Open Access | Research Article
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Leucine metabolite β-hydroxy-β-methyl butyrate (HMB) supplementation on muscle mass during resistance training in older subjects: meta-analysis
# These authors contributed equally to this work.
* Correspondence: Wei Gao, MD, PhD.
Mailing address: Department of Geriatrics, Sir Run Run Hospital, Nanjing Medical University, Nanjing 211166, Jiangsu, China.
Email: gaowei84@njmu.edu.cn
* Correspondence: Xiang Lu, MD, PhD
Mailing address: Department of Geriatrics, Sir Run Run Hospital, Nanjing Medical University, Nanjing 211166, Jiangsu, China.
Email: luxiang66@njmu.edu.cn
Received: 06 January 2022 / Accepted: 09 March 2022
DOI: 10.31491/APT.2022.03.079
Abstract
Background: Aging, which is accompanied by loss of muscle mass, strength, and function, may contribute to the development of frailty and fractures in older people. Interventions such as β-hydroxy-β-methyl butyrate (HMB) treatment and resistance exercise training (RET) have been well established independently to attenuate muscle loss in previous researches. Nevertheless, no consensus exists on whether the combination of HMB intervention and RET could obtain an additional benefit to the older population. Our aim was to systematically quantify whether HMB supplementation combined with RET has a synergistic effect on improving muscle mass, strength, and function in older adults.
Methods: A systematic search was performed using the electronic databases Medline, Embase, Cochrane Library, and Web of Science from inception of the study until Oct 30, 2021. The articles included were all randomized controlled trials and met the inclusion. A fixed or randomized (if data were heterogeneous) effects metaanalysis was performed using Stata.
Results: A total of 256 articles were screened, with eight studies matching the eligibility criteria, which enrolled 333 subjects (≥ 65 years old). A meta-analysis was conducted, and the results showed no significant difference between the groups in lean mass, fat mass, or physical performance. In the subgroup analysis regarding the differences in muscle strength between appendicular muscles, HMB supplementation combined with RET
contributed to significantly improving the muscle strength of the lower limbs (n = 6, SMD: 0.55, 95% confidence interval: 0.06 to 1.04).
Conclusion: A combination of HMB supplementation and RET in older people has an additional benefit for muscle strength, especially in the lower limbs, instead of muscle function and physical performance. Further
studies are needed to demonstrate the mechanism.
Keywords
PHMB, muscle mass, resistance exercise training, elderly
Introduction
The number of elderly people is estimated to reach 2 billion by 2050. Age-related muscle loss accompanied by a decrease in muscle strength and function, which leads
to multiple adverse consequences, including disability,
frailty, morbidity, and mortality is an important clinical
problem in elderly people [1]. The prevalence of sarcopenia, which is characterized by muscle loss and dysfunction
reported by the European Working Group on Sarcopenia
in Older People (EWGSOP), has reached 9.9–40.4% in
older adults [2]. Methods to relieve sarcopenia have been
intensively researched, including resistance training, nutritional supplements, hormones, drug treatments, and so
on. For the elderly, especially disabled elderly, because of
the decline in physical function, the interventions mentioned above are greatly restricted in clinical conduction.
Therefore, for high-risk groups for sarcopenia, and the
elderly, providing intervention as soon as possible, to delay progression and to explore efficient and multi-target
strategies is the key clinical priorities.
Muscle atrophy is mainly due to a dynamic imbalance between muscle protein synthesis (MPS) and muscle protein
breakdown. Resistance exercise and nutrition therapy are
two promising ways to maintain protein balance [3, 4].
However, some elderly people experience some difficulty
in implementing and persisting in resistance exercise,
particularly during acute illness and disability. In addition, the elderly need professional guidance to carry out
resistance training to reduce the risk of sports injuries and
falls. Furthermore, nutritional supports, including protein
and amino acid supplements, are considered safe and
convenient. However, their efficiency depends on the appetite and digestive functions of the elderly. For this reason, novel nutrients those are more available and efficient
should be investigated.
β-Hydroxy-β-methyl butyrate (HMB) is a metabolite of
the amino acid leucine. Studies have confirmed its role
in relieving and promoting muscle synthesis and relieving muscle atrophy [5, 6]. HMB stimulates protein synthesis through increasing growth hormone/insulin-like
growth factor I activity (IGF-1) axis in skeletal muscle;
downregulating eukaryotic initiation factor 2αeIF2α and
upregulating the mammalian target of rapamycin(mTOR)/
p70 ribosomal protein S6 kinase (S6K1) pathway; and
activating mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK) and
phosphatidylinositol-3-OH kinase (PI3K)/Akt pathways,
facilitated by IGF-I. The possible mechanisms of HMB
inhibition of protein degradation are as follows. First,
HMB inhibits protein kinase C (PKC)-signaling, which
diminishes the ubiquitin-proteasome protein hydrolysis pathway. Second, HMB decreases mitochondria-associated cystathionase activity and subsequent reactive oxygen species (ROS) production. These effects lead to the
inhibition of apoptosis in myonuclear cells. Third, HMB
increases cell membrane integrity via cholesterol synthesis to reduce tissue injury-induced protein hydrolysis. Finally, HMB may act as a structural component within cell
membranes, but its role remained unclear. The molecular
mechanism is illustrated in Figure 1 [7]. HMB may also
enhance the stability of the cell membrane by undergoing
polymerization. In clinical studies, the role of HMB combined with resistance exercise training (RET) treatment
has not yet reached consensus because of study-specific
characteristics. Researchers have found both gains and no
changes in strength with HMB supplementation. Whether
HMB supplementation combined with RET is more effective than RET alone, which component focuses on muscle
mass, strength, and function, is unclear in the elderly [8, 9]. Hence, we conducted a meta-analysis to assess the
influence of HMB supplementation combined with RET
on lean mass, body fat mass (FM), muscle strength, and
muscle function in older people.
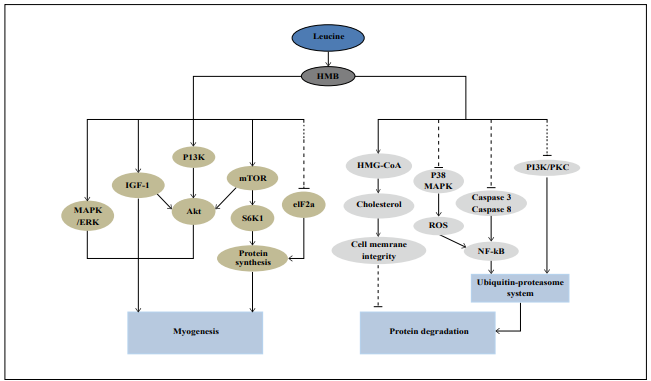
Figure 1. Possible mechanisms for HMB to stimulate myogenesis and inhibit protein degradation in skeletal muscle. S6K1: p70 ribosomal protein S6 kinase, MAPK: Mitogenactivated protein kinase, ERK: extracellular signal-regulated protein kinase, IGF-I: Insulin-like growth factor I, PI3K: Phosphoinositide-3-OH kinase, eIF2α: Eukaryotic initiation factor 2α, ROS: Reactive oxygen species, NF-κB: Nuclear factor-κB, mTOR: Mammalian target of rapamycin, PKC: Protein kinase C, HMG-CoA: β-hydroxy-β-methyl glutarylCoA. Dashed lines indicate suppression, solid lines indicate enhancement.
Methods
Study inclusion/exclusion criteria
Studies that met the following criteria were eligible for inclusion according to the participant, intervention, control, outcome measures, and study design strategy (Table 1).
Table 1
Inclusion and exclusion criteria used to evaluate studies for the
meta-analysis.
| Inclusion criterion | Description |
|---|---|
| Participants | Aged 65 or older. |
| Intervention | HMB oral supplementation in addition to resistance training. |
| Control | Participants were not provided with HMB supplementation (controls or placebo). |
| Outcome | Body composition, muscle strength, muscle function. |
| Study design | Randomized controlled trial. |
Data sources and searches
A literature search was conducted by searching relevant databases to investigate the effects of HMB combined with resistance exercise on body composition and muscle strength and function in older adults. Relevant articles from the earliest year to 2000 were searched. Search terms were used, including (HMB or beta-hydroxy-beta-methyl butyrate or β-hydroxy-β-methyl butyrate) and (exercise or training or “resistance exercise”) and (“older adults” or elderly or elder) and (“muscle mass” or “muscle strength” or “sarcopenia”). Search electronic libraries included PubMed, Web of Science, Cochrane Library, and Embase, with keywords used in various combinations, and maximum search results have been reached. The search covered original papers written in English and published before Oct 30, 2021, in these databases. Trials were conducted in humans.
Data extraction and outcome measures
The data in the papers were extracted by 2 independent and parallel investigators. First, all the papers were downloaded by the two researchers. Second, duplicates were removed, and titles and abstracts were screened to identify studies that met the eligibility criteria. Thereafter, the full texts were assessed. We manually searched the references meeting the inclusion criteria for further analysis. The following data were extracted for each study: authors, year of publication, sample size, gender, mean age, RET intervention, placebo/control information, body composition results, information on muscle strength and muscle function, and any other noteworthy information (e.g., source of bias/conflict of interest). The original data were obtained from the corresponding authors when the required data were missing in the articles. Otherwise, the data were imputed according to the methods given in the Cochrane Handbook. For studies in which no standard deviation (SD) was given, the post-intervention SD could be derived from the sample size (95% confidence interval [CI]).
Risk of bias analysis
The methodological quality of the included articles was assessed by the two investigators according to the Cochrane Collaboration risk-of-bias tool [10], including seven separate areas: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessments; (5) incomplete outcome data; (6) selective reporting and (7) other sources of bias. When the two researchers disagreed on study eligibility, data extraction, and risk-of-bias assessment, a third investigator was available to arbitrate. Judgments for each entry involved assessing the risk of bias as “low risk,” “high risk,” and “unclear,” the latter indicating a lack of information or uncertainty about potential bias.
Data analysis
The outcomes of interest in this paper included the effect on body composition, strength, and physical function. If the dependent variable had multiple time points, only the pre-intervention and post-intervention values were selected. Meta-analysis was performed on the extracted data with Stata-SE. For the included studies, given the different ways used to measure muscle mass and strength, effect sizes were expressed as standardized mean differences (SMDs) with a 95% CI. SMD values of 0.2, 0.5, and 0.8 were defined as small, medium, and large effect sizes, respectively [11]. The heterogeneity of the included studies was determined by I2 (<50% was considered low, 50%–74.9% was considered moderate, and 75%-100% was considered high heterogeneity). The fixed-effects model was used when I2 was less than 50%; otherwise, the random-effects model was used.
Results
Study selection
A total of 256 studies were identified from the search strategy and other searches. After eliminating duplicates, 215 records were available for title and abstract screening. A total of 37 articles were screened for full text, and after further screening based on our selection criteria, 10 of these articles were reviewed for inclusion. After further exclusion based on our selection criteria, eight randomized controlled trials met the inclusion criteria and underwent a final analysis. Figure 2 shows the PRISMA flow diagram.
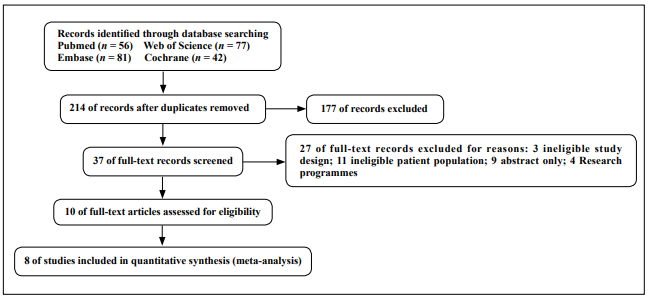
Figure 2. Flow through of articles through the search and review process.
Study characteristics
The eight eligible randomized controlled trials involved a total of 333 older adults: 159 were included in the experience group, and 174 were assigned to the control group. Because of unavailable data in two studies [12, 13], the authors were contacted to provide additional data by e-mail but no reply was received. The characteristics of these studies are shown in Table 2 and individual study outcomes included in the meta-analysis are shown in Table 3. The studies were conducted in healthy older adults with an average age of over 65 years. No studies mentioned the race of the subjects. The duration of the intervention varied widely, from 6 weeks to 12 months, and the frequency was two to three times per week. The HMB dose varied between 1.5 g/day (n = 2) and 3.0 g/day (n = 6).
Table 2
Study characteristics of included trials.
| Author, Year | Subjects | No. (C; T) | Age. (C; T) | Training Regimen | HMB Supplementation | |||||
| Length | Frequency | Intensity (Reps) | Sets | Loading | Daily dose | Control | ||||
| Stout, 2013 | Man, woman; healthy | NC = 20; NT = 16 |
73.0 ± 1.0; 73.0 ± 1.0 |
21 (wk) | 3/wk | 8-12 at 80% 1-RM | 66 (77.6%) | Mixture | 3 g HMB/d | Placebo |
| Stout, 2015 | Man; healthy | NC = 12; NT = 12 |
72.1 ± 5.7; 72.1 ± 5.7 |
12 (wk) | 3/wk | N.R | 6 (54.54%) | Mixture | 3 g HMB/d | Placebo |
| Din, 2019 | Man; healthy | NC = 8; NT = 8 |
768.5 ± 1.0; 67.8 ± 1.15 |
6 (wk) | 3/wk | 6-8 at 75% 1-RM | 7 (87.5%) | Mixture | 3 g HMB/d | Placebo |
| Vukovich, 2000 | Man, woman; healthy | NC = 17; NT = 14 |
70.0 ± 1.0; 70.0 ± 1.0 |
8 (wk) | 2/wk | 10-12 at 70% 1-RM | 4 (80%) | Capsules | 3 g HMB/d | Placebo |
| Berton L, 2016 | Women; healthy | NC = 33; NT = 32 |
69.5 ± 5.3; 69.5 ± 5.3 |
8 (wk) | 2/wk | Mild fitness | 7 (87.5%) | Drink | 1.5 g ca-HMB/d | Standard diet |
| Deutz, 2013 | Men, women; healthy | NC = 11; NT = 8 |
67.1 ± 1.7; 67.4 ± 1.4 |
8 (wk) | 3/wk | 8-10 at 80% 1-RM | 4 (80%) | Sachet | 3 g HMB/d | Placebo |
| Rathmacher, 2020 | Man, woman; healthy | NC = 34; NT = 30 |
67.7 ± 0.7; 67.2 ± 0.7 |
12 (mon) | 3/wk | 60 minutes of supervised resistance | 4.8+6.5, [0.04-30] | Capsules | 3 g ca-HMB/dplus vitamin D3 (2,000 IU/day) | Placebo |
| Osuka, 2021 | Woman | NC = 39; NT = 39 |
71.8± 4.1; 73.5 ± 4.2 |
12 (wk) | 2/wk | 50 min resistance training | N.R | Powder | 1.5 g ca-HMB/d | Placebo |
C: control; T: treatment; No: number; NC: number of control; NT: number of treatment; PL: Placebo; ca-HMB: calcium beta-hydroxy-beta-methylbutyrate; N.R: not reported; 1-RM: one repetition maximum.
Table 3
Individual study outcome included in the meta-analysis.
| Author, Year | Subjects | No. (C; T) | Age. (C; T) | Training Regimen |
|---|---|---|---|---|
| Stout, 2013 | DXA | Total lean mass, kg Leg lean mass, kg Total fat mass, kg |
Extensor 180° • s−1 Flexor 180° • s−1 Extensor 60° • s−1 Flexor 60° • s−1Hand grip strength, kg |
Get up and go, s |
| Stout, 2015 | DXA | Abdominal fat mass, kg | ||
| Din, 2019 | DXA | Thigh lean muscle mass, gThigh fat free mass, g | MVC, Nm1-RM: Nm | |
| Vukovich, 2000 | DXA | Body fat (%)Fat-free mass, kg | Upper body strength (%)Lower body strength (%) | |
| Berton L, 2016 | DXApQCTRadial pQCTTibial pQCT | ASMMIAbdominal fat mass, kgFat-free mass, kgMuscle area, mm2Fat area, mm 2Muscle area, mm 2Fat area, mm2 | PT isokinetic Nm: PT isokinetic ext, Nm:PT isokinetic flex, Nm:Handgrip strength, kgHandgrip endurance, s | Chair stand times, s6 MWT, mWalking time, sBalance test, scoreSPPB |
| Deutz, 2013 | DXA | Leg Lean, kgTotal body fat mass, kgTotal lean Mass, kg | Knee extensor (60°) strength, NmKnee extensor (180°) strength, Nm | |
| Rathmacher, 2020 | DXA | Lean Mass, kgBody fat (%) | Hand grip strength, kg | Get up and go, sGet up (reps) |
| Osuka, 2021 | DXA | Appendicular lean mass, kg Fat-free mass, kgSkeletal muscle index, kg/m 2Fat mass, kg Upper-extremity lean mass, kgLower-extremity lean mass, kg | Knee extensor strength, N Hip adductor strength, NHandgrip strength, kg | Usual gait speed, m/s Maximal gait speed, m/s Timed up-and-go, s Five-repetition sit-to-stand, s |
DXA: dual X-ray absorptiometry; SPPB: short physical performance battery; MVC: maximal voluntary contraction; 1-RM: one repetition maximum.
Quality of included studies and risk of bias
No study was considered to have a low risk of bias in all categories. Scout showed a high risk of selective reporting because of a difference from the pre-registered trial [12]. The smallest biases were found in allocation concealment and blinding of outcome assessments. Figure 3 indicates the risk of bias assessment. The heterogeneity of the included studies was determined by I2 (< 50% was considered low, 50–74.9% was considered moderate, and 75–100% was considered high heterogeneity). The fixedeffects model was used when I2 was less than 50%. Otherwise, a random-effects model was used.
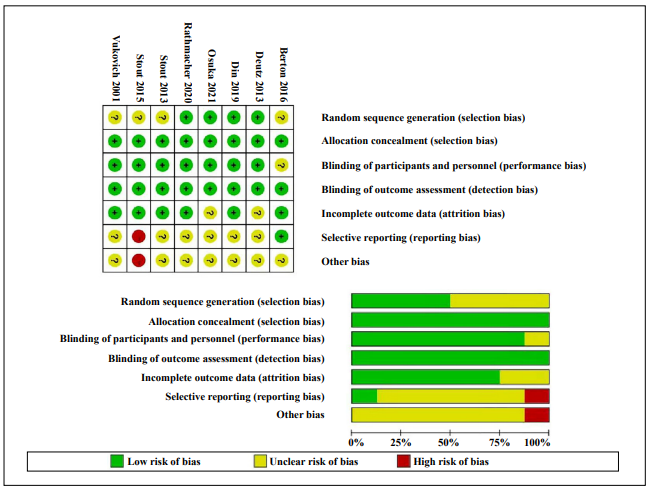
Figure 3. Risk-of-bias summary for all studies and outcomes, drawing by Review Manager 5.3.
Main outcomes
All studies included reported measurements of body composition, including FM and fat-free mass, which is based on dual X-ray absorptiometry (DXA) or computed tomography, with a measurement cycle ranging from 6 weeks to 12 months. Five studies reported the effects of HMB on lower body strength, including knee flexion/extension by isokinetic, isometric, one maximum repetition, maximal voluntary contraction, peak torque isometric and isokinetic strength of the lower limbs, and hip adductor strength. Four studies reported the effects on upper-body strength muscle strength, including handgrip strength and handgrip endurance [13-16], and one study reported a percentage change in upper and lower body strength [17]. Four studies reported the effects on muscle function, including short physical performance battery (SPPB), get up and go, 4-meter walk time, 6-second walk distance, usual gait speed, and five-repetition sit-to-stand [13-16]. The SMD of variables at the end of the intervention period between the HMB groups and the placebo combined with resistance exercise was used for analysis.
All included studies were tested for heterogeneity: FM, fat-free mass, lower-body strength, upper-body strength, and muscle function, and the results revealed high heterogeneity. Therefore, random-effects models were applied to the meta-analysis. Eight studies were included in the meta-analysis, revealing evidence that HMB or supplements containing HMB improved lower body strength compared with controls, but with a moderate to large effect size (SMD = 0.55; 95% CI: 0.06, 1.04; P = 0.000; I2 = 86.9%, Figure 4).
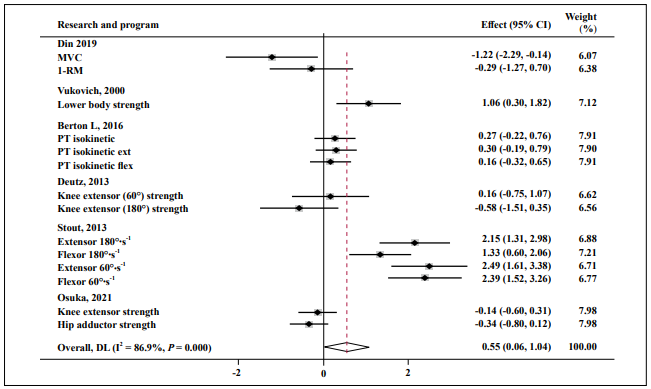
Figure 4. Forest plot of the results of a random-effects meta-analysis of HMB shown as pooled standard mean differences with 95% CIs on lower-body
strength. Data were analyzed by stata 15. Vertical lines and diamonds indicate the overall measure of effects and confidence intervals.
Note: Weights are from random- ffects model.
HMB showed no significant effect on upper body strength (SMD = 0.27; 95% CI: -0.55, 1.09; P = 0.000; I2 = 92.0%, Figure 5).
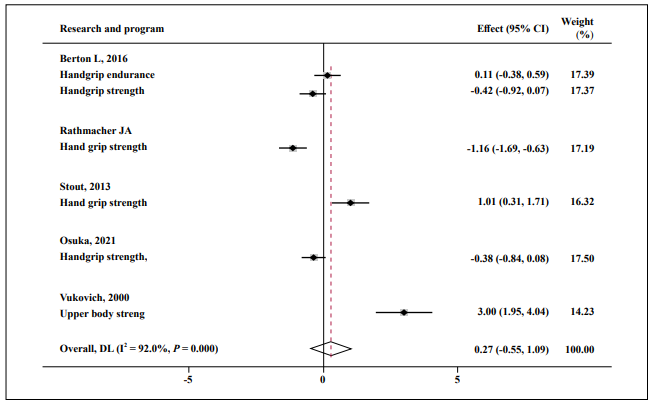
Figure 5. Forest plot of the results of a random-effects meta-analysis of HMB shown as pooled standard mean differences with 95% CIs on upper-body
strength. Data were analyzed by stata 15. Vertical lines and diamonds indicate the overall measure of effects and confidence intervals.
Note: Weights are from random- ffects model.
Almost no effect was found in FM (SMD = 0.25; 95% CI: -0.18, 0.75; P = 0.001; I2 = 82.1%, Figure 6), fat-free mass (SMD = 0.04; 95% CI: -0.26, 0.33; P = 0.000; I2 = 76.3%, Figure 7), and muscle function (SMD = 0.15; 95% CI: -0.21, 0.51; P = 0.000; I2 = 83.7%, Figure 8).
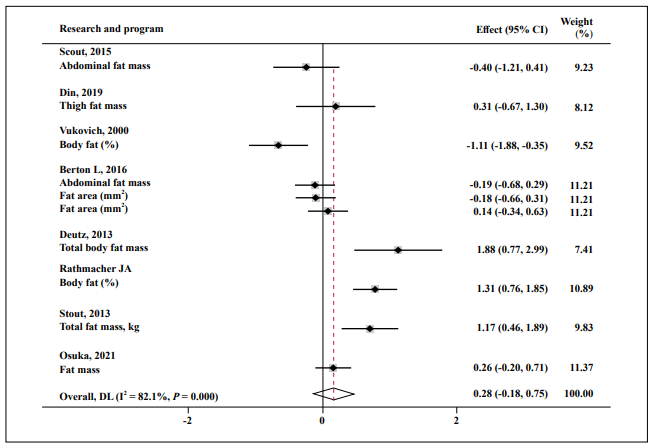
Figure 6. Forest plot of the results of a random-effects meta-analysis of HMB shown as pooled standard mean differences with 95% CIs on fat mass. Data
were analyzed by stata 15. Squares are effect sizes. Vertical lines and diamonds indicate the overall measure of effects and confidence intervals.
Note: Weights are from random- ffects model.
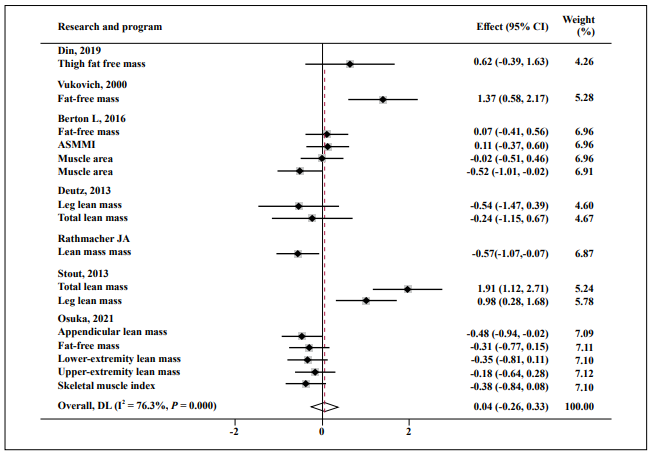
Figure 7. Forest plot of the results of a random-effects meta-analysis of HMB shown as pooled standard mean differences with 95% CIs on fat-free mass.
Data were analyzed by stata 15. Squares are effect sizes. Vertical lines and diamonds indicate the overall measure of effects and confidence intervals.
Note: Weights are from random- ffects model.
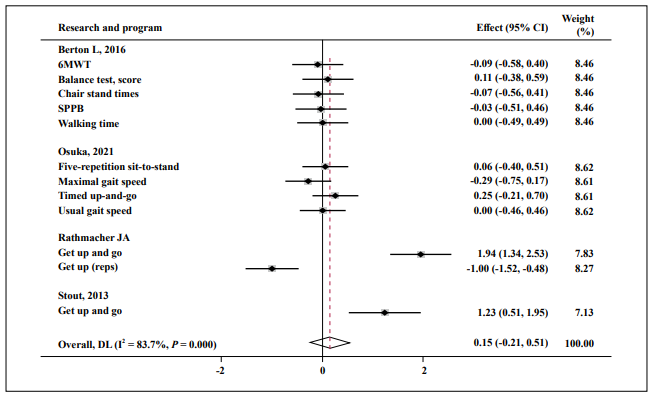
Figure 8. Forest plot of the results of a random-effects meta-analysis of HMB shown as pooled standard mean differences with 95% CIs on muscle function.
Data were analyzed by stata 15. Squares are effect sizes. Vertical lines and diamonds indicate the overall measure of effects and confidence intervals.
Note: Weights are from random- ffects model.
Sensitivity analyses and publication bias
Following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, statistical tests to build funnel plot asymmetry were not undertaken owing to the use of SMDs in the meta-analysis.
Discussion
This study was conducted to compare the effects of interventions of HMB supplementation in addition to RET
and RET alone on the muscle mass, strength, and function of healthy community-dwelling elder adults. HMB
supplementation and RET are well-recognized effective
treatments for sarcopenia, and the synergistic effects of
the intervention methods mentioned above have been previously evaluated [18, 19]. However, no consensus was
reached. Jakubowski concluded that HMB and RET treatment has a small effect on total body mass gain, but this
effect was not seen in free FM (FFM), muscle strength,
or decreases in (FM) [20]. Rowlands and Thomson determined a synergistic effect in leg strength gains in previously untrained men by supplementing them with HMB
during resistance training, but this effect in trained lifters
was not significant [18]. Another recent study in untrained
young adults showed that additional supplementation with HMB combined with resistance exercise resulted in segmental lower extremity FFM growth when compared with
resistance exercise alone [21]. These different results may
relate to the heterogeneity of the participants included
and the interventions used. Although the additional effect
of muscle gains from the combination of HMB and RET
is unknown, drawing a final conclusion was too early,
and more updated research should be included for evaluation. Our analysis, which included the latest clinical
randomized controlled trial (RCT) studies, showed that
HMB supplementation combined with RET significantly
enhanced the muscle strength of the lower limbs in the elderly, but no positive effect was noted for muscle strength
of the upper limbs, lean mass, FM, and muscle function
during the entire study. Here, we found that in the elderly
population, supplementing HMB on the basis of resistance training could achieve additional positive effects
on muscle strength of lower limbs. This conclusion was
similar to the previous study conducted among untrained
lifters.
With the aging of the population intensifying, we conducted this study mainly for the elderly, who often suffer
multiple chronic diseases. The synergy between HMB
supplementation and RET in the elderly is not surprising.
Physical inactivity and malnutrition, which interact with
each other, are common conditions in older adults. Nutritional interventions are important for promoting physical
activity. Exercise also helps improve appetite and promote
nutrient absorption. Although a recent meta-analysis suggested that HMB supplementation in addition to physical
exercise has a low impact on improving muscle strength
in adults aged 50–80 years [22], we found preserved muscle strength in the intervention group compared with the
gradual loss experienced in the control group. This might
be due to the findings of our meta-analysis being mainly
based on older adults who were untrained or who lightly
exercised.
Although studies have shown that RET is an effective way
to prevent frailty and sarcopenia, nutritional supplementation remains a safer, simpler, and more feasible intervention for older adults than RET. HMB has been widely
recognized as a nutrient ingredient for the treatment of
sarcopenia by activating the major signaling pathways
leading to protein synthesis [23, 24]. In several preclinical models, HMB has been shown to improve MPS by
activating mTOR. To explore the exact role of HMB intervention during RET treatment, we conducted subgroup
analyses and found that HMB combined with RET mainly
improved lower limb muscle strength rather than the upper limbs. Notably, the strength of the lower limb muscles
is more important than the upper ones to improve the selfcare ability and stability of the elderly. This novel result
may provide optimized strategies to prevent frailty and
sarcopenia. The findings of our analysis showed blunted
effects of additional HMB supplementation in muscle
mass. This result may be due to four reasons. First, the
analysis included studies of healthy older adults who were
probably not malnourished. The effect of the HMB intervention may be diminished in older adults who habitually
consume adequate nutrients. Second, in contrast to the
young, the elderly may not achieve the expected effects of
the conventional dose of HMB because of the degradation
of digestion and absorption functions. In addition, because
of the imbalance in muscle protein metabolism in older
adults, long-term nutritional supplementation is needed,
whereas short-term supplementation may not show obvious effects. Moreover, inflammation, immobilization,
and chronic comorbidities could further lead to muscle
loss. Thus, the different results may indicate that different
populations respond differently to HMB supplementation
combined with RET .
Through the results of this study, we confirmed that HMB
supplementation combined with RET had a positive effect
on the muscle improvement of the elderly living in the
community, although this positive effect was limited to
the muscle strength of the lower limbs. Among the elderly
in the community, the molecular mechanism of HMB supplementation on the basis of RET to improve the muscle
strength of the lower limbs needs to be further studied.
Further research is needed to explore the appropriate timing, intensity, and prognosis of interventions.
Conclusion
The combination of HMB supplementation and RET in older people has an additional benefit for muscle strength, especially in the lower limbs, instead of muscle function and physical performance. Further studies are needed to demonstrate the mechanism.
Declarations
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Guangqin Zou, Quan Wang, Xiang Lu, Wei Gao; Performed data acquisition, as well as providing administrative, technical, and material support: Guangqin Zou, Hua Wan, Wei Gao.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by grants from the National Key Research and Development Plan of China (No. 2020YFC2008505 to Xiang Lu), the National Natural Science Foundation of China (No. 81970218 to Xiang Lu and No. 81970217 to Wei Gao), the Six Talent Peaks Project of Jiangsu Province (No. WSN‐175 to Wei Gao), and Jiangsu Geriatrics Society (JGS2019ZXYY06 to Xiang Lu).
Conflicts of interest
Wei Gao is a member of the Youth Editorial Board of Aging Pathobiology and Therapeutics. All authors declare no conflict of interest and were not involved in the journal’s review or decisions related to this manuscript.
Ethical approval and consent to participate
Not applicable.
References
1. Cruz-Jentoft A J, Sayer A A. Sarcopenia. The Lancet, 2019, 393(10191): 2636-2646.
2. Mayhew A J, Amog K, Phillips S, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age and ageing, 2019, 48(1): 48-56.
3. Bischoff-Ferrari H A, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. Jama, 2020, 324(18): 1855-1868.
4. Nilsson M I, Mikhail A, Lan L, et al. A five-ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients, 2020, 12(8): 2391.
5. Gepner Y, Varanoske A N, Boffey D, et al. Benefits of β-hydroxy-β-methylbutyrate supplementation in trained and untrained individuals. Research in Sports Medicine, 2019, 27(2): 204-218.
6. He X, Duan Y, Yao K, et al. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids, 2016, 48(3): 653-664.
7. Duan Y, Li F, Li Y, et al. The role of leucine and its metabolites in protein and energy metabolism. Amino acids, 2016, 48(1): 41-51.
8. Weihrauch M, Handschin C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochemical pharmacology, 2018, 147: 211-220.
9. Teixeira F J, Matias C N, Monteiro C P, et al. Leucine Metabolites Do Not Enhance Training-induced Performance or Muscle Thickness. Medicine and Science in Sports and Exercise, 2019, 51(1): 56-64.
10. Cochrane handbook for systematic reviews of interventions. 2011.
11. Cohen J. The analysis of variance and covariance. Statistical power analysis for the behavioural sciences, 1988.
12. Stout J R, Fukuda D H, Kendall K L, et al. β-Hydroxy-βmethylbutyrate (HMB) supplementation and resistance exercise significantly reduce abdominal adiposity in healthy elderly men. Experimental gerontology, 2015, 64: 33-34.
13. Osuka Y, Kojima N, Sasai H, et al. Effects of exercise and/ or β-hydroxy-β-methylbutyrate supplementation on muscle mass, muscle strength, and physical performance in older women with low muscle mass: a randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition, 2021, 114(4): 1371-1385.
14. Berton L, Bano G, Carraro S, et al. Effect of oral betahydroxy-beta-methylbutyrate (HMB) supplementation on physical performance in healthy old women over 65 years: an open label randomized controlled trial. PloSone, 2015, 10(11): e0141757.
15. Stout J R, Smith-Ryan A E, Fukuda D H, et al. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+ yrs: a randomized, double-blind pilot tria. Experimental gerontology, 2013, 48(11): 1303-1310.
16. Rathmacher J A, Pitchford L M, Khoo P, et al. Long-term effects of calcium β-hydroxy-β-methylbutyrate and vitamin D3 supplementation on muscular function in older adults with and without resistance training: A randomized, double-blind, controlled study. The Journals of Gerontology: Series A, 2020, 75(11): 2089-2097.
17. Vukovich M D, Stubbs N B, Bohlken R M. Body composition in 70-year-old adults responds to dietary β-hydroxy β-methylbutyrate similarly to that of young adults. The Journal of nutrition, 2001, 131(7): 2049-2052.
18. Rowlands D S, Thomson J S. Effects of β-hydroxy-βmethylbutyrate supplementation during resistance training on strength, body composition, and muscle damage in trained and untrained young men: A metaanalysis. The Journal of Strength & Conditioning Research, 2009, 23(3): 836-846.
19. Bear D E, Langan A, Dimidi E, et al. β-Hydroxy-βmethylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. The American journal of clinical nutrition, 2019, 109(4): 1119-1132.
20. Jkubowski J S, Nunes E A, Teixeira F J, et al. Supplementation with the Leucine Metabolite β-hydroxy-βmethylbutyrate (HMB) does not Improve Resistance Exercise-Induced Changes in Body Composition or Strength in Young Subjects: A Systematic Review and Meta-Analysis. Nutrients, 2020, 12(5): 1523.
21. Stahn A C, Maggioni M A, Gunga H C, et al. Combined protein and calcium β-hydroxy-β-methylbutyrate induced gains in leg fat free mass: a double-blinded, placebocontrolled study. Journal of the International Society of Sports Nutrition, 2020, 17(1): 1-12.
22. Courel-Ibáñez J, Vetrovsky T, Dadova K, et al. Health benefits of β-Hydroxy-β-Methylbutyrate (HMB) supplementation in addition to physical exercise in older adults: a systematic review with meta-analysis. Nutrients, 2019, 11(9): 2082.
23. Duncan N, Fuller J C, Baier S M, et al. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, doubleblind, placebo-controlled study. European Journal of Applied Physiology, 2014, 114, 1217–1227.
24. Kraemer W J, Hatfield D L, Volek J S, et al. Effects of amino acids supplement on physiological adaptations to resistance training. Medicine & Science in Sports & Exercise, 2009, 41(5): 1111-1121.