Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Sex-dependent lifespan extension of ApcMin/+ FAP mice by chronic mTOR inhibition
* Corresponding author: Manish Parihar
Mailing address: Greehey Children's Cancer Research Institute
University of Texas Health San Antonio, 8403 Floyd Curl Dr,
San Antonio, TX 78229, USA.
E-mail: parihar@uthscsa.edu
Received: 30 November 2020 / Accepted: 15 December 2020
DOI: 10.31491/APT.2020.12.039
Abstract
Background: ApcMin/+ mice model familial adenomatous polyposis (FAP), a disease that causes numerous colon polyps leading to colorectal cancer. We previously showed that chronic treatment of ApcMin/+ females with
the anti-aging drug, rapamycin, restored a normal lifespan through reduced polyposis and anemia prevention.
Lifespan extension by chronic rapamycin in wildtype UM-HET3 mice is sex-dependent with females gaining
the most benefit. Whether ApcMin/+ mice have a similar sex-dependent response to chronic mTOR inhibition is
not known.
Methods: To address this knowledge gap and gain deeper insight into how chronic mTOR inhibition prevents intestinal polyposis, we compared male and female ApcMin/+ mice responses to chronic treatment with a
rapamycin-containing diet. Animals were fed a diet containing either 42 ppm microencapsulate rapamycin or
empty capsules, one group was used to determine lifespan and a second group with similar treatment was harvested at 16 weeks of age for cross-sectional studies.
Results: We found that the survival of males is greater than females in this setting (P < 0.0197). To explore
the potential basis for this difference we analyzed factors affected by chronic rapamycin. Immunoblot assays
showed that males and females exhibited approximately the same level of mTORC1 inhibition using phosphorylation of ribosomal protein S6 (rpS6) as an indirect measure. Immunohistochemistry assays of rpS6
phosphorylation showed that rapamycin reduction of mTORC1 activity was on the same level, with the most
prominent difference being in intestinal crypt Paneth cells in both sexes. Chronic rapamycin also reduced crypt
depths in both male and female ApcMin/+ mice (P < 0.0001), consistent with reduced crypt epithelial cell proliferation. Finally, chronic rapamycin prevented anemia equally in males and females.
Conclusions: In males and females, these findings link rapamycin-mediated intestinal polyposis prevention
with mTORC1 inhibition in Paneth cells and concomitant reduced epithelial cell proliferation.
Keywords
Rapamycin, small intestine, polyposis, mTORC1, Paneth cells, crypt stem cells
Introduction
Adenomatous polyposis coli (APC), a tumor suppressor gene, encodes an inhibitor of the canonical Wnt-β-catenin
pathway. APC mutations in the germline cause familial
adenomatous polyposis (FAP) [1-3], which, if untreated,
leads to colorectal cancer in humans at an early age. Somatic defects in APC function and Wnt signaling are also
observed in a majority of colorectal adenomas and carcinomas [4]. Currently, the standard of care for FAP patients
is colectomy before the polyps develop [5]. Although this
strategy reduces mortality, it significantly deteriorates the
quality of life [6]. Hence, there is a clear need to develop
better preventative strategies for patients with this class of intestinal cancer.
The ApcMin/+ mouse, an established model to study FAP,
presents with multiple adenomas in the intestine, intestinal
bleeding, severe anemia, and early death [7]. Previously
we showed that the mTOR (mammalian or mechanistic
target of rapamycin) inhibitor rapamycin in a targeted
enteric release formulation (eRapa) reduced the number
of adenomas in the small intestine of female ApcMin/+ mice
leading to a five-fold extension in their mean survival
[8]. In addition to the reduction in the number of polyps,
eRapa also restored life-long normal hematocrits. Although inhibition of mTORC1 has a role in the reduction
of polyposis in Apcfl/fl mice [9], the exact mechanism underlying rapamycin effects on adenomas in ApcMin/+ mice
is unknown.
We previously studied female ApcMin/+ mice since rapamycin trials by the ITP showed a stronger response for life
span extensions in females [10]. In humans, daily aspirin
administration for more than five years prevented distant
metastasis and reduced deaths due to colorectal cancers.
Although aspirin also inhibits mTOR [11-13], it was
found to only increase the lifespan of male mice in the genetically heterogeneous UM-HET3 strain [14]. How male
ApcMin/+ will respond to chronic mTOR inhibition is an
important unknown.
Intestinal polyps in Apcfl/fl mice are believed to originate
from the Lgr5+
stem cells of the crypts [15]. The selfrenewal of stem cells is mediated by mTORC1, which is
sensitive to both rapamycin and caloric restriction [16]. To
maintain homeostasis in the intestinal crypts, these cells
form a niche with the neighboring Paneth cells that are
interspersed between the stem cells [17, 18]. Paneth cells
not only secrete bactericides to protect the intestinal cell
lining but also regulate stem cell function by niche signaling. In wild-type mice, Paneth cells respond to rapamycin
treatment as measured by a reduction in phosphorylation
of ribosomal protein S6 (rpS6) [16].
A twofold purpose of our study was to compare survival
and polyposis preventive effects of chronic rapamycin in
ApcMin/+ male and female mice and to obtain additional
insights into its mechanism of action in tumor prevention by an anti-aging drug. Surprisingly, our data show
that chronic rapamycin improves the lifespan of ApcMin/+
males more than in females. Our results also suggest that
rapamycin prevents tumors in this model by suppressing
mTORC1 activity in the Paneth cells to a similar extent
in both sexes leading to a reduction in intestinal crypt
length. Additionally, chronic rapamycin prevents anemia
in ApcMin/+ comparably in both sexes.
Methods
Mouse husbandry and diets
We treated and used animals according to Institutional Animal Care and Use Committee and NIH guidelines. We ad libitum fed male and female ApcMin/+ mice (purchased from Jackson Laboratories Stock No. 002020) our 42 ppm microencapsulated rapamycin or empty Eudragit capsule (control) diets. Diets were started on four-week-old mice. For longevity experiments, they were allowed to live out their lifespans and euthanasia was performed only on those mice that could not eat or drink or were unable to respond to gentle prodding. The second set of similarly treated animals was sacrificed at 16 weeks of age for cross-sectional studies, and tissue and blood were harvested. We used a 16-week time point for this study based on our previous observations of polyposis in the ApcMin/+ mice. This harvested tissue was used for immunoblots and histological analysis. Blood concentration of rapamycin was determined as previously described [19].
Hematocrit measurement
We collected approximately 75 μL of whole blood into a heparinized micro-hematocrit capillary tube (Fisherbrand cat. 22-362-566) by submandibular bleed during tissue harvest. The capillary tubes were centrifuged to pack the cells in the blood and the percentage of packed cell volume (PCV) was measured.
Immunoblots
We performed these assays as previously described [20].
Immunohistochemistry
Harvested tissue was fixed in 10% buffered formalin and
embedded in paraffin for sectioning. The sections were
heated at 95-100 ℃ immersed in antigen retrieval buffer
containing 10mM sodium citrate and 0.05% tween 20 (pH
adjusted to 6.0). Endogenous peroxidases were inhibited
by hydrogen peroxide and the sections were blocked using
5% normal goat serum for 1 hour. Tissue sections were
then incubated overnight with the primary antibodies at 4
℃. For colorimetric assays, the Signal Stain Boost IHC
Detection reagent and Signal Stain DAB Substrate Kit
(Cell Signaling Technology, CST 8059) were used for detecting the IHC signal. DNA was stained by hematoxylin
counterstain (CST 14166) and an Echo Revolve microscope or a Nikon Eclipse 80i microscope was used to take
pictures. Antibodies used were rabbit anti-rps6 (1:600;
CST 2217) and rabbit anti-phospho-rps6 ser240/244
(1:2000; CST 5364). For immunofluorescence assays, the
sections were incubated with secondary antibodies for 2
hours at room temperature in a dark, humidified chamber
chamber and mounted using DAPI medium (Vectashield
H-1200). Antibodies used were: goat anti-cKit (1:50;
R&D AF1356), donkey anti-goat Alexa 594 (1:500, Invitrogen A32758), goat anti-rabbit Alexa 488 (1:500, Invitrogen A11034).
For measuring the crypt lengths, sections were stained
with H&E and the lengths of 40-50 crypts were measured
from at least three mice/group from images taken on an
Echo Revolve microscope.
Statistical analysis
Lifespan data for the groups was analyzed using the Logrank (Mantel-Cox) test. The polyp counts and %PCV were compared using a one-way ANOVA with Tukey’s multiple comparisons. All western blot data and the crypts lengths were compared using the Student’s t-test. A P-value of<0.05 was considered significant.
Results
Lifespan: Chronic eRapa increases ApcMin/+ male survival longer than females
Chronic rapamycin treatment has previously been shown to improve the survival of ApcMin/+ females [8]. Survival results showed that eRapa extended the lifespans of both males and females compared to controls (Figure 1, P < 0.0001 for both sexes, n=5). There was no difference in the survival of Eudragit-treated (control) male and female ApcMin/+ animals. Interestingly, our analysis showed that ApcMin/+ males chronically treated with eRapa had a significantly longer lifespan than females (Figure 1, P = 0.0197).
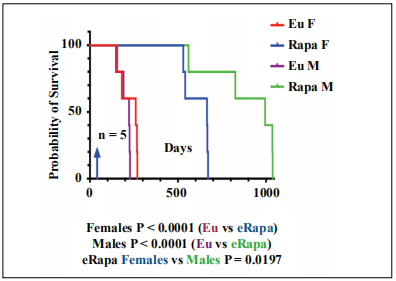
Figure 1. Chronic rapamycin extends longevity of male ApcMin/+ mice greater than females. Blue arrow indicates the start of eRapa 42 ppm and control (Eudragit) diets at 4 weeks of age. Lifespan statistics for the goups were calculated using the Log-rank (Mantel-Cox) test. P values are below the graph.
Polyp number and hematocrits: eRapa reduced polyp numbers and restored hematocrits similarly in both sexes
Since chronic eRapa had a sexually dimorphic effect on
longevity in ApcMin/+ mice, we ask if there was a sex difference in rapamycin prevention of polyposis. For this
purpose, we treated 5 males and 5 females with 0 or 42
ppm eRapa diets (Figure 2A). At 16 weeks of age, we
sacrificed the animals and counted the number of polyps
in the small intestine. Chronic eRapa significantly reduced
the number of polyps in both females (P = 0.0004) and
males (P < 0.0001) to an equal level (P = 0.999), Figure
2B. Polyp reduction being close to the same in both males
and females treated with eRapa, it is unlikely to account
for the longer lifespan in males.
Since ApcMin/+ mice primarily die from anemia, which
chronic eRapa prevents in females [8], we next asked
if a sex difference in hematocrit response by rapamycin
could account for the difference in longevity effects. At tissue harvest, the percentage of packed cell volume
(%PCV) of the eRapa treated ApcMin/+ mice (both sexes)
was significantly improved (P = 0.0024 for males and P =
0.0219 for females), with no difference between the male
and the female eRapa treated animals (P = 0.95), Figure
2C. These data show that reduction in tumor number and
anemia amelioration are mostly responsible for the extension in the lifespan of rapamycin-treated male and female
ApcMin/+ mice. However, they do not provide clues as to
why chronic inhibition of mTOR results in longer-lived
ApcMin/+ males compared to females (Figure 1). We next
investigated mTORC1 status for potential changes in response to eRapa treatment.
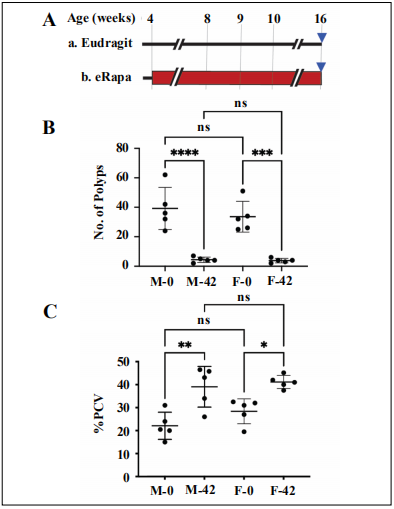
Figure 2. Rapamycin reduces small intestine polyposis in female and male ApcMin/+ mice.(A) Experimental design for cross-section experiments. Red rectangle indicates rapamycin treatment period. Blue triangles indicate the age at which we collected tissues. (B) Graph of the number of polyps for females (F) and males (M) on the indicated diets (0 or 42 ppm eRapa). (C) Rapamycin prevents anemia in female and male ApcMin/+ mice. PCV = packed cell volume. Graph showing packed cell volume (PCV) percentages for females and males on the indicated diets. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001.
Status of mTORC1. Chronic eRapa decreases mTORC1 activity in small intestine tissue lysates
Previously, it has been documented that chronic rapamycin brings about a reduction of mTORC1 activity in
C57BL/6 female small intestine [8]. To address the question of mTORC1 status in ApcMin/+ males and females,
we used immunoblot assays of small intestine lysates in
cross-section experiments. As expected, chronic treatment
of male ApcMin/+ fed 42 ppm eRapa diet resulted in a reduction of phosphorylation.
(Ser240/244)-dependent intensity values relative to
phosphorylation state-independent intensity (total rpS6
protein) in the small intestine lysates of males show a
reduction with rapamycin treatment (Figure 3A and 3B).
Rapamycin treatment also raised the levels of rpS6 signal
relative to GAPDH (Figure 3C), a response not observed
in C57BL/6 intestine [8] or colon [20]. Immunoblot assays of female small intestine lysates also demonstrate a
similar reduction of rpS6 phosphorylation (Figure 3D and
3E) and an increase in rpS6 protein signals (Figure 3F).
As determined by immunohistochemistry, polyps in the
small intestine of control animals (Figure 3G) showed a
markedly higher rpS6 phosphorylation which was absent
in the small polyp in the eRapa group (Figure 3H). These
data indicate mTORC1 inhibition in the small intestine in
both sexes in response to rapamycin treatment.
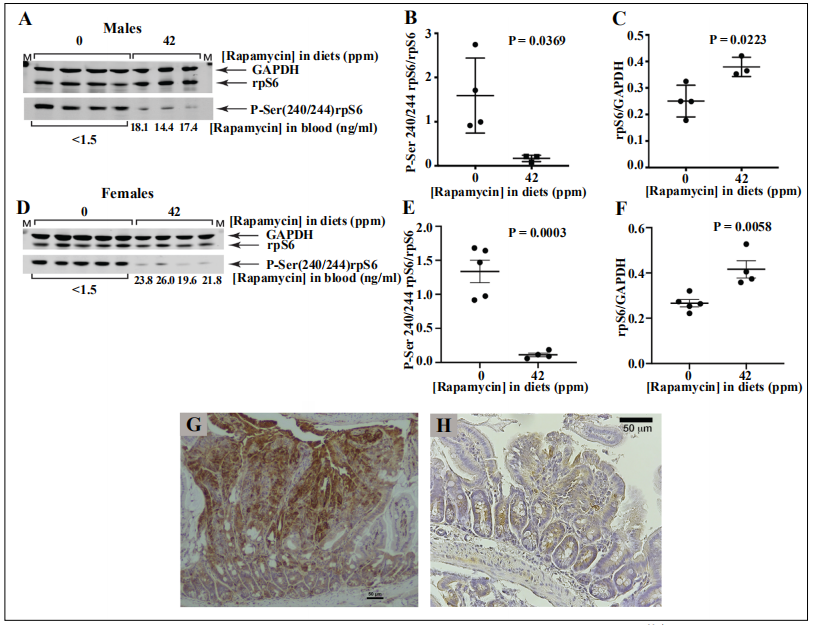
Figure 3. eRapa effects on mTORC1. Representative immunoblot assay of small intestine lysates prepared from ApcMin/+ male (A) and female (D) mice respectively. Diets indicated above the blots, antibodies to the right of each image, and rapamycin concentrations in blood are below the blots. Graphs show the quantification of the immunoblot data as measured by the ratio of the intensity values for phosphorylation state-dependent signal (P-Ser 240/244 rpS6) to phosphorylation state–independent (rpS6) signal (B, E) and rpS6 intensity values relative to GAPDH (C, F). Phosphorylated rpS6 expression in small intestine of control (G) and eRapa treated (H) mice.
mTORC1 Crypt Status. Chronic eRapa reduces mTORC1 activity in Paneth cells in ApcMin/+ mice
Yilmaz et al. [16] linked a calorie restriction-associated
increase in the renewal of the small intestine crypt stem
cells in C57BL/6 mice with the repression of mTORC1
in Paneth cells. We asked: what effect would chronic
eRapa diets have on mTORC1 status in ApcMin/+ Paneth
cells? This is an important question for two reasons: 1)
Paneth cells constitute a niche for intestinal crypt cells
[21]; 2) Paneth cell mTORC1 plays a critical role in nutrient and rapamycin responses for stem cell renewal in the
niche [16]. In both sexes, immunohistochemistry using
an antibody specific for phosphorylation of Ser-240/244
in rpS6 demonstrated eRapa -mediated suppression of
staining prominently in crypt cells (Figure 4, females and
Figure 5, males). Thus, at the cellular level, there was no
discernable sex difference in chronic rapamycin-mediated
mTORC1 suppression.
We postulated that chronic rapamycin reduced rpS6 phosphorylation (and mTORC1 activity) in Paneth cells. As
a test, we used an antibody specific for cKit in immunofluorescence assays of small intestine tissue sections. cKit
receptor tyrosine kinase and its ligand, stem cell factor
(SCF), are known to play important roles in various mammalian organs through several signaling pathways including PI3 kinase [22]. It is also known to be specifically
expressed in intestinal crypt Paneth cells [23]. Supporting our postulate, a representative panel of microscopic images shows co-localization of fluorescence generated by
antibodies specific for P-Ser(240/244)rpS6 and cKit in
Figure 6A, females, and Figure 6B, males. Consistent
with previous assays for mTORC1 responses to chronic
rapamycin, we could not detect any sex difference in Paneth cells.
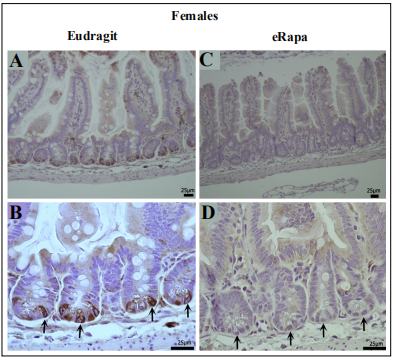
Figure 4. Chronic eRapa represses mTORC1 activity in female intestinal crypt niche primarily in Paneth cells.(A, B). Intestinal preparations from Eudragit fed females. Arrows in panel B point to prominent staining of crypt cells with an antibody specific for P-Ser(240/244)-rpS6. In eRapa fed specimens (C, D), no signal is detected in the crypt niches (arrows in panel D). Panels A and C show lower magnification of villi and crypts in mice fed Eudragit and eRapa diets respectively.
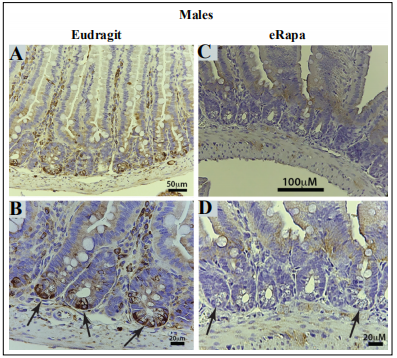
Figure 5. Chronic eRapa represses mTORC1 activity in male intestinal crypt niche primarily in Paneth cells. (A, B). Intestinal preparations from Eudragit fed females. Arrows in panel B point to prominent staining of crypt cells with an antibody specific for P-Ser(240/244)-rpS6. In eRapa fed specimens (C, D), no signal is detected in the crypt niches (arrows in panel D). Panels A and C show lower magnification of villi and crypts in mice fed Eudragit and eRapa diets respectively.
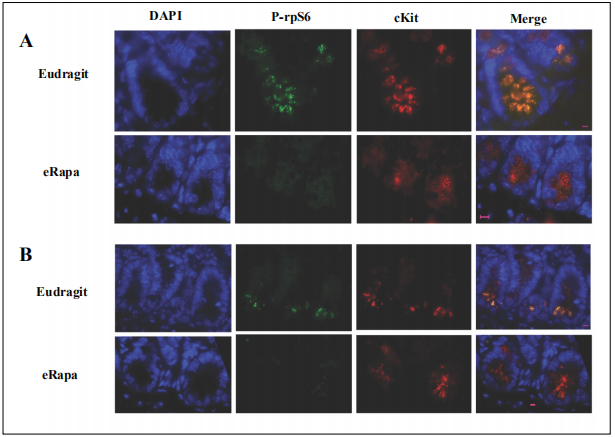
Figure 6. Co-localization of cKit and reduced phosphorylated rpS6 by chronic rapamycin in intestinal crypts. Panels A and B show results using tissue sections from females and males respectively, which were prepared from mice fed eRapa diets (42 ppm rapamycin) or Eudragit controls. DAPI identifies nuclei (blue). Antibodies specific for P-Ser(240/244)rpS6 are green and those specific for cKit are red. Magnification bars are 5 μm.
eEF2 Kinase: Evidence that chronic eRapa reduced translation elongation
In a liver regeneration setting, rapamycin preferentially inhibits S6 kinase 1(S6K1) over 4E-BP1 [24] suggesting that the mTORC1-4E-BP1 pathway might not be a limiting pathway in polyp promotion. Translation initiation control by mTORC1 has been extensively studied, while mTOR’s effect on translation elongation has gotten less scrutiny. Eukaryotic elongation factor 2 kinase (eEF2K) is a key node in the regulation of translation elongation. In anabolic settings, S6K1 phosphorylates eEF2K to inhibit its activity [25]. In conditions where mTORC1 (and S6K1) are inhibited, dephosphorylation of these residues frees eEF2K to phosphorylate eEF2, which slows elongation of translation [25]. Relevant to our study, Faller et al. showed that translation elongation by way of S6K1 is a significant factor for tumor growth in Apcfl/fl mice in addition to mTORC1 effects on protein synthesis [26]. To explore the effects of chronic eRapa on this axis, we used an immunoblot assay of eEF2K levels. In both males and females, rapamycin increased the levels of eEF2K significantly in small intestine lysates (Figure 7 A-D).
Crypt Lengths: Chronic eRapa reduced intestinal crypt lengths
Faller et al. reported that rapamycin and cycloheximide reduced intestinal crypt size in Apcf/f mice [26]. Would this be the case for rapamycin-treated ApcMin/+ mice? Compared to ApcMin/+ mice fed Eudragit diets, both female and male mice on rapamycin diets had significantly reduced intestinal crypt depths (P < 0.0001), Figure 8.
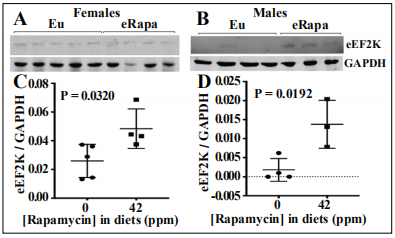
Figure 7. eRapa effects on eukaryotic elongation factor 2 kinase (eEF2K). Representative immunoblot assay of small intestine lysates prepared from ApcMin/+ female (A) and male (B) mice respectively. Diets indicated above the blots, antibodies to the right of the lanes. Graphs showing the quantification of the immunoblot data as measured by the ratio of the intensity values for eEF2K signal relative to GAPDH signal (C, D).
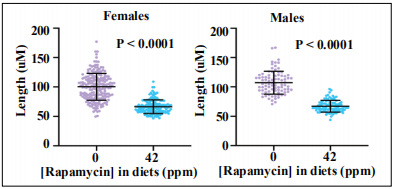
Figure 8. Rapamycin reduced crypt depths in female and male intestinal crypt measurements.
Discussion
Our experiments to determine if there were sex differences in response to chronic rapamycin in ApcMin/+ mice revealed an unexpected and important difference; males had greater survival benefits than females. Our other assessments of mTORC1 effects in each sex revealed no other detectable
differences. In earlier rapamycin trials by the Intervention Testing Program (ITP), UM-HET3 females fared significantly better than males in a dose-dependent manner [10]. If cancer prevention played a role in longevity extension by chronic rapamycin treatments in this setting, the ITP results would have predicted outcomes opposite to what we found. Flurkey et al.[27] reported no difference in a
lifespan extension of UM-HET3 females and males by diet restriction, which reduces the activity of mTORC1,
although in a circadian-dependent manner [28]. Combined, these data point to a significant difference in how rapamycin extends lifespan compared to diet restriction, and also different in cancer-prone models. For example,
Livi et al. [29] found no interaction of sex with longevity extension by chronic rapamycin in the Rb1+/- cancer-prone model.
Why chronic eRapa works better in males in the ApcMin/+ cancer-prone model is a mystery. Solving this mystery
will require additional studies perhaps focusing on Wnt/β-catenin signaling in other organs such as adipose. Curiously, it has been proposed that rapamycin feminizes
males [30, 31] as a possible reason for the sexual dimorphism in its effect on longevity. Again, we cite Livi et al.’s
rapamycin Rb1+/- report [29] and Flurkey et al.’s UMHET3 diet restriction study [27] showing no sex differences as counterarguments. Thus, the interaction of sex in the rapamycin longevity effect, like diet restriction, probably depends on the experimental setting including mouse
genotype.
This is the second demonstration that delivery of rapamycin to the location of polyp formation in the small intestine is an effective strategy for the prevention of tumor development in females, and now for the first time in males.
To gain an initial understanding of how chronic rapamycin
achieves this effect, we used immunoblots and immunolocalization assays to determine the status of a mTORC1
downstream effector. Our data indicate that Paneth cells in
the crypts have the most prominent reduction in rpS6 Ser
240/244 phosphorylation. However, what does this mean
in terms of the prevention of polyposis? Lgr5+ intestinal
crypt stem cells (ICSCs) originate polyps in Apcfl/fl mice
[15]. Paneth cells are thought to be supporting cells for
ICSCs in the crypt niche [21, 32], although were recently
found to be dispensable resulting in a remodeled crypt
with enteroendocrine and tuft cells supporting Lgr5+
stem
cells [33]. Assuming that ICSCs are the cells-of-origin for
polyps in ApcMin/+ mice, what could be the mechanism by
which Paneth cells mediate the prevention of polyposis by
chronic mTORC1 inhibition? In wild-type mice, Yilmaz
et al. provided an important clue by showing that inhibition of mTORC1 in Paneth cells by diet restriction or rapamycin increased intestinal crypt stem cell renewal [16].
Yilmaz et al. attributed this effect to an increase in Bst1,
an ectoenzyme that converts NAD+ to cyclic ADP ribose (cADPR). As a paracrine effector, cADPR promotes
proliferation (or self-renewal in stem cells) by activating
calcium signaling. In the ApcMin/+ intestine, our attempts to
assay Bst1 in response to chronic rapamycin were inconsistent leaving this possibility open. Importantly, we did
observe a reduction in crypt depth by chronic rapamycin
suggesting an increase in ICSCs renewal and reduction in
transit-amplifying cells of the crypt. Whatever the reason
our major finding remains that chronic rapamycin prevents polyposis and extends the health span of an accepted
model of FAP.
In addition to S6K1→rpS6, mTORC1 regulates translation elongation through the S6K1→eEF2K→eEF2 pathway. In our studies of DSS-induced colon cancer in Ap- c
Min/+ mice, we observed that chronic rapamycin resulted
in increased levels of eEF2K and elevated levels of Thr
56 phosphorylation of eEF2 in colonic crypts indicating a
reduction in elongation and protein synthesis [19]. These
data were consistent with the idea that chronic rapamycin prevents tumorigenesis in this setting by a mTORC1
reduction of protein synthesis via two pathways,
S6K1 → rpS6, and S6K1 → eEF2K → eEF2. Thus, we
expected that chronic rapamycin would have the same effect on the eEF2 pathway in the small intestine of ApcMin/+
mice. Indeed, we observed a significant increase in eEF2K
levels by immunoblot assays consistent with our expectations. These data suggest that the rpS6 and eEF2 pathways have a combined role in preventing small intestine
polyposis. However, there is a caveat to this interpretation
since the results of our immunohistochemistry assays of
Thr 56 phosphorylation in crypt eEF2 were inconsistent
despite repeated attempts under varying conditions. Thus,
the effects of chronic rapamycin on translation elongation
remain to be fully tested.
Contrary to long-standing beliefs, chronic rapamycin appears to be safe and effective as an anti-cancer or anti-aging intervention. It remains to be determined if localized
delivery of rapamycin on polyposis in FAP patients will
be as effective as it is in mice. It is clear that minor adverse effects associated with chronic rapamycin compared
with the potential benefits strongly suggest that its use
would be worth the risk if it works as well in FAP patients
(and perhaps other colitis driven diseases in people) as it
does in mice. Since eRapa is effective in preclinical trials
of wild type mice by the ITP, including those started late
in life [34], we suggest that chronic rapamycin would be a
good candidate for the prevention of polyposis and other
colorectal cancers in post colectomy patients at risk for
another duodenal adenomatosis [35], and lastly in older
patients who are under surveillance by colonoscopy.
Declarations
Acknowledgements
The authors thank Drs. Teresa Marple and Valerie B. Holcomb for their assistance with tissue harvesting, and Greg Friesenhahn for rapamycin measurements. The authors also thank San Antonio Nathan Shock Aging Animals Models and Longevity Assessment Core for animal care and the UT Health San Antonio Histology and Immunohistochemistry Core for preparing tissue for histology.
Financial supports
ZDS: National Institutes of Health R01- CA193835; PH: National Institute of Health R01 CA188032-01, P01AG017242-17A1; RS: U01 AG022307; P30 AG013319; and a Senior Research Career Scientist Award from the Department of Veterans Affairs Office of Research and Development.
Potential financial conflict of interest
The University of Texas Health Science Center at San Antonio has applied for a patent, U.S. Patent Application No. 13/128,800, by inventors Zelton Dave Sharp, Randy Strong, and Paul Hasty for an encapsulated rapamycin formulation used in this paper. Under a licensing agreement between Emtora Biosciences (formerly Rapamycin Holdings, Inc.) and the University of Texas Health Science Center San Antonio, R. Strong, Z.D. Sharp, P. Hasty, and the University are entitled to milestone payments and royalty on sales of microencapsulated rapamycin. The university has a plan for managing conflicts of interest under its "Policy and Procedures for Promoting Objectivity in Research by Managing, Reducing, or Eliminating Conflicts of Interest."
References
1. Burt R W, Bishop D T, Lynch H T, et al. Risk and surveillance of individuals with heritable factors for colorectal cancer. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bulletin of the World Health Organization, 1990, 68(5): 655.
2. Kinzler K W, Nilbert M C, Su L K, et al. Identification of FAP locus genes from chromosome 5q21. Science, 1991, 253(5020): 661-665.
3. Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science, 1991, 253(5020): 665-669.
4. Borras E, San Lucas F A, Chang K, et al. Genomic landscape of colorectal mucosa and adenomas. Cancer Prevention Research, 2016, 9(6): 417-427.
5. Brenner H, Chang-Claude J, Seiler C M, et al. Protection from colorectal cancer after colonoscopy: a populationbased, case–control study. Annals of internal medicine, 2011, 154(1): 22-30.
6. Wallace M H, Phillips R K S. Upper gastrointestinal disease in patients with familial adenomatous polyposis. British journal of surgery, 1998, 85(6): 742-750.
7. Moser A R, Pitot H C, Dove W F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science, 1990, 247(4940): 322-324.
8. Hasty P, Livi C B, Dodds S G, et al. eRapa restores a normal life span in a FAP mouse model. Cancer Prevention Research, 2014, 7(1): 169-178.
9. Fujishita T, Aoki K, Lane H A, et al. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcΔ716 mice. Proceedings of the National Academy of Sciences, 2008, 105(36): 13544-13549.
10. Miller R A, Harrison D E, Astle C M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging cell, 2014, 13(3): 468-477.
11. Sun D, Liu H, Dai X, et al. Aspirin disrupts the mTORRaptor complex and potentiates the anti-cancer activities of sorafenib via mTORC1 inhibition. Cancer letters, 2017, 406: 105-115.
12. Henry W S, Laszewski T, Tsang T, et al. Aspirin suppresses growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer research, 2017, 77(3): 790-801.
13. Yue W, Yang C S, DiPaola R S, et al. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer prevention research, 2014, 7(4): 388-397.
14. Strong R, Miller R A, Astle C M, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging cell, 2008, 7(5): 641- 650.
15. Barker N, Ridgway R A, Van Es J H, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature, 2009, 457(7229): 608-611.
16. Yilmaz Ö H, Katajisto P, Lamming D W, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature, 2012, 486(7404): 490- 495.
17. Roth S, Franken P, Sacchetti A, et al. Paneth cells in intestinal homeostasis and tissue injury. PloS one, 2012, 7(6): e38965.
18. Tetteh P W, Basak O, Farin H F, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell stem cell, 2016, 18(2): 203-213.
19. Parihar M, Dodds S G, Hubbard G, et al. Rapamycin Extends Life Span in ApcMin/+ Colon Cancer FAP Model. Clinical Colorectal Cancer, 2020.
20. Dodds S G, Livi C B, Parihar M, et al. Adaptations to chronic rapamycin in mice. Pathobiology of Aging & Agerelated Diseases, 2016, 6(1): 31688.
21. Sato T, Van Es J H, Snippert H J, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature, 2011, 469(7330): 415-418.
22. Rönnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cellular and Molecular Life Sciences CMLS, 2004, 61(19-20): 2535-2548.
23. Schmitt M, Schewe M, Sacchetti A, et al. Paneth cells respond to inflammation and contribute to tissue regeneration by acquiring stem-like features through SCF/cKit signaling. Cell reports, 2018, 24(9): 2312-2328. e7.
24. Jiang Y P, Ballou L M, Lin R Z. Rapamycin-insensitive regulation of 4e-BP1 in regenerating rat liver. Journal of Biological Chemistry, 2001, 276(14): 10943-10951.
25. Thoreen C C. The molecular basis of mTORC1-regulated translation. Biochemical Society Transactions, 2017, 45(1): 213-221.
26. Faller W J, Jackson T J, Knight J R P, et al. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature, 2015, 517(7535): 497-500.
27. Flurkey K, Astle C M, Harrison D E. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2010, 65(12): 1275-1284.
28. Tulsian R, Velingkaar N, Kondratov R. Caloric restriction effects on liver mTOR signaling are time-of-day dependent. Aging (Albany NY), 2018, 10(7): 1640.
29. Livi C B, Hardman R L, Christy B A, et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging (Albany NY), 2013, 5(2): 100.
30. Estep III P W, Warner J B, Bulyk M L. Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PloS one, 2009, 4(4): e5242.
31. Tyshkovskiy A, Bozaykut P, Borodinova A A, et al. Identification and application of gene expression signatures associated with lifespan extension. Cell metabolism, 2019, 30(3): 573-593. e8.
32. Barker N, Van Es J H, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 2007, 449(7165): 1003-1007.
33. van Es J H, Wiebrands K, López-Iglesias C, et al. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proceedings of the National Academy of Sciences, 2019, 116(52): 26599-26605.
34. Harrison D E, Strong R, Sharp Z D, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. nature, 2009, 460(7253): 392-395.
35. Bülow S, Alm T, Fausa O, et al. Duodenal adenomatosis in familial adenomatous polyposis. International journal of colorectal disease, 1995, 10(1): 43-46.