Open Access | Case Report
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Spontaneous chordoma: a case report on a female UM-HET3 mouse from the SLAM study
* Corresponding author: Rafael de Cabo
Mailing address: Translational Gerontology Branch, National
Institute on Aging, National Institutes of Health, Baltimore, MD
21224, USA.
E-mail: decaboRa@mail.nih.gov
Received: 01 December 2020 / Accepted: 16 December 2020
DOI: 10.31491/APT.2020.12.043
Abstract
A female UM-HET3 mouse from the Study of Longitudinal Aging in Mice (SLAM) was euthanized at 164 weeks of age due to hind limb weakness. Necropsy and histological analysis revealed that the most probable cause of the clinical finding was the compression of the thoracolumbar segment of the spinal cord by herniated intervertebral disks. In addition, a spontaneous chordoma was incidentally found in the coccygeal bones. Given the rarity of this type of tumor, bio-clinical annotations acquired throughout lifespan, detailed histopathological assessment, and comparative clinical-pathological correlations for this mouse are presented and discussed.
Keywords
Geropathology, geroscience, study of longitudinal aging in mice (SLAM), spontaneous neoplasm, tumor burden, chordoma
Introduction
The Study of Longitudinal Aging in Mice (SLAM) is a large mouse population study instituted within the Intramural Research Program at the National Institute on Aging (NIA/NIH) [1] to comprehensively characterize normal aging in two commonly used strains of mice and to validate their usefulness as preclinical models for human aging and age-related diseases. Here, we report the case of a particularly long-lived SLAM female mouse in which a spontaneous chordoma was incidentally found during the necropsy.
Case Report
A 164-week-old female UM-HET3 mouse was humanely
euthanized via carbon dioxide asphyxiation following veterinarian advice due to persistent hind limb weakness. The
body was formalin-fixed and a complete necropsy was
performed by the Division of Veterinary Resources (DVR/NIH, Bethesda, MD). The mouse was very lean, with
muscle mass adequate for an aged mouse. Macroscopically, the liver was diffusely pale, as were the kidneys
which also appeared to be smaller than normal. The major
organs, gastro-intestinal tract, brain, and spinal cord were
saved for histologic examination. Whole slide images
of hematoxylin and eosin (H&E)-stained tissue sections
were acquired with a high-resolution Zeiss Axio Scan Z1
digital slide scanner (Zeiss, Oberkochen, Germany), and
analyzed by a pathologist.
Microscopically, diffuse mild to moderate amyloid deposits were present in the heart (Figure 1A), liver (Figure 1B), spleen, small intestines (Figure 2B), mesentery, and
subcutaneous fat. Both kidneys had moderate diffuse expansion of the glomerular mesangium, with pale eosinophilic deposits also present within glomerular capillary
tufts. In addition, the right kidney had a severe tubular
atrophy with >90% of the nephrons being affected (Figure 1C). The lungs displayed a mild, multifocal acidophil
macrophage Epneumonia (Figure 1D). In the duodenum,
a pedunculated polyp projecting into the lumen was observed (Figure 2A), with mucosal cells forming dysplastic
crypts (Figure 2B). Liver and spleen had aggregates of
macrophages with pale gray granular pigment and scant
dark brown polarizable granules. The brain had prominent
gliosis and rare spheroids in large white matter tracts of
the cerebrum. Bilateral symmetrical foci of gitter cells
were present in the rostral ventrolateral aspect of the hippocampus. The deep cerebellar nuclei had rare swollen
axons. Spinal nerve roots had dilated myelin sheaths and myelomacrophages. Disks of the thoracolumbar segment
of the vertebral column were herniated and severely compressed the spinal cord. Thoracic spinal cord cranial to
this compressed section had multifocal spheroids. Vertebrae and long bones showed thinning of cortical bone and
widened lacunae with foci of replacement of bone marrow
by new woven bone and fibrovascular connective tissue
were present. Hematopoietic cells of the bone marrow had
an increased proportion of plasma cells, some of which
with large, atypical nuclei, suggesting a multiple myeloma. Finally, coccygeal bones displayed marrow cavities
with normal fat replaced by sheets of markedly vacuolated
polygonal cells (i.e. physaliferous cells) with small, round
randomly located hyperchromatic nuclei (Figure 2C, D).
Mitotic figures were not seen. This finding is consistent
with an intramedullary chordoma, a very rare tumor of
embryonic intervertebral disk cells. While the most probable cause of hindlimb weakness was the spinal cord compression, the reasons for symmetrical encephalomalacia
and gliosis remain uncertain.
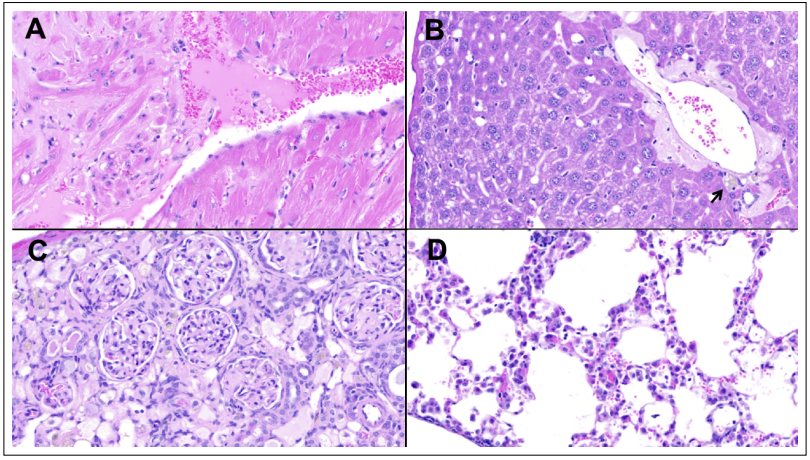
Figure 1. Selected non-neoplastic lesions. (A) Moderate amyloid deposits separating and replacing myocardial cells in the left ventricle, at the base of a papillary muscle. (B) Moderate amyloid deposits surrounding a portal region in the liver (arrow pointing at macrophages scant with dark brown polarizable granules). (C) Right kidney cortex showing a segmental, diffuse expansion of the glomerular mesangium and severe diffuse tubular atrophy. (D) Lungs displaying a mild, acidophil macrophage pneumonia. Hematoxylin and Eosin, original magnification 400x.
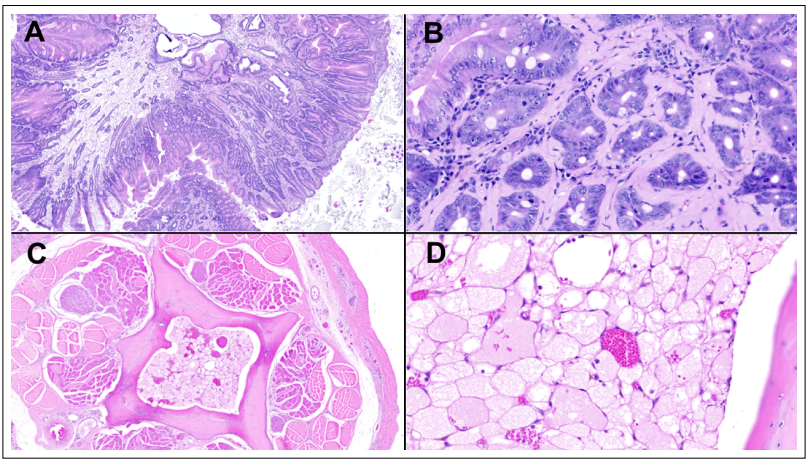
Figure 2. Selected neoplastic lesions. (A) Pedunculated duodenal polyp projecting into the lumen. (B) Amyloid deposits surrounding the stalk of the polyp dysplastic crypts. (C) Coccygeal bones marrow cavities replaced by an intramedullary chordoma, which is pathognomonically featured by markedly vacuolated polygonal cells (i.e. physaliferous cells) with small, round randomly located hyperchromatic nuclei. (D) Hematoxylin and Eosin, original magnification 40x in A, C; and 400x in B, D.
Discussion
SLAM was conceived as the mouse counterpart of the
NIA Baltimore Longitudinal Study of Aging (BLSA), one
of the world’s oldest and still ongoing studies on healthy
normative aging in humans [2]. Similar to BLSA, SLAM
aims at defining individual mouse chronological normative aging, which is surprisingly lacking despite mice
having been used for decades as a model of human aging, and to test preclinically lifespan extending compounds.
Currently, most of the proposed metrics of aging stem
from cross-sectional studies and rely on associations with
chronological age that often fail to accurately predict mortality. Together with this, variables such as sex and age
at administration of a given intervention have hindered
the effectiveness of anti-aging strategies in mice and their
translational applicability into humans [3]; hence the
Geroscience research calls for a true model of functional
or phenotypic normative mouse aging. Two strains of
mice – the inbred C57BL/6J and outbred HET3 – of both
sexes are employed in the SLAM study to account for
genetic heterogeneity, sex- and strain-specific aging phenotypes, pathologies, and mortality patterns. To date, 2800
mice (700 mice per sex and strain, logistically separated
into 14 cohorts of 200 mice) have been enrolled into the
study, longitudinally evaluated for functional, phenotypic
and biological health, and for collection of biospecimens
throughout their lifespan and post-mortem. Within the
longitudinal cohorts, a cross-sectional arm of the study
has also been implemented to collect tissues and to generate a vast biorepository of serum, urine and feces, as well
as flash frozen and formalin-fixed tissues [1].
The mouse of the present case report (SLAM794) belonged to Cohort 7 and was group-housed in a cage of
four animals. They were ad libitum fed the open formula
mouse diet, NIH-31 (3.0 kcal/g) (Envigo), with free access to water. The body weight of SLAM794 peaked (41
g) at 78 weeks, while still housed with two other cagemates (Figure 3A). The subsequent progressive decline of
body weight was synchronous to the presence of only one cagemate and then being single-housed. Motor function
tests, including cage top and rotarod [4], were performed
at 24-week intervals starting from 16 weeks of age. Both
tests showed a reduction in the latency time, with the
steepest decline from 16 to 40 weeks for cage top assessment and from 40 to 64 weeks for the rotarod test (Figure 3B). As expected, frailty index scores [4], assessed three
times starting from 53 weeks, increased as the mouse
(SLAM794) aged.
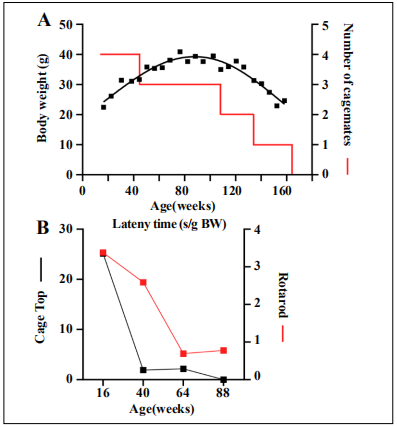
Figure 3. Longitudinal and cross-sectional measurements assessed for mouse SLAM794. (A) Bodyweight trajectory and housing density over the mouse lifespan. (B) Motor function tests were performed at 24- week intervals starting from 16 weeks of age: Cage top (black symbols) and rotarod (red symbols)
The examination of individual frailty index features that
contributed to this increase showed that loss of whiskers
and loss of vision occurred early and became permanent
over time. Some impairment of forelimb grip strength
was seen from 98 weeks onward. Kyphosis, hearing loss,
tail stiffening, menace reflex impairment, and moderately
high mouse grimace scale scores were observed at 132
weeks. The comparison of survival of this mouse to the
full SLAM cohort showed that 99.7% of all mice were
dead by the time it was euthanized at 164 weeks. In addition, more than 98.8% of HET3 female mice were dead
at this time point. Upon necropsy and histopathological
analyses, the disease burden, defined as the amount of
histologic disease present at end of life in an individual
[5], paralleled well with the clinical findings and was
quite typical for a very long-lived mouse. As a matter of
fact, instead of a highly lethal malignant tumor, such as
lympho-reticular tumors, fibrosarcoma, and mammary
adenocarcinoma, which would have killed the mouse at a
younger age, a collection of slowly developing degenerative diseases (i.e. multiorgan amyloidosis, osteoporosis,
encephalomalacia, and gliosis) and slowly-growing localized neoplastic diseases (i.e. duodenal dysplastic polyp,
coccygeal chordoma, spinal multiple myeloma, for a total
tumor burden of 3) was observed. In this regard, the spine
alteration detected while assessing the frailty index was
an early sign of the osteoporotic process that culminated
in the disc herniation/spinal cord compression and chronic
limb weakness requiring euthanasia. Along the same line,
the intestinal and liver amyloidosis very likely contributed
to the progressive weight loss.
Focusing on neoplasms, the coccygeal chordoma was a
surprising incidental finding, being predominantly composed of pathognomonic physaliferous cells comparable
to the typical form of chordomas described in humans.
The lack of mitotic figures and morphological signs of
invasion of the surrounding tissues supported a biologically indolent behavior. Chordomas are rare bone malignant neoplasms believed to be derived from notochord
remnants that may persist anywhere along with the axial
skeleton [6]. In humans, they can be sporadic or familial and account for 1-4% of all bone malignancies, with
an incidence of 0.08 per 100,000 in the United States
[7]. They are generally slow growing, locally invasive,
chemotherapy-resistant and radiotherapy-resistant tumors.
However, due to their location, chordomas are a highly
morbid and potentially fatal disease that requires complex
medical decisions and tailored patient care. Despite having been reported in many vertebrate species, including
fish and numerous mammals (i.e. dog, cat, ferret, mink,
Sprague-Dawley and Fischer 344 rat), epidemiological data are scarce due to their rarity. Recently, an interesting
high prevalence of chordomas was reported among the
captive population of the endangered Perdido Key Beach
Mouse (PKB mouse, Peromyscus polionotus trissyllepsis)
[8]. With 38 out of 88 mice (43%) bearing a chordoma,
without any significant sexual dimorphism differences,
a genetic predisposition was the most likely explanation
for this unusually high prevalence, especially when considering the known bottlenecks and inbreeding that have
occurred in these captive PKB mice. In humans, both
the sporadic and the familial forms of chordomas have
been linked to the tbxt gene (6q27), which encodes the Tbox transcription factor T or brachyury homologue. This
protein is important in the development of the notochord
and is highly expressed in chordoma cells. Duplication
of the tbxt gene has been shown to be a predisposing factor for familial chordoma [9]. It is thus possible that PKB
mice carry a similar or alternative germline mutation,
that, coupled with founder effect, predisposes the captive
population to chordoma and ensures it is the prevalent
cause of mortality as they age (chordoma prevalence
was 50% and 71% in mice 4 and 5 years old) [8]. On the
other hand, the chordoma described in the present report
appears more similar to the human sporadic form of chordoma as it was found in a heterogeneous mouse strain, HET3, that typically has a varied spectrum of age-related
mortality causes. As the collection and in-depth analysis
of data generated by SLAM move forward, it will be very
interesting to see how rarely reported pathologies, like the
chordoma discussed here, will cluster in relation to strain,
sex, age, and subsequent clinicopathological correlations,
providing a strong foundation for translational research.
Declarations
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
References
1. Palliyaguru D L, Vieira Ligo Teixeira C, Duregon E, et al. Study of Longitudinal Aging in Mice: Presentation of Experimental Techniques (SLAM POET). The Journals of Gerontology: Series A, 2020.
2. Shock N W. A New Research Building for Gerontology. The Gerontologist, 1964, 4(4): 185-189.
3. Gonzalez-Freire M, Diaz-Ruiz A, Hauser D, et al. The road ahead for health and lifespan interventions. Ageing Research Reviews, 2020: 101037.
4. Bellantuono I, de Cabo R, Ehninger D, et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nature protocols, 2020, 15(2): 540-574.
5. Snyder J M, Ward J M, Treuting P M. Cause-of-death analysis in rodent aging studies. Veterinary pathology, 2016, 53(2): 233-243.
6. Walcott B P, Nahed B V, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. The lancet oncology, 2012, 13(2): e69-e76.
7. McMaster M L, Goldstein A M, Bromley C M, et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes & Control, 2001, 12(1): 1-11.
8. Taylor K R, Garner M M, Russell K, et al. Chordomas at high prevalence in the captive population of the endangered Perdido Key beach mouse (Peromyscus polionotus trissyllepsis). Veterinary pathology, 2016, 53(1): 163- 169.
9. Yang X R, Ng D, Alcorta D A, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nature genetics, 2009, 41(11): 1176-1178.