.11 ± 8.70 |
| Laboratory data at baseline Serum albumin (g/L) Alanine aminotransferase (U/L) Aspartate aminotransferase (U/L) Total bilirubin (μmol/L) |
34.50 ± 5.87 18 [12, 30] 25 [18, 39] 8 [6, 12] |
Note: a Values are No. (%) or median [min, max] or mean ± SD.
The Pearson correlation analysis showed that voriconazole PPB was positively correlated with plasma albumin concentration (R = 0.664, P < 0.001). A scatter plot of the correlation between voriconazole PPB and plasma albumin concentration is shown in Figure 1. However, no significant correlations with voriconazole PPB were found for ALT (R = 0.03, P > 0.05), AST (R = 0.059, P > 0.05), or TBil (R = -0.051, P > 0.05).
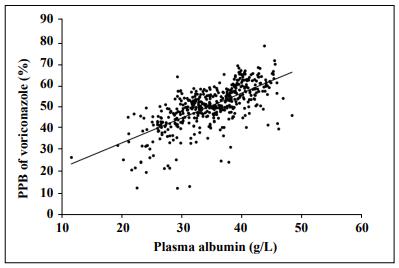
Figure 1. Correlation between the voriconazole plasma protein binding rate and plasma albumin concentrations (R = 0.664, P < 0.001).
In addition, we divided the included patients into two
groups according to hepatic function. There were no
significant differences in voriconazole PPB between the
patients with normal ALT/AST/TBil levels and those with
abnormal levels (P > 0.05).
Based on these results, an equation describing the relationship between voriconazole PPB and plasma albumin
concentration was established by using the general linear
model below:
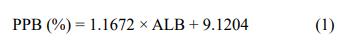
Where PPB is PPB rate, and ALB is plasma albumin concentration (g/L).
Moreover, a modification of the measured voriconazole
concentration was needed for voriconazole TDM and adjusted according to equation 2:
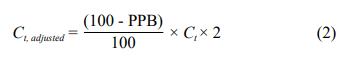
Where Ct is the measured total voriconazole plasma concentration, and Ct, adjusted is the adjusted voriconazole concentration. The rate of voriconazole PPB is approximately 50% in most patients [6], and the current therapeutic target of voriconazole is defined as a trough concentration of 1–4.5 mg/L [7]. Thus, the range for the unbound voriconazole concentration is 0.5–2.25 mg/L. However, patients with different albumin levels will have different unbound voriconazole concentrations even if their measured total concentrations are the same. Converting the measured total concentration into an adjusted concentration using equation 2 provides a measurement more suitable for judging whether voriconazole is within the therapeutic target (1–4.5 mg/L). Together with the above-mentioned equations, the data presented in Figure 2 shows the results of a variety of adjusted voriconazole concentrations under different plasma albumin concentrations.
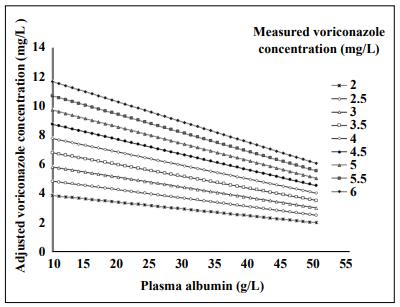
Figure 2. Adjusted voriconazole concentrations based on plasma albumin concentrations.
Discussion
This study investigated the impact of plasma albumin levels on voriconazole PPB and demonstrated that voriconazole PPB was positively correlated with plasma albumin
levels. In patients with hypoproteinemia, the change in
plasma protein levels has a great influence on the concentration of an unbound form of a drug if the drug has a
high PPB rate. For drugs that do not have a high PPB rate,
the increased unbound form caused by decreased plasma
protein has little effect on clinical efficacy or safety [10,
11], because for drugs with linear pharmacokinetics, the
increase in the unbound form is limited and can be rapidly
eliminated [2]. However, while voriconazole is a drug
with a moderate protein binding rate [5], the increase in its
unbound form caused by decreased plasma albumin level
cannot be quickly eliminated because it has a nonlinear
pharmacokinetics profile. In addition, voriconazole has a
narrow therapeutic window, and its saturated metabolism
profile is hypothesized to result in an increased risk for
toxic adverse events in patients with hypoproteinemia.
However, even when these patients receive TDM, their
test results have the potential to be misinterpreted when
the measured total concentration of voriconazole is compared with the therapeutic target. Therefore, the impact
of fluctuations in plasma albumin levels on voriconazole
PPB should be considered when interpreting the TDM
results for voriconazole, especially in patients with hypoproteinemia.
We constructed a formula to describe the correlation
between voriconazole PPB and plasma albumin concentration using a general linear model through which
the adjusted voriconazole concentration under different
plasma albumin levels could be attained and directly used
to judge whether Ct, adjusted is within the therapeutic target.
Previous studies have reported formulas for correcting
the measured plasma concentrations of two antiepileptics
(phenytoin sodium and valproate sodium) with a high
protein binding rate (PPB > 70%) and saturated metabolism [12, 13], which is of great significance in accurately evaluating the pharmacokinetic/pharmacodynamic PKPD
attainment of drugs.
While we can obtain the unbound form of a drug through
equilibrium dialysis or other separation technology, the
process to measure the concentration of an unbound form
takes a long time. Based on the current study, we recommend using equation 2 to correct the measured concentration of voriconazole as this equation can help interpret the
influence of plasma albumin levels on the PPB of voriconazole.
We also investigated the effect of ALT, AST, and TBil on
voriconazole PPB and found no significant correlations
between these and voriconazole PPB. Patients with decompensated cirrhosis often show a decrease in plasma albumin, which decreases the unbound fraction of voriconazole. In the current study, the proportion of patients with
hepatic insufficiency was low, which may have been related to the fact that the clinicians chose an alternative drug
for patients with hepatic insufficiency due to concerns that
voriconazole might aggravate liver function damage. In
addition, we also investigated the impact of concomitant
medications on voriconazole PPB. Drugs with high protein binding rates can excrete other drugs from albumin,
resulting in an increase in the unbound fraction [14]. In
this study, we did not find a significant impact from concomitant high PPB medications on voriconazole PPB,
which may be because only 11 of the 470 samples in this
study included concomitantly administered aspirin (PPB >
99%), sodium valproate (PPB > 80%), or warfarin (PPB >
98%).
There are great individual differences in the pharmacokinetics of voriconazole, and factors such as age, weight,
liver function, and gastrointestinal status affect its metabolism [15-20]. Voriconazole is prone to causing adverse reactions, such as hepatotoxicity and neurotoxicity, as it has
a narrow therapeutic window. Therefore, TDM is a crucial
tool for avoiding the adverse effects of voriconazole while
ensuring its efficacy, especially in elderly patients who
have higher voriconazole concentrations due to their relatively insufficient liver function. This study found that it
was insufficient to evaluate efficacy and safety based only
on measured voriconazole concentrations as voriconazole
PPB was significantly affected by plasma albumin levels.
Estimating adjusted voriconazole plasma concentrations
based on plasma protein concentrations helps to reduce
the risk of voriconazole-related adverse reactions.
Declarations
Authors’ contributions
Xuben Yu and Haina Zhang conceptualized and planned the work that led to the manuscript; Yexuan Wang, Fangmin Xu, Xiaoshan Zhang, Junhui Yu, Liwen Zhang, Xuben Yu, and Haina Zhang collected and analyzed the data; Yexuan Wang, Fangmin Xu, and Xuben Yu drafted the manuscript. The final submitted version of manuscript was reviewed and approved by all the authors.
Availability of data and materials
The data that support the findings of study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by grants from the Wenzhou Science and Technology Project of Public Welfare and Social Development (Medical Health) (Y20190654) and the General Scientific Research Project of Zhejiang Provincial Education Department (Y202045533).
Conflicts of interest
There are no financial relationships with any organizations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work. All authors have no conflicts of interest to disclose.
Ethical approval and consent to participate
This retrospective, observational study was designed in accordance with the Declaration of Helsinki and was approved by the Ethical Committees of the First Affiliated Hospital of Wenzhou Medical University, China ([2021]069).
References
1. Ulldemolins M, Roberts JA, Wallis SC, Rello J, & Lipman J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J Antimicrob Chemother, 2010, 65(8): 1771-1778. [Crossref]
2. Ulldemolins M, Roberts JA, Rello J, Paterson DL, & Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet, 2011, 50(2): 99-110. [Crossref]
3. Yamasaki K, Chuang VT, Maruyama T, & Otagiri M. Albumin-drug interaction and its clinical implication. Biochim Biophys Acta, 2013, 1830(12): 5435-5443. [Crossref]
4. Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, et al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother, 2013, 57(12): 6165-6170. [Crossref]
5. Vanstraelen K, Wauters J, De Loor H, Vercammen I, Annaert P, Lagrou K, et al. Protein-binding characteristics of voriconazole determined by high-throughput equilibrium dialysis. J Pharm Sci, 2014, 103(8): 2565-2570. [Crossref]
6. Theuretzbacher U, Ihle F, & Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet, 2006, 45(7): 649-663. [Crossref]
7. Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, & Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother, 2014, 69(5): 1162-1176. [Crossref]
8. Vanstraelen K, Wauters J, Vercammen I, de Loor H, Maertens J, Lagrou K, et al. Impact of hypoalbuminemiaon voriconazole pharmacokinetics in critically ill adult patients. Antimicrob Agents Chemother, 2014, 58(11): 6782-6789. [Crossref]
9. Pauwels S, Vermeersch P, Van Eldere J, & Desmet K. Fast and simple LC-MS/MS method for quantifying plasma voriconazole. Clin Chim Acta, 2012, 413(7-8): 740-743. [Crossref]
10. Musteata FM. Calculation of normalized drug concentrations in the presence of altered plasma protein binding. Clin Pharmacokinet, 2012, 51(1): 55-68. [Crossref]
11. D’Arcy PF, McElnay JC. Drug interactions involving the displacement of drugs from plasma protein and tissue binding sites. Pharmacol Ther, 1982, 17(2): 211-220. [Crossref]
12. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci, 2005, 97(4): 489-493. [Crossref]
13. Dager WE, Inciardi JF, & Howe TL. Estimating phenytoin concentrations by the Sheiner-Tozer method in adults with pronounced hypoalbuminemia. Ann Pharmacother, 1995, 29(7-8): 667-670. [Crossref]
14. Johnson GJ, Kilpatrick CJ, Bury RW, Fullinfaw RO, & Moulds RF. Unbound phenytoin plasma concentrations in patients comedicated with sodium valproate--the predictive value of plasma albumin concentration. Br J Clin Pharmacol, 1989, 27(6): 843-849. [Crossref]
15. Han K, Bies R, Johnson H, Capitano B, & Venkataramanan R. Population pharmacokinetic evaluation with external validation and Bayesian estimator of voriconazole in liver transplant recipients. Clin Pharmacokinet, 2011, 50(3): 201-214. [Crossref]
16. Han K, Capitano B, Bies R, Potoski BA, Husain S, Gilbert S, et al. Bioavailability and population pharmacokinetics of voriconazole in lung transplant recipients. Antimicrob Agents Chemother, 2010, 54(10): 4424-4431. [Crossref]
17. Liu P, Mould DR. Population pharmacokinetic analysis of voriconazole and anidulafungin in adult patients with invasive aspergillosis. Antimicrob Agents Chemother, 2014, 58(8): 4718-4726. [Crossref]
18. Muto C, Shoji S, Tomono Y, & Liu P. Population pharmacokinetic analysis of voriconazole from a pharmacokinetic study with immunocompromised Japanese pediatric subjects. Antimicrob Agents Chemother, 2015, 59(6): 3216-3223. [Crossref]
19. Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, et al. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin Infect Dis, 2012, 55(3): 381-390. [Crossref]
20. Wang T, Chen S, Sun J, Cai J, Cheng X, Dong H, et al. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J Antimicrob Chemother, 2014, 69(2): 463-470. [Crossref]