Open Access | Research Article
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Clinical diagnosis and treatment of primary small intestinal lymphoma
*Corresponding author: Guitian Huang
Mailing address: Department of Geriatrics, Guangzhou First Peoples’s Hospital, Guangzhou, Guangdong Province,
510000,China.
E-mail: 1647733071@qq.com
Received: 22 February 2021 / Accepted: 14 April 2021
DOI: 10.31491/APT.2021.09.066
Abstract
Background: To report experiences in the diagnosis and treatment of primary lymphoma of the small
intestine
(PSIL). Lymphoma, non-Hodgkin, primary small intestine lymphoma (PSIL),
diagnosis, therapy
Primary small intestinal lymphoma (PSIL) is a rare malignant
tumor of the gastrointestinal tract. Due to its insidious
onset and lack of specific clinical manifestations, it is
easily misdiagnosed. We aimed to explore the diagnosis
and clinical treatments of PSIL by analyzing the clinical
manifestations and computed tomography (CT) characteristics
of 15 patients who were admitted to our hospital
between January 2015 and July 2019.
There were 15 cases in this group, comprising 9 men and
6 women. This study was approved by the Ethics Review
Committee at Guangzhou First People’s Hospital, and
we obtained the clinical information of the patients after
obtaining their informed consent. The age of PSIL onset
ranged from 18 to 73 years, and the median age was 51.6
years. Eleven cases (73.3%) were over the age of 40 years.
The patients’ history of symptoms ranged from 1 week to
1.5 years. The main clinical manifestations included abdominal
pain, abdominal mass, intestinal obstruction, gastrointestinal
bleeding, and wasting. Serum tumor indexes
(carcinoembryonic antigen, CAl9-9) were normal in all
patients. All cases met the Dawson diagnostic criteria as
follows [1] :
All 15 cases were examined by abdominal spiral CT, and
the findings included intestinal wall thickening, intestinal
luminal mass, and mesenteric lymph node enlargement.
Secondary manifestations included intussusception, intestinal
obstruction, and small amounts of peritoneal effusion.
The intestinal lumen was in an “aneurysm-like”
dilated state in 9 cases, and the lumen of the intestine was
mildly stenosed with incomplete intestinal obstruction
in 1 case. Fifteen cases showed soft tissue density on the
CT scan, irregular low-density necrotic areas were seen
within the lesion, and the lesion was mildly enhanced on
enhanced scans. The fat surrounding the intestinal wall
disappeared in 7 cases. The CT findings of 9 cases showed
mesenteric lymphadenopathy. There were 15 cases diagnosed
by CT, of which 12 cases were accurately diagnosed
(Figure 1: a-c), 1 case was misdiagnosed as intestinal
adenocarcinoma,
and 1 case was misdiagnosed as a stromal
tumor; the diagnosis of 1 case was still uncertain.
Before surgery, 11 cases underwent barium gastrointestinal
examination. One case exhibited the disappearance
of mucosal folds in the intestinal wall and dilatation of
the intestinal lumen, which was suggestive of lymphoma.
One case exhibited disappearance of mucosal folds in the
intestinal wall, and multiple fine niches were seen, which
led to a misdiagnosis of limited enteritis. Two cases had
results suggestive of external pressure changes, and seven
patients had no obvious abnormalities on the barium gastrointestinal
examination.
Of the 15 patients, 5 cases had tumors in multiple sites, 3
had tumors in the ileocecal region, 1 had tumors involving
both the stomach and duodenum, 1 had tumors involving
both the duodenum and jejunum, 7 had tumors involving
the ileum, 2 had tumors involving the jejunum, and 1
had tumors involving the duodenum. Ileal lymphoma was
defined as a lesion involving the terminal ileum and the
ileocecal valve, cecum, or appendix.
All patients received surgical treatment, including 9
cases who underwent radical resection, 3 cases who underwent
tumor reduction surgery due to the discovery of
extensive metastases in the abdominal cavity, and 3 cases
who underwent short-circuiting and biopsy due to severe
abdominal adhesions or tumor with invasion of the large
retroperitoneal vessels. After surgery, 11 cases received 4
to 8 cycles of adjuvant chemotherapy with the CHOP (cyclophosphamide,
epirubicin, vincristine, and prednisone)
regimen.
The 15 PSIL patients were confirmed by pathological examination
to have non-Hodgkin lymphoma (NHL). Eight
(53.3%) patients were classified as having diffuse large Bcell
lymphoma (DLBCL), 5 (33.3%) as having mucosaassociated
lymphoid tissue B-cell lymphoma (33.3%), and
2 (13.4%) as having enteropathy-associated T-cell lymphoma.
We followed up with 14 patients for a mean duration of
30 months (range 6-52 months). One case of stress ulcer
occurred after the operation, and there were no perioperative
deaths. However, 6 patients died due to tumor metastasis
or recurrence. The 1- and 3-year survival rates were
85.7% and 57.1%, respectively.
Primary small intestinal lymphoma (PSIL) is a rare malignant
tumor of the gastrointestinal tract, which accounts for
19% to 38% of malignant tumors of the small intestine,
and 20% to 30% of all primary gastrointestinal lymphomas
[2, 3] . Small
intestinal
lymphomas can be classified
as primary or secondary. The former occurs in the submucosal
lymphatic tissue of the small intestine, which grows
as solitary nodules and does not infiltrate the surrounding
tissues for a long time; the prognosis of primary small
intestinal lymphoma is good. The secondary type refers to
small bowel disease as a component of systemic lymphoma,
and autopsies have revealed that 50% of lymphoma
patients had small bowel invasion [4]. The causes of PSIL
are not exactly known, but research has reported that it is
related to environmental factors, viral infections, genetics,
immunodeficiency, some intestinal diseases, and drugs [5,
6]. PSIL can occur in any part of the small intestine, but
the lymphatic-rich distal ileum has the highest incidence.
PSIL often manifests as intermittent abdominal pain, abdominal
masses, unexplained gastrointestinal bleeding
and obstruction, and decline of body mass, but has no specific
clinical manifestations [7]. Therefore, patients with
the above clinical manifestations should undergo a small
bowel examination. The author contributed solely to
the article. All authors declared that there are no conflicts of interest. This study was approved by the Ethics Review Committee at Guangzhou
First People’s Hospital, and we obtained the clinical
information of the patients after obtaining their informed
consent. 1. Dawson I M, Cornes J S, Morson B C. Primary malignant
lymphoid tumours of the intestinal tract. Report of 37
cases with a study of factors influencing prognosis. British
Journal of Surgery, 1961, 49: 80-89. 2. Tamura H, Ogata K, Kondo A, et al. Double balloon endoscopy
as a useful tool for the diagnosis and treatment of
four cases of primary small intestinal lymphoma. Rinsho
Ketsueki, 2007, 48(6): 510-513. 3. Foukas P G, de Leval L. Recent advances in intestinal lymphomas.
Histopathology,
2015, 66(1): 112-136. 4. Nakamura S, Matsumoto T, Iida M, et al. Primary gastrointestinal
lymphoma in Japan: a clinicopathologic
analysis of 455 patients with special reference to its time
trends. Cancer, 2003, 97(10), 2462-2473. 5. Grande B M, Gerhard D S, Jiang A, et al. Genome-wide
discovery of somatic coding and noncoding mutations
in pediatric endemic and sporadic Burkitt lymphoma.
Blood, 2019, 133(12): 1313-1324. 6. Terai S, Iijima K, Kato K, et al. Long-term outcomes of
gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. The
Tohoku
Journal of Experimental Medicine, 2008, 214(1):
79-87. 7. Feng L, Zhang G, Hu Z, et al. Diagnosis and treatment of
81 patients with primary gastrointestinal lymphoma.
Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2009, 34(7): 582-
588. 8. Kobayashi H, Nagai T, Omine K, et al. Clinical outcome
of non-surgical treatment for primary small intestinal
lymphoma diagnosed with double-balloon endoscopy.
Leukemia & Lymphoma, 2013, 54(4): 731-736. 9. Yang C B, Yu N, Jian Y J, et al. Spectral CT Imaging in the
Differential Diagnosis of Small Bowel Adenocarcinoma
From Primary Small Intestinal Lymphoma.Academic Radiology,
2019, 26(7): 878-884. 10. Beaton C, Davies M, Beynon J. The management of primary
small bowel and colon lymphoma--a review. International
Journal of Colorectal Disease, 2012, 27(5): 555-
563. 11. Gonzalez Q H, Heslin M J, Dávila-Cervantes A, et al. Primary
colonic lymphoma.American Surgeon, 2008, 74(3):
214-216.
Methods: The clinical data of 15 patients with PSIL treated from January 2015 to July 2019
at Guangzhou First
People’s Hospital were investigated retrospectively. Among the 15 patients, 9 were male, and 6 were female,
with ages ranging from 18 to 73 years, with a median age of 51.6 years. Data relating to gender, age, clinical
manifestation, laboratory examination, imaging, diagnosis, and treatment of the patients were
reviewed.
Results: The most common clinical manifestations were abdominal pain, abdominal lump,
bowel obstruction,
gastrointestinal hemorrhage, and athrepsy. Serum tumor markers were checked and found to be normal. In
all 15 cases, tumors were found by spiral computed tomography (CT), and 12 cases were diagnosed as PSIL.
Eleven cases were given barium meal examinations, and positive results were found in 4 cases, with only 1
case considered to be PSIL. All 15 patients underwent surgery. All patients were diagnosed as having non-
Hodgkin lymphoma by postoperative pathology (8 patients with diffuse large B-cell lymphoma, 5 with mucosa
associated lymphoid tissue type B-cell lymphoma and 2 with enteropathy-type intestinal T-cell lymphoma).
There were no cases of perioperative deaths. Ten patients received adjuvant chemotherapy with the CHOP
(cyclophosphamide, epirubicin, vincristine, and prednisone) regimen after the operation. Fourteen cases were
followed up for a mean duration of 30 months (range of 6-52 months). The 1- and 3-year survival rates were
85.7% and 57.1%, respectively.
Conclusion: PSIL has no specific clinical manifestations. The
diagnostic rate with barium study is low,
whereas
spiral CT is a promising diagnostic method for PSIL. Surgery combined with chemotherapy is important for
the treatment of PSIL.
Keywords
Introduction
Materials and methods
General clinical information
(1) There was no pathological superficial lymph node enlargement
when the patient was first seen.
(2) Chest X-rays did not show any mediastinal lymph
node enlargement.
(3) There were no naive or abnormal cells in the peripheral
blood.
(4) The tumor was mainly located in the small intestine or
invaded nearby lymph nodes via lymphatic vessels.
(5) There was no invasion of the liver or spleen
(except for the direct spread of adjacent lesions).
Spiral CT examination
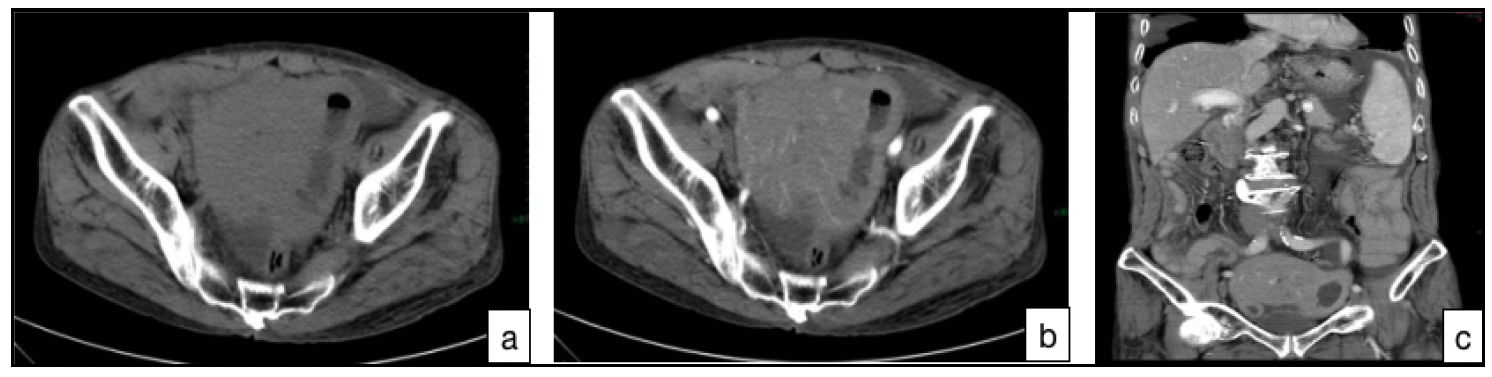 Figure 1. (CT images (a-c) of a 74-year-old man who was considered
as possibly
having small intestinal lymphoma because his small intestine
wall was evidently unevenly thickened and soft tissue masses had formed. a: CT non-contrast
enhanced
scan. b: CT contrast enhanced scan. c:
coronal reconstruction.).
Figure 1. (CT images (a-c) of a 74-year-old man who was considered
as possibly
having small intestinal lymphoma because his small intestine
wall was evidently unevenly thickened and soft tissue masses had formed. a: CT non-contrast
enhanced
scan. b: CT contrast enhanced scan. c:
coronal reconstruction.).
Barium meal examination of the digestive tract
Tumor site
Surgical modality and adjuvant treatment
Results
Histological type
Follow-up and prognosis
Discussion
The clinical manifestations of PSIL are unspecific, and
it is hard to diagnose by endoscopic and barium meal
examination. In the past, PSIL was diagnosed by postoperative
pathological examination [8]. With the popularity
of multi-slice computed tomography (MSCT) and the
development of 3D reconstruction technology, these have
become the most important and valuable examination
methods for the current-day diagnosis of small bowel tumors
[9].
PSIL originates from the lamina propria of the small
intestine
mucosa or lymphatic tissue in the submucosa. It
often grows in the lamina propria or submucosa along the
long axis of the organ, and then invades into and out of
the cavity, and the lesions can be widespread or multiple
in the early stage. Barium meal examination can only
show lesions in the intestinal cavity and indirect signs
of extracavitary lesions, and it is often not easy to detect
smaller mucosal lesions. When PSIL is accompanied by
ulceration, it is often difficult to distinguish it from adenocarcinoma.
Therefore, barium meal examination, as
well as enteroscopy, lacks specificity for its diagnosis.
When small intestinal lymphoma is not accompanied by
ulceration, it is difficult to make a correct diagnosis, as
endoscopic lesions are not clear, and they often show generalized
inflammation and erosion. The diagnostic rate of
pathological biopsy is low, as the biopsy often does not
extend deep into the mucosa, and it is also greatly limited
in its ability to diagnose tumor infiltration of extracavity
tissue.
The performance of spiral CT in diagnosing PSIL is mainly
characterized by its ability to identify different forms of intestinal wall thickening, and the
following
characteristics
can provide an important basis for diagnosis:
(1) The intestinal wall is thickened, and the
intestinal
cavity
is dilated. Normal small intestinal wall thickness is <3 mm, and the normal intestinal cavity width
is
<30 mm.
This standard can be used as a reference for intestinal wall thickening and expansion, which can
manifest as
symmetrical or eccentric thickening. The thickening of the intestinal wall is mainly due to thickening
of
the submucosa and muscle layers thickening. Most of the intestinal tube is above 3/4 weeks of diameter.
(2)
Analysis of the images of the same level of lesions in different phases shows that most of the lesions
have
variable intestinal morphology and still maintain a certain degree of expansion and flexibility. This
may be
related to the absence of factors that induce fibroblast responses in lymphoma.
(3) The lesions
are less
invasive.
(4) Analysis of the enhancement CT values of each stage reveals that the difference
before and
after
enhancement is 20–35 HU, suggesting that PSIL is a mildly or moderately enhanced tumor. PSIL needs to be
differentiated from small-bowel adenocarcinoma and small-bowel Crohn’s disease when the main
manifestation
is thickening of the bowel wall. MSCT and its post-processed images can not only show intra-intestinal
lesions, but also submucosal and extraintestinal lesions. It has unique advantages in the diagnosis of
PSIL
and is a better diagnostic method.
Malignant lymphoma is a tumor that is sensitive to radiotherapy
and chemotherapy. The current consensus is
that malignant lymphoma should be treated surgically
followed by the use of adjuvant treatment [10, 11]. The
surgical resection and the scope of lesion cleanup should
be based on the tumor location, tumor size, and the range
of its invasiveness. It is easier to separate and remove
the tumor from the surrounding tissues during surgery, as
intestinal lymphoma normally grows non-invasively. We
advocate active radical surgery for intestinal malignant
lymphoma if it cannot be cured to prevent complications
such as perforation and bleeding during chemotherapy;
palliative surgery should be considered. Early diagnosis is
vital for improving the prognosis of PSIL. Hence, to avoid
delays in the timing of surgery, an exploratory laparotomy
should be performed decisively for patients who have surgical
indications.
Declarations
Authors’ contributions
Conflicts of interest
Ethical approval and consent to participate
References