Open Access | Case Report
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
A fornix infarction causing post-operative anterograde amnesia
* Corresponding author: Shyh Poh Teo
Mailing address: Raja Isteri Pengiran Anak Saleha (RIPAS)
Hospital, Jalan Putera Al-Muhtadee Billah, Bandar Seri Begawan BA1710, Brunei Darussalam.
E-mail: shyhpoh.teo@moh.gov.bn
Received: 13 May 2021 / Accepted: 29 May 2021
DOI: 10.31491/APT.2021.06.055
Abstract
A 65-year-old woman underwent elective hip replacement surgery and postoperatively developed significant anterograde amnesia, without other significant neurological deficits. Magnetic resonance imaging (MRI) revealed an area of infarction in the anterior fornix and genu of the corpus callosum. Neuropsychological testing revealed extremely poor learning performance and immediate memory, as well as delayed memory, consistent with anterograde amnesia. This case illustrates the relationship between the anterior fornix and memory function and the role of hypoperfusion brain injury in ischemic strokes.
Keywords
Anterograde amnesia, brain fornix, ischaemic stroke, postoperative period
Introduction
A stroke following total joint arthroplasty is rare, occurring in around 0.2% of patients [1]. The first 2 weeks
after arthroplasty are high risk in terms of developing
an ischemic stroke, with almost a five times greater risk
compared to that in an age-adjusted population. The risk
remains high for about 6 weeks, with the greatest risk occurring in the immediate postoperative period [2]. Cerebral hypoperfusion, marrow embolization, and anesthetic
effects on the cardiovascular system have been cited as
possible mechanisms underlying this ischemic risk [1,2].
Ischemic strokes affecting the anterior fornix are also an
infrequent occurrence, with just a few case reports published [3-8]. All these case reports demonstrated a link
between acute amnesia and fornix infarcts. The role of
the anterior fornix in memory function is related to its
anatomical placement within the Papez circuit, which
comprises the fornix, hippocampus, mammillary bodies,
anterior thalamus, and cingulate gyrus [9,10]. The anterior fornix acts as the output tract from the hippocampus,
primarily to the mammillary bodies. The primary function of the Papez circuit, which was first described in 1937, involves encoding and recall of new, episodic information.
Memory function is more reliant on the structural integrity
of the circuit as a whole rather than the integrity of each
of the individual components [9,10].
In this paper, a case of a patient who developed a post
-
operative fornix infarct and anterograde amnesia is
described, followed by a discussion of the relationship
between the anterior fornix and memory function and the
role of hypoperfusion brain injury in ischemic strokes.
Case Report
Ms. O was an independent 65-year-old admitted for an
elective right total hip replacement for osteoarthritis. She
had a background of hypertension and hyperlipidemia and
was receiving alpha-blocker, beta-blocker, and statin therapies. There were no other risk factors for strokes. During
the intraoperative and immediate postoperative period,
she became hypotensive with lowest intraoperative blood
pressure being 80/40 despite cessation of antihypertensive
drugs, and required fluid resuscitation. During the first
24 hours post-surgery, she appeared confused and disoriented, without focal neurological deficits. She repeatedly
asked where she was and why she was in hospital, even
after she was reorientated by nursing staff. A computed
tomography (CT) brain (Figure 1A) 48 hours after surgery
showed hypodensity in the medial aspect of the frontal
lobe, appearing to involve the anterior corpus callosum.
Brain magnetic resonance imaging (MRI) (Figure 1B)
revealed an area of increased signal in fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) sequences in the beginning of the corpus callosum, suggesting acute diffusion impairment in the genu of
the corpus callosum, extending into the fornix and septum
pellucidum. A diagnosis of an acute fornix infarction was
made.
A neuropsychological assessment, including the Repeatable Battery of the Assessment of Neuropsychological
Status, revealed extremely low performance in two areas:
learning and immediate memory and delayed memory.
These results were incongruent with the remainder of the
patient’s test scores of average or higher for premorbid
ability, attention, processing speed, visuospatial and constructional ability, fluency, and insight. The patient was
later transferred to an inpatient brain injury rehabilitation
service and required 3 months of multidisciplinary brain
rehabilitation before discharge to the community.
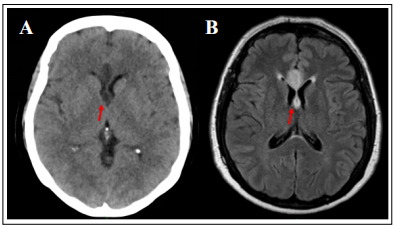
Figure 1. Brain imaging showing the fornix infarct (A: CT; B: MRI FLAIR).
Discussion
The case of a patient who developed postoperative anterograde amnesia due to a fornix infarct was described. This
case reveals the relationship between the anterior fornix
and memory function, as well as the role of hypoperfusion
brain injury in ischemic strokes. Acute fornix infarcts are
rare, and previous case reports describe acute amnesia as
the main presenting symptom [3-8]. Two of these cases
were due to an isolated fornix infarction [3,4], while the
other cases [5-8], including the one described herein, were
associated with ischemia of nearby structures, including
the corpus callosum and cingulate gyrus. Interestingly, the
case of the isolated left anterior fornix injury [4] was not
clinically different to the case with the isolated bilateral
fornix infarction [6], suggesting that isolated fornix lesions can cause amnesia and a lack of laterality to fornix
function. This may be due to the dual role of the fornix as
a tract from the thalamus to the basal forebrain and as a
commissure between the left and right hemispheres [9].
Multiple pathologies can damage the anterior fornix, either on their own or as part of a diffuse process. These include congenital absences, neoplastic diseases, infections,
autoimmune diseases, metabolic diseases, infarctions,
and traumas. Isolated fornix infarcts almost exclusively occur in the anterior columns. This is likely due to the
proximity of the remaining fornix to the choroidal arterial
supply aiding collateral perfusion and protecting against
infarctions [9]. Blood to the anterior fornix is supplied by
branches of the anterior communicating artery (ACoA) [9],
which accounts for the high rate of postoperative amnesia
in ACoA aneurysm surgery [11,12]. Other variations to
the vascular supply that can cause anterior fornix infarctions include occlusion of the subcallosal artery [8] or the
anterior cerebral artery via short medial central arteries [3].
Approximately 20–50% of poststroke patients have subjective complaints of memory difficulties, which may
be accounted for by nonanatomical etiologies, such as
depression, fatigue, medication side-effects, or sleep disorders. However, lesions within memory-associated structures or amnesic lesions and lesions impairing memory
processing (process lesions) should be considered [10].
Pathological processes affecting hippocampal and diencephalic connections are consistently more likely to
impair memory function than fornix lesions [10,13]. Interestingly, in a case study on 142 epileptic patients who
underwent bilateral anterior fornix transection, no patients
experienced long-term memory impairment [14].
In the present case, the fornix infarct occurred during the
perioperative period. Hypoperfusion of the fornix during
the period of hypotension contributed to the infarct in this
case. The combination of systemic hypotension and preexisting extracranial arterial occlusive disease caused a
reduced blood supply, which led to a hemodynamic stroke
[15]. In this patient, carotid Doppler ultrasound revealed
50–69% stenosis in the right internal carotids. Although
this level of stenosis is considered only mild-to-moderate,
the concurrent hypotension likely contributed to the hypoperfusion and the hemodynamic stroke. Hypotension is a
known cause of ischemic strokes, with a high risk among
those with symptomatic orthostatic hypotension and cardiac failure [15]. Intraoperative blood pressure has been
shown to be directly related to the neurological outcome
following cardiac surgery, with almost 50% of patients
undergoing cardiac surgery having MRI evidence of bilateral ischemic lesions in “border zone” areas between the
main brain vascular supplies, which are vulnerable areas
to ischemic injury. [16] The risk of watershed infarcts was
higher in patients with an intraoperative fall in systolic
blood pressure of 10 mmHg or more [16]. Another study
found radiological evidence of strokes in 7.2% of patients with intraoperative mean arterial pressure of 50–60
mmHg as compared to 2.4% when mean arterial pressure
was maintained between 80 and 100 mmHg [17]. Thus,
maintaining blood pressure and brain perfusion during the
intraoperative and postoperative periods is important to
reduce the risk of ischemic strokes.
In the present case, the patient required an intensive multidisciplinary assessment and treatment at an inpatient
specialized acute brain injury rehabilitation service. The
patient was discharged after 3 months of inpatient rehabilitation. Early initiation of postacute rehabilitation treatment in brain injury is beneficial and increases the likelihood of a functional improvement [18]. The mechanism underlying such recovery is likely multimodal, ranging
from controllable factors (motivation, family support systems, and rehabilitation service quality) to uncontrollable
factors (age, sex, injury severity and site, and premorbid
cognitive reserve) [19].
The long-term cognitive outcome after acute fornix infarcts is uncertain. One study described a patient who
developed amnesia and confabulation following astrocytoma removal, which disrupted the right anterior fornix.
[13] This patient made a good recovery over a 17-month
follow-up period, suggesting that recovery is possible in
the long term. Compensatory measures by the left anterior
fornix likely account for recovery in such patients [13].
However, a remaining functional fornix is not a prerequisite for memory recovery, as observed in patients who
improved cognitively after bilateral fornix transection [20].
Conclusion
A case of a fornix infarct resulting in anterograde amnesia was described. The fornix infarct was likely due to a hemodynamic stroke caused by perioperative hypotension and hypoperfusion. The patient required brain rehabilitation and recovered sufficiently to be discharged after 3 months. However, the long-term outcome in terms of cognitive recovery remains unclear.
Declarations
Authors’ contributions
The author contributed solely to the article.
Conflict of interest
The author declared that there are no conflicts of interest.
Consent for Publication
Written informed consent for publication of de-identified case details and radiological images were obtained from the patient (witnessed by the son).
References
1. Mortazavi S M J, Kakli H, Bican O, et al. Perioperative stroke after total joint arthroplasty: prevalence, predictors, and outcome. JBJS, 2010, 92(11): 2095-2101.
2. Lalmohamed A, Vestergaard P, Cooper C, et al. Timing of stroke in patients undergoing total hip replacement and matched controls: a nationwide cohort study. Stroke, 2012, 43(12): 3225-3229.
3. Moudgil S S, Azzouz M, Al-Azzaz A, et al. Amnesia due to fornix infarction. Stroke, 2000, 31(6): 1418-1419.
4. Korematsu K, Hori T, Morioka M, et al. Memory impairment due to a small unilateral infarction of the fornix. Clinical neurology and neurosurgery, 2010, 112(2): 164-166.
5. Renou P, Ducreux D, Batouche F, et al. Pure and acute Korsakoff syndrome due to a bilateral anterior fornix infarction: a diffusion tensor tractography study. Archives of neurology, 2008, 65(9): 1252-1253.
6. Rizek P, Pasternak S, Leung A, et al. Acute-onset anterograde amnesia caused by isolated bilateral fornix infarction. Canadian journal of neurological sciences, 2013, 40(5): 738-739.
7. Saito Y, Matsumura K, Shimizu T. Anterograde amnesia associated with infarction of the anterior fornix and genu of the corpus callosum. Journal of Stroke and Cerebrovascular Diseases, 2006, 15(4): 176-177.
8. Moussouttas M, Giacino J, Papamitsakis N I H. Amnestic syndrome of the subcallosal artery: a novel infarct syndrome. Cerebrovascular Diseases, 2005, 19(6): 410.
9. Lim C, Alexander M P. Stroke and episodic memory disorders. Neuropsychologia, 2009, 47(14): 3045-3058.
10. Thomas A G, Koumellis P, Dineen R A. The fornix in health and disease: an imaging review. Radiographics, 2011, 31(4): 1107-1121.
11. Gade A. Amnesia after operations on aneurysms of the anterior communicating artery. Surgical neurology, 1982, 18(1): 46-49.
12. Hattingen E, Rathert J, Raabe A, et al. Diffusion tensor tracking of fornix infarction. Journal of Neurology, Neurosurgery & Psychiatry, 2007, 78(6): 655-656
13. Ruggeri M, Sabatini U. Recovery from amnesic confabulatory syndrome after right fornix lesion. Neurorehabilitation and neural repair, 2008, 22(4): 404-409.
14. Garcia-Bengochea F, Friedman W A. Persistent memory loss following section of the anterior fornix in humans. A historical review. Surgical neurology, 1987, 27(4): 361- 364.
15. Klijn C J M, Kappelle L J. Haemodynamic stroke: clinical features, prognosis, and management. The Lancet Neurology, 2010, 9(10): 1008-1017.
16. Gottesman R F, Sherman P M, Grega M A, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke, 2006, 37(9): 2306-2311.
17. Gold J P, Charlson M E, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass: a randomized trial comparing intraoperative high versus low mean arterial pressure. The Journal of thoracic and cardiovascular surgery, 1995, 110(5): 1302-1314.
18. Hayden M E, Plenger P, Bison K, et al. Treatment effect versus pretreatment recovery in persons with traumatic brain injury: a study regarding the effectiveness of postacute rehabilitation. PM&R, 2013, 5(4): 319-327.
19. Wilson B A. Brain injury: recovery and rehabilitation. Wiley Interdisciplinary Reviews: Cognitive Science, 2010, 1(1): 108-118.
20. Mazarakis N K, Summers F, Murray A D, et al. Partial recovery from amnesia following bilateral surgical fornix transection is correlated with cortical plasticity. British journal of neurosurgery, 2011, 25(5): 658-661.