Open Access | Commentary
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Geropathology. An inside view of biological aging
* Correspondence author: Warren Ladiges
Mailing address: Department of Comparative Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA.
Email: wladiges@uw.edu
Received: 17 March 2022 / Accepted: 21 March 2022
DOI: 10.31491/APT.2022.03.078
Abstract
The geropathology concept assumes all age-related lesions are relevant, which allows the ability to grade each lesion in an organ with a severity score resulting in a quantitative value. Because aging pet cats have similar age-related diseases as older humans, knowledge of histopathology occurring during aging would be invaluable to determine how age-related lesions progress with increasing age and the connection with comorbidities. The ability to use the severity of specific organ geropathology to predict biological aging would provide new approaches to study pathways of aging and their role in the development of age-related diseases in animal models.
Keywords
Geropathology, age-related lesions, standard lesion curve, age-related diseases, pathways of aging, Alzheimer’s disease, domestic cats
Geropathology is an upcoming concept within the geroscience field. It is based on the same goal: to understand the relationship between aging and disease, but from a pathological perspective [1]. By definition, geropathology assumes all age-related lesions are relevant, which allows the ability to grade each lesion in an organ with a severity score resulting in a quantitative value. We have developed a geropathology grading platform for the laboratory mouse [2] and are currently working on a platform for laboratory rats, nonhuman primates, and domestic cats. Because aging pet cats have similar age-related diseases as older humans [3], knowledge of histopathology occurring during aging would be invaluable to determine how age-related lesions progress with increasing age and the connection with comorbidities. The ability to use the severity of specific organ geropathology to predict biological aging would open a new window of opportunity to study pathways of aging and their role in the development of age-related diseases in animal models.
The concept is based on a computational approach to develop a standard lesion curve (SLC) to integrate increasing lesion severity with increasing age based on average organ lesion scores at different ages. By using pathology records from autopsies of domestic cats at the University of California Davis (graciously provided by Dr. Denise Imai) and the University of Pennsylvania (graciously provided by Dr. Molly Church) a presumptive SLC was constructed for four major organs (Figure 1) without accounting for sex, breed or other variables, but the exercise is useful to demonstrate the power of the SLC. It can be appreciated that the liver and kidney have increasingly high lesion scores compared to the heart and brain. Therefore, as defined by age-related lesions, liver and kidney age more rapidly than heart and brain and thus are biologically older with increasing chronological age.
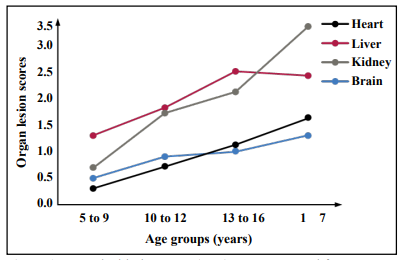
Figure 1. A standard lesion curve (SLC) was constructed from average organ lesion scores of cats autopsied at the University of Pennsylvania and University of California Davis Veterinary schools. N = 44 at 5-9 yrs, 26 at 10-12 yrs, 25 at 13-16 yrs, 16 at 17 + yrs.
Furthermore, the SLC can be used to compare where the organ lesion score (OLS) from an individual cat of a specific age falls along the curve. For example, a kidney OLS of 3.1 for an individual 15-year-old cat can be plotted on the kidney SLC under the 13 to 16 age group to show that this cat’s kidney is actually closer to 17 years reflecting an estimate of biological age. The biological age of a specific organ can then be correlated with readout data from specific pathways of aging in comparison to the chronological age (Table 1). In this example, correlation analysis of digital imaging immunohistochemistry staining intensity with biological organ age suggests that different aging pathways are involved in the aging of different organs, and some pathways may play a more prominent role than others depending on the organ.
Table 1
Example of how biological age of each organ can be correlated
with increase or decrease in digital imaging immunohistochemistry staining
intensity of aging pathway proteins.
| Organs | CA | BA | DNS | AIC | LOA | EPG |
|---|---|---|---|---|---|---|
| Brain | 15 | 13 | - | - | + | + |
| Heart | 15 | 13 | - | - | + | + |
| Lungs | 15 | 15 | nc | nc | nc | nc |
| Liver | 15 | 17 | + | + | - | - |
| Kidney | 15 | 17 | + | + | - | - |
Increase: "+"; Decrease: "-"
CA: Chronological age; BA: Biological age; DNS: Deregulated nutrient
sensing; AIC: Altered intercellular communication; LOA: Loss of
autophagy; EPG: Epigenetic alterations.
In addition to addressing questions on the role of aging pathways in individual organ aging, the SLC can be used to investigate the role of aging pathways in the pathology of age-related diseases. For example, the brains of some domestic cats exhibit the presence of Alzheimer’s disease (AD) lesions including both Aβ42 plaques and tau fibrillary tangles [4]. The NIA-AA has developed guidelines for grading the severity of AD lesions in human brains in an ABC format [5, 6, 7, 8]. These guidelines can be used to score the severity of AD lesions in cat brains. To show how the role of aging pathways can be studied, brain OLS’s and AD ABC scores can be compared between two 15-year-old cats (Table 2). Cat number 2 has an AD ABC summary score of 6 (2 for A, 3 for B, and 1 for C), with a brain OLS of 1.3, while cat number 1 has an AD ABC score of 0, and a brain OLS of 0.8. Therefore, cat number 1 has a biologically younger brain. The difference in digital imaging immunohistochemistry staining expression of aging pathway proteins in the brains of the two cats suggests specific pathways of aging might be associated in some way with the neuropathology of AD.
Table 2
Example of how biological age and the AD neuropathology grade
of cat brains can be correlated with increase or decrease in digital imaging
immunohistochemistry staining intensity of aging pathway proteins.
| Cat | OLS | ABC | CA | BA | DNS | AIC | LOA | EPG |
|---|---|---|---|---|---|---|---|---|
| Cat #1 | 0.8 | 0 | 15 | 11 | - | - | + | + |
| Cat #2 | 1.3 | 6 | 15 | 17 | + | + | - | - |
Increase: "+"; Decrease: "-"
OLS: Organ lesion score; ABC: Alzheimers disease neuropathology
score; CA: Chronological age; BA: Biological age; DNS: Deregulated
nutrient sensing; AIC: Altered intercellular communication; LOA: Loss of
autophagy; EPG: Epigenetic alterations.
Declarations
Authors’ contributions
The author contributed solely to the article.
Financial support and sponsorship
Partially supported by R24 AG047115 (Ladiges, PI) and R01 AG067193 (Ladiges, PI).
Availability of data and materials
Not applicable.
Conflicts of interest
Warren Ladiges is a member of the Editorial Board of Aging Pathobiology and Therapeutics. The author declares no conflict of interest.
Ethical approval and consent to participate
Not applicable.
References
1. Ladiges W, Ikeno Y, Niedernhofer L, et al. The Geropathology Research Network: an interdisciplinary approach for integrating pathology into research on aging. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2016, 71(4): 431-434..
2. Snyder J M, Snider T A, Ciol M A, et al. Validation of a geropathology grading system for aging mouse studies. Geroscience, 2019, 41(4): 455-465.
3. Ladiges W. The unrecognized potential of pet cats for studying aging and age-related diseases. Aging Pathobiology and Therapeutics, 2021, 3(4): 134-135.
4. Klug J, Snyder J M, Darvas M, et al. Aging pet cats develop neuropathology similar to human Alzheimer’s disease. Aging Pathobiology and Therapeutics, 2020, 2(3): 120-125.
5. Thal D R, Rüb U, Orantes M, et al. Phases of Aβdeposition in the human brain and its relevance for the development of AD. Neurology, 2002, 58(12): 1791-1800.
6. Hyman B T, Phelps C H, Beach T G, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & dementia, 2012, 8(1): 1-13.
7. Montine T J, Koroshetz W J, Babcock D, et al. Recommendations of the Alzheimer’s disease–related dementias conference. Neurology, 2014, 83(9): 851-860.
8. Braak H, Thal D R, Ghebremedhin E, et al. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology, 2011, 70(11): 960-969.