Open Access | Research Article
This work is licensed under a Creative
Commons Attribution-ShareAlike 4.0 International License.
Di-2-pyridylketone 4, 4-dimethyl-3-thiosemicarbazone effectively induces human colorectal carcinoma cell apoptosis via mTOR pathway
# These authors contributed equally to this work and should be considered co-first
authors.
* Corresponding to: Yong Zhang
Mailing address: Department of Orthopaedics,
Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030,
China.
Email: zhangyong2018@hust.edu.cn
Received: 02 August 2021 / Accepted: 03 September 2021
DOI: 10.31491/APT.2021.09.063
Abstract
Background: To investigate the anticancer mechanisms of di-2-pyridylketone
4,4-dimethyl-3-thiosemicarbazone
(Dp44mT) in human colon cancer cells. Human colorectal carcinoma (HCC) is one of the most commonly
diagnosed cancers in both males and females. Current studies have found that iron chelators can be used as
novel anticancer drugs; however, the anticancer activity of iron chelators and their target genes in HCC has
been rarely reported.
Dp44mT, cell apoptosis, mTOR, DNA damage
Colorectal cancer is the fourth most common cause of cancer-related deaths worldwide, and annual deaths have
increased to approximately 700,000 [1]. Surgical resection
and chemotherapy are the primary treatments. However,
the risk of resection surgery and the side effects of chemotherapy,
including hair loss and neuropathy, has urged
researchers to develop a new target for colorectal cancer.
A major limitation of cytotoxic chemotherapy is drug resistance
caused by P-glycoprotein (Pgp) [2], which greatly
restricts the effects of chemotherapy.
Dp44mT was synthesized and characterized using standard
procedures [14]. Dp44mT was dissolved in DMSO
as storage concentration of 10 mM. Stock solutions of 100
mg/mL FAC (Sigma-Aldrich, St Louis, MO, USA) were
prepared in deionized water. Both solutions were stored at
˗20 °C and diluted to the proper concentrations before use.
In this experiment, the highest level of DMSO in media
was 0.05% (v/v).
The human colon carcinoma cell lines HT29 and SW480
were obtained from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in 10% FBS supplemented
RPMI medium with L-glutamine and maintained
at 37 °C with 5% CO2.
Apoptosis of HT29 and SW480 cells was detected by flow cytometry with annexin V-FITC/PI double staining.
Approximately 2 x 106 HT29 and SW480 cells were collected,
washed twice with cold PBS, and added with binding
buffer with annexin V-FITC/PI (10 mM Hepes/NaOH
at pH 7.4, 0.14 M NaCl, 2.5 mM CaCl2). Finally, the
cells were and incubated at room temperature and under
dark conditions for 15 min. Flow cytometry was used to
detect cell apoptosis. Annexin V-FITC/PI double staining
showed double positive in late apoptotic or necrotic cells [15].
HT29 and SW480 cells were collected fixed with 70%
ethanol for 30 s, and washed with PBS three times. Approximately
2 μg/mL Hoechst 33258 dye solution was
then added , and the cells were incubated at room temperature
for 30 minutes. Finally, the cells were observed
under a fluorescence microscope.
The cells were collected and then washed with cold PBS.
The cells were lysed on ice with lysate with protease inhibitor
and phosphatase inhibitor, and the protein supernatant
was collected after centrifugation at 16,000 × g for
40 min at 4 °C. The protein concentrations were measured
using standard Bradford assays, and an equal amount of
the protein was collected and then transferred to a nitrocellulose
membrane, which was then closed with 5% milk
and incubated with primary antibodies against p-Histones
H2A.X, mTOR, p-mTOR, and GAPDH. The membrane
was washed after incubation with the primary antibodies.
The membrane was then incubated with horseradish
peroxidase-bound secondary antibody. Finally, the protein
was exposed using a ChemiDoc MP imager. All antibodies
were obtained from Santa Cruz Biotech (Santa Cruz,
CA, USA). The item numbers of the antibodies against p-
Histones H2A.X, mTOR, p-mTOR, and GAPDH were
SC-517348, SC-517348, SC-293133, and SC-47724, respectively.
Finally, density analysis was performed using
the ChemiDoc Image Lab software (BioRad).
Data are expressed as mean ± standard deviation (SD) of
the three experiments. Experimental data are compared
using one-way ANOVA. Results are considered statistically
significant when P < 0.05.
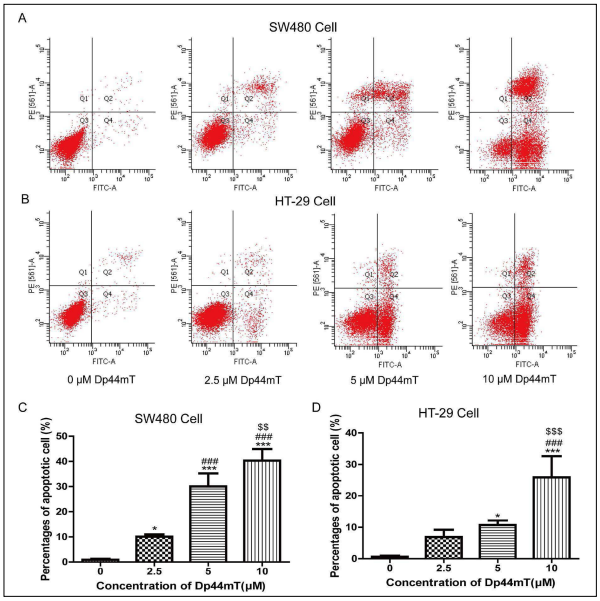
Annexin V-FITC/PI double staining and Hoechst 33258
staining were used to evaluate the effect of Dp44mT on
the apoptosis of SW480 and HT-29 cells. The results of
flow cytometry showed that Dp44mT could promoted
apoptosis more strongly than that in the 0 μM-treated
group (Figures 1A and 1B). The apoptotic percentages in
the different concentrations of Dp44mT-treated groups
were significantly higher than those in the 0 μM-treated
group (P < 0.05, Figures 1C and 1D). The effect of Dp44mT on the
apoptosis of SW480 and HT-29 cells is dose
dependent. Treatment with 10 μM Dp44mT showed the strongest effect on the apoptosis of SW480 and HT-
29 cells. Therefore, 10 μM Dp44mT was selected for the
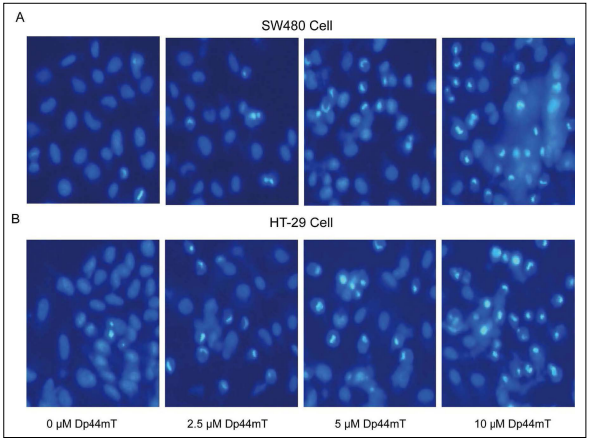
subsequent experiments. After staining with Hoechst 33258, the SW480 and HT-29
cells in the 0 μM Dp44mT group had normal morphology
and complete round nuclei (Figure 2), whereas cells
treated with Dp44mT became rare, with small nuclei,
widespread bubbles, intense fluorescent spots, and pyrosis.
These findings indicated chromatin concentration and
the presence of apoptotic bodies. Consistent with these
results of flow cytometry, Dp44mT induced SW480 and
HT-29 cell apoptosis in a dose-dependent way.
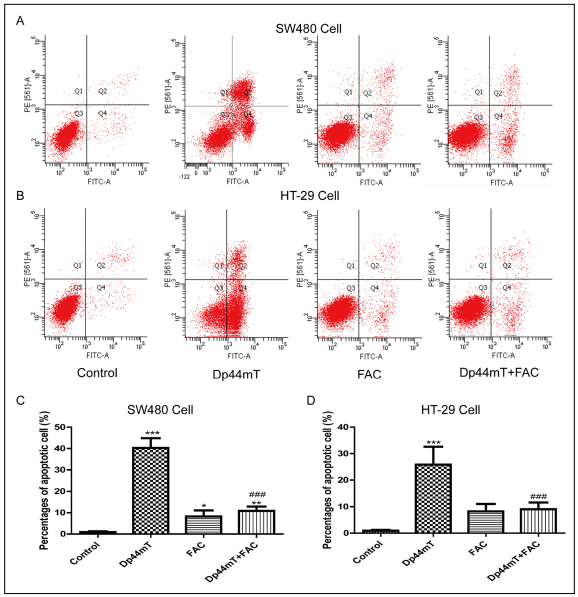
The salvage effect of FAC on DP44MT-induced apoptosis
of SW480 and HT-29 cells was detected by flow cytometry.
In this experiment, 10 μM Dp44mT was chosen to
induce cell apoptosis, and the percentages of apoptotic
cells in the untreated control group, Dp44mT group, FAC
group (100 mg/ml), and Dp44mT + FAC group were compared
(Figure 3). The results showed that co-treatment
with FAC could significantly inhibit the cell apoptosis induced
by Dp44mT. Similar results were observed in both
SW480 and HT-29 cells.
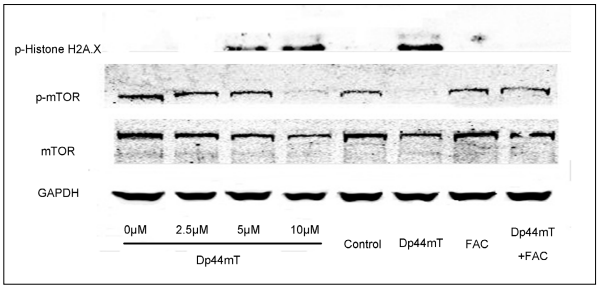
The effects of different concentrations of Dp44mT and FAC on the protein expressions of p-Histone H2A.X, mTOR,
and p-mTOR in SW480 cells were investigated.
As shown in Figure 4, Dp44mT induced the protein expressions
of p-Histone H2A.X and inhibited the phosphorylation
of mTOR in a dose-dependent way. Cells
treated with Dp44mT and FAC showed lower expression
of p-Histone H2A.X and higher expression of mTOR
and p-mTOR compared with that in the Dp44mT-treated
cells. These results indicated that FAC inhibited Dp44mTinduced
p-Histone H2A.X expression and recovered the
phosphorylation of mTOR in SW480 cells.
Increased drug resistance to standard treatment among
cancers has lead to the investigation of new therapeutic
strategies. As a result, the implication of the Dp44mT and
its analogues was emerged as new anticancer therapeutics,
as suggested by their broad anti-tumoractivities [6, 16, 17]
their effects on drug resistance [18] and tumor metastasis
[19], and their oral bioavailability and tolerability. Di-
2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazon
is a kind of Dp44mT analogues that has been used in
entered a multi-center clinical trial to treat advanced and
drug-resistant tumors in early 2016 [11]. Qianqian Fu, Shengli Wang and
Chuyun Zhu performed the experiments. Shengli Wang
and Zhenlong Zhou analyzed the data. Qianqian Fu and
Shengli Wang wrote the paper. Yong Zhang and Xiaojuan
Yu were responsible for study supervision. All authors approved
the final manuscript. All authors declared that there are no conflicts of interest. 1. Brody H. Colorectal cancer. Nature, 2015, 521(7551): S1. 2. Gottesman MM, Fojo T, Bates SE, et al. Multidrug resistance
in cancer: role of ATP-dependent transporters. Nat
Rev Cancer, 2002, 2(1): 48-58. 3. Chaston TB, Lovejoy DB, Watts RN, et al. Examination of
the antiproliferative activity of iron chelators: multiple
cellular targets and the different mechanism of action of
triapine compared with desferrioxamine and the potent
pyridoxal isonicotinoyl hydrazone analogue 311. Clinical
cancer research, 2003, 9(1): 402-414. 4. Le NTV, Richardson DR. The role of iron in cell cycle
progression and the proliferation of neoplastic cells.
Biochimica Et Biophysica Acta-reviews On Cancer, 2002,
1603(1): 31-46. 5. Lovejoy DB, Jansson PJ, Brunk UT, et al. Antitumor Activity
of Metal-Chelating Compound Dp44mT Is Mediated
by Formation of a Redox-Active Copper Complex That
Accumulates in Lysosomes. Cancer research, 2011,
71(17): 5871-5880. 6. Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridylderived
iron chelators with marked and selective antitumor
activity: in vitro and in vivo assessment. Blood,
2004, 104(5): 1450-1458. 7. Whitnall M, Howard J, Ponka P, et al. A class of iron chelators
with a wide spectrum of potent antitumor activity
that overcomes resistance to chemotherapeutics.
Proceedings of the National Academy of Sciences of the
United States of America, 2006, 103(40): 14901-1496. 8. Kovacevic Z, Chikhani S, Lovejoy DB, et al. Novel thiosemicarbazone
iron chelators induce up-regulation and
phosphorylation of the metastasis suppressor N-myc
down-stream regulated gene 1: a new strategy for the
treatment of pancreatic cancer. Molecular pharmacology, 2011, 80(4): 598-609. 9. Jansson PJ, Yamagishi T, Arvind A, et al. Di-2-pyridylketone
4,4-dimethyl-3-thiosemicarbazone (Dp44mT)
overcomes multidrug resistance by a novel mechanism
involving the hijacking of lysosomal P-glycoprotein (Pgp).
Journal Of Biological Chemistry, 2015, 290(15): 9588-
9603. 10. Seebacher NA, Lane DJ, Jansson PJ, et al. Glucose Modulation
Induces Lysosome Formation and Increases Lysosomotropic
Drug Sequestration via the P-Glycoprotein
Drug Transporter. Journal Of Biological Chemistry, 2016,
291(8): 3796-3820. 11. Kalinowski DS, Stefani C, Toyokuni S, et al. Redox cycling
metals: Pedaling their roles in metabolism and their use
in the development of novel therapeutics. Biochimica Et
Biophysica Acta-molecular Cell Research, 2016, 1863(4):
727-748. 12. Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl–
derived iron chelators with marked and selective antitumor
activity: in vitro and in vivo assessment. Blood,
2004, 104(5): 1450-1458. 13. Merlot AM, Shafie NH, Yu Y, et al. Mechanism of the induction
of endoplasmic reticulum stress by the anti-cancer
agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone
(Dp44mT): Activation of PERK/eIF2α, IRE1α,
ATF6 and calmodulin kinase. Biochemical Pharmacology,
2016, 109: 27-47. 14. Richardson DR, Sharpe PC, Lovejoy DB, et al. Dipyridyl
thiosemicarbazone chelators with potent and selective
antitumor activity form iron complexes with redox activity.
Journal of medicinal chemistry, 2006, 49(22): 6510-
6521. 15. Park JJ, Lim KH, Baek KH. Annexin-1 regulated by HAUSP
is essential for UV-induced damage response. Cell death
& disease, 2015, 6(2): e1654-e1654. 16. Guo ZL, Richardson DR, Kalinowski DS, et al. The novel
thiosemicarbazone, di-2-pyridylketone 4-cyclohexyl-
4-methyl-3-thiosemicarbazone (DpC), inhibits neuroblastoma
growth in vitro and in vivo via multiple mechanisms.
Journal of hematology & oncology, 2016, 9(1):
1-16. 17. Gutierrez EM, Seebacher NA, Arzuman L, et al. Lysosomal
membrane stability plays a major role in the cytotoxic
activity of the anti-proliferative agent, di-2-pyridylketone
4,4-dimethyl-3-thiosemicarbazone (Dp44mT). Biochimica
Et Biophysica Acta, 2016, 1863(7): 1665-1681. 18. Seebacher NA, Richardson DR, Jansson PJ. A mechanism
for overcoming P-glycoprotein-mediated drug resistance:
novel combination therapy that releases stored doxorubicin from lysosomes via lysosomal permeabilization
using Dp44mT or DpC. Cell death & disease, 2016,
7(12): e2510-e2510. 19. Wangpu X, Lu J, Xi R, et al. Targeting the Metastasis Suppressor,
N-Myc Downstream Regulated Gene-1, with
Novel Di-2-Pyridylketone Thiosemicarbazones: Suppression
of Tumor Cell Migration and Cell-Collagen Adhesion
by Inhibiting Focal Adhesion Kinase/Paxillin Signaling.
Molecular pharmacology, 2016, 89(5): 521-540. 20. Greene BT, Thorburn J, Willingham MC, et al. Activation
of caspase pathways during iron chelator-mediated
apoptosis. Journal Of Biological Chemistry, 2002,
277(28): 25568-25575.
Methods: Dp44mT was used to treat two colorectal tumor cell lines, SW480 and HT-29. The
proapoptotic effects
of different concentrations of Dp44mt were measured using flow cytometry and Hoechst 33258 staining.
Ferric ammonium citrate (FAC) was used as an additional iron donor to inhibit the effects of Dp44mT. Apoptosis
and DNA damage-related proteins were examined by Western blot analysis.
Results: In this study, we found that the iron chelators Dp44mT could induce the apoptosis in two
colorectal
tumor cell lines SW480 and HT-29, upregulate the expression level of p-histone H2A.X, and inhibit the
phosphorylation
level of mTOR in a dose-dependent way. Those effects could be reversed by the additional iron donor
FAC.
Conclusion: These data indicate that iron depletion and/or the presence of iron can modulate the HCC
apoptosis progression in vitro, which may be a potential target for future HCC therapy.
Keywords
Introduction
Iron is an indispensable trace element for cell metabolism.
It plays a crucial role in deoxyribonucleic acid (DNA)
synthesis, oxygen transport and electron transport, and
energy metabolism pathways related to adenosine triphosphate
(ATP) production. Iron-chelating agents exhibit
highly efficient antitumor activities [3]. Iron-chelating agents have a
stronger inhibitory effect on tumor cell
proliferation activity [3]. Iron is necessary for tumor cells
because it plays a key role in the protein activity sites,
and tumor cells highly expressing TRF13, iron-containing
enzymes, and ribonucleic acid reductase (RR), which are
involved in DNA synthesis [4]. As a potential anticancer
agent, iron chelators can be combined with other anticancer
drugs to enhance their anticancer activity.
As a novel class of anti-tumor agents, the iron chelator,
di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone
(Dp44mT) has recently been extensively studied [5]. The
antitumor mechanism of Dp44mT is related to its ability
to bind, to deplete cellular iron, and to generatecytotoxic
radicals [6]. In addition, Dp44mT demonstrates potent and
selective anti-tumor activity [6-8], and can overcome the
Pgp-related multidrug resistance by directly utilizing lysosomal
Pgp-transport activity [9, 10]. This drug can significantly
inhibit tumor growth and metastasis and can overcome
resistance to currently used chemotherapy drugs;
thus, it can be usedin clinical treatment [11]. Dp44mT induces
cancer cell apoptosis by increasing the expressionof
the apoptotic proteins Caspase-3 8 and 9, Bax protein and
cleaved PARP in different cancer cells [12, 13] .
Notably, Dp44mT leads to the induction of various of
apoptotic markers, such as cleaved caspase 3, caspase 4,
cleaved PARP and so on, in different cancer cell types
[12, 13]. However, the
antitumor effects of Dp44mT on
colorectal tumor cells have not yet been examined. In
this research, we investigated the proapoptotic effects of
Dp44mT on different kinds of colorectal tumor cell lines.
Dp44mT induces colorectal tumor cell apoptosis in a
dose-dependent manner, and the antagonist of Dp44mT,
ferric ammonium citrate (FAC), could reverse those effects.
These results indicated that cellular iron might
be a possible target for colorectal tumor treatment, and
Dp44mT might be a potential therapy.
Materials and methods
Reagents
Cell Culture
Flow cytometry
Hoechst 33258 staining
Protein extraction and western blotting analysis
Statistical analysis
Results
Dp44mT induces SW480 and HT-29 cells apoptosis
FAC inhibited Dp44mT-induced SW480 and HT-29 cell apoptosis
Dp44mT induced SW480 and HT-29 cells apoptosis via the mTOR pathway
Discussion
In the present study,
the effects of different concentrations of
Dp44mT on colorectal cancer cell apoptosis were explored.
The results indicated that Dp44mT could induce
cell apoptosis in a dose-dependent way. FAC, which was
usually used as the agonist of Dp44mT, could significantly
inhibited Dp44mT-induced cell apoptosis. The exploration
of mechanisms indicated that the proapoptotic effects
of Dp44mT on colorectal cancer cells were related to the
promoted expression of p-Histone H2A.X and the inhibition
of mTOR and p-mTOR. The rescued effects of FAC
partially contributed to its inhibition on Dp44mT-induced
p-Histone H2A.X expression and recovery of phosphorylation
of mTOR.
The iron-chelating agent can be used as an anticancer
agent to induce the apoptosis of cancer cells mainly by
activating mitochondrial apoptosis induced by the caspase
pathway; therefore, the proliferation of cancer cells can be
inhibited by regulating apoptosis [20]. Dp44mT is a very
effective antitumor-chelating agent, and studying its effect
on apoptosis is of great significance.
The ability of a chelator to bind cellular iron leads to
apoptosis [20]. If iron chelators lead to tumor cell death
by influencing the apoptotic pathway, then regulating
apoptosis becomes important in inhibiting cancer cell
proliferation. Given that Dp44mT was the most effective
chelator yet screened for antitumor activity, it was crucial
to assess its ability to induce apoptosis.
In this study, we found that Dp44mT could promote the
expression of p-Histone H2A.X, indicating double-strand
DNA damage. The PI3K/AKT/mTOR pathway plays a
critical role in the growth and progression of colorectal
cancer. As the downstream protein of PI3K/Akt pathway,
mTOR plays an important role in cell proliferation and
apoptosis. In our study, we investigated the influence
of Dp44mT on the phosphorylation level of mTOR and
found that Dp44mT could significantily inhibit its activation.
This founding may partialy explain the antitumor effects
of Dp44mT and related iron chelators.
In conclusion, we explored the anti-tumor effects of the
iron chelator Dp44mT on colorectal tumor cells using
various assays. We found that Dp44mT could promote
tumor cell apoptosis upregulate the expression level of p-Histone H2A.X, and inhibit the phosphorylation level
of mTOR in a dose-dependent way. These data indicate that
iron depletion could modulate the human colorectal carcinoma
(HCC) apoptosis progression in vitro, which may be
a potential target for future HCC therapy.
Declarations
Authors’ contributions
Conflicts of interest
References