Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Interfering with miR-24 alleviates rotenone-induced dopaminergic neuron injury via enhancing autophagy by up-regulating DJ-1
* Corresponding author: Fengrui Li
Mailing address: College of Basic Medicine and Forensic Medicine, Baotou Medical College, 31 Jianshe Rd, Baotou 014060,
People’s Republic of China.
E-mail: rui6826@qq.com
Received: 18 December 2019 / Accepted: 20 December 2019
DOI: 10.31491/APT.2019.12.004
Abstract
Background: To investigatw the mechanism of miR-24 on dopaminergic neuron injury in parkinson’s disease (PD).
Methods: In vivo and in vitro PD model was established using rotenone. All rats were received behavioral assessments using open field test and rotarod test. MN9D cell viability was measured using MTT assay. The relationship between miR-24 and DJ-1 was detected using dual-luciferase reporter assay.
Results: MiR-24 expression was increased in the brain tissues from PD rats and rotenone-induced MN9D cells,
while DJ-1 expression, the LC3-II/LC3-I ratio and the protein expression of Beclin 1 was decreased. Rotenone
reduced MN9D cell viability, but interfering with miR-24 changed the inhibition effect of rotenone. Moreover,
interfering with miR-24 enhanced the LC3-II/LC3-I ratio and the protein expression of Beclin 1 by increasing
DJ-1 expression, and then relieved rotenone-induced dopaminergic neuronal cell injury. These findings were
also proved in PD rat model.
Conclusions: Interfering with miR-24 alleviated rotenone-induced dopaminergic neuron injury via enhancing
autophagy by increasing DJ-1.
Keywords
Parkinson’s disease, autophagy, miR-24, DJ-1, dopaminergic neuron
Introduction
As the second most common neurodegenerative disease,
the incidence of Parkinson’s disease (PD) in elderly patients is 2% and can reach 5% in people over 85 years old [1]. The main pathological changes of PD are necrosis of
dopaminergic neurons in the compact part of the substantia nigra (SN) and the formation of Lewy bodies [2]. The
pathogenesis of PD is complicated and not clearly understood. Recently, it has been reported that autophagy is involved in PD [3]. Autophagy is widespread in eukaryotic
cells and is a pathway for lysosomes to degrade their own
organelles and other macromolecules. Autophagy dysfunction has been implicated in the pathogenesis of many
neurodegenerative disorders, including PD [4].
DJ-1 is a member of the peptidase C56 family encoded
by the PARK7 gene, a PD-related pathogenic gene, which
can protect neurons from oxidative stress and cell death [5]. DJ-1 defciency is related to the development of PD [6]. De Miranda et al. [7] reported that overexpression of
DJ-1 protected against rotenone-induced neurotoxicity in
a rat model of PD. In addition, it has been reported that
DJ-1 can act as a regulator of autophagy in PD. Xu et al. [8] found that knockdown of DJ-1 aggravated α-synuclein
aggregation by inhibiting the activation of chaperonemediated autophagy and then promoted the development
of PD. Although many studies have investigated the role
of DJ-1 in PD, the molecular mechanism of DJ-1 in autophagy in PD is not completely understood.
MicroRNAs (miRNAs) are a class of noncoding RNAs
with a length between 18 and 25 nucleotides, which play
an important role in various cellular biology processes [9]. Recently, growing evidence has revealed that multiple miRNAs are involved in the development of PD.
For example, serum miR-221 can act as a PD biomarker [10], and miR-30e can regulate neuroinflammation in PD
by targeting Nlrp3 [11]. Moreover, several studies have
demonstrated that miRNAs can regulate autophagy in PD.
Wen et al. [12] showed that overexpression of miR-185
inhibited the autophagy of dopaminergic neurons by regulating the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) signaling pathway
in PD. MiR-24 is a well-known miRNA that is correlated
with tumorigenesis; abnormal miR-24 expression has
been found in many cancers [13]. In an expression profle
study, miR-24 was found to be highly expressed in PD [14]. However, its role in PD has not been reported. In addition, miR-24 is known to be related to autophagy. Tong
et al. [15] found that overexpression of miR-24 induced
autophagy by increasing the level of sirtuin 1 (SIRT1)
in uterine sarcoma. However, the relationship between
miR-24 and autophagy in PD is not clear. Bioinformatics
analysis with TargetScan shows that miR-24 has binding
sites with DJ-1. Therefore, we speculated that the role of
miR-24 in autophagy in PD might be related to DJ-1.
Rotenone, a plant-derived insecticide, can cause specifc
injury to dopaminergic neurons and induce Parkinson’s
disease–like symptoms [16]. In this study, we investigated
the mechanism of miR-24 in autophagy in PD using rotenone-induced injury of dopaminergic neurons in rats and
cultured cells, aiming to provide a possible target for the
prevention and treatment of PD.
Methods
Animals
Twenty-four adult Wistar rats (200-250 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All rats were housed under a 12/12-h light/dark cycle at 25 ± 2 °C. The study was approved by the Ethics Committee of Baotou Medical College.
PD rat model
The expressions of miR-152, MKK7 mRNA, and MKK7 mRNA in cells were detected using quantitative real-time PCR (qRT-PCR). Total RNAs were extracted from the tumor tissues and cells using Trizol reagent (Invitrogen, Waltham, MA, US). The TaqMan Reverse Transcription Kit (Applied Biosystems, Foster City, CA, US) was used to reversely transcribe RNAs into cDNAs. The qRT-PCR was conducted using the SYBR Premix Ex TaqTM II Kit (Takara, Dalian, Liaoning, China) on an ABI 7500 RealTime PCR system (Applied Biosystems, Waltham, MA, US). U6 was used as the internal control for miR-152, and GAPDH was used as the internal control for mRNAs. The relative expressions were calculated using the 2−ΔΔCt method.
Cell transfection
The PD rat model was established as previously described [17]. Briefly, rats received one subcutaneous injection of rotenone (in a dose of 1.5 mg/kg dissolved in dimethyl sulfoxide [DMSO]) every other day for a total of 21 days.
Animal grouping
The rats were divided into 4 groups of 6 rats: (1) Sham group: rats received one subcutaneous injection of DMSO (0.1 mL/100 g) every other day; (2) PD group: the model group; (3) miR-24 inhibitor group: rats were anesthetized by intraperitoneal injection of 6% chloral hydrate (6 mL/ kg). An adenovirus vector–mediated miR-24 inhibitor (5 μL; Genechem, Shanghai, China) was injected into the SN of rats at the following stereotactic coordinates: -5.2 mm from the bregma, 2.0 mm to the left of the midline, and 7.5 mm below the subdural matter [18]; (4) negative control (NC) group: rats were anesthetized by intraperitoneal injection of 6% chloral hydrate (6 mL/kg). Negative control of adenovirus vector–mediated miR-24 inhibitor (5 μL; Genechem) was injected into the SN of rats at the same site. After 4 weeks of injections, the rats in the miR-24 inhibitor group and the NC group were injected with rotenone in a dose of 1.5 mg/kg at the same site. At the end of the experiments, all rats underwent behavioral assessment using an open feld test and a rotarod test. They were subsequently euthanized, and their brains were obtained for further experiments.
Tyrosine hydroxylase (TH) staining
TH staining was used to evaluate the histopathological changes in rats. Briefly, tissue sections were pre-incubated for 30 min in 5% normal bovine serum and then incubated with diluted TH antibody (abcam, Cambridge, UK) overnight at 4 °C. The next day, the corresponding biotinylated secondary antibody was added and incubated for 1-2 h, then added avidin/biotin reagent (Dako, High Wycombe, UK) to continue incubation, and stained with diaminobenzidine-HCl (DAB).
Open feld test
The open feld test was performed as previously described [18]. A square wooden box (80 × 80 × 40 cm) with red walls and a white smooth polished floor divided by black lines into 16 equal squares of 4 × 4 cm was used. The rats were placed in the central area of the open field and allowed to freely explore it for 3 min. A video camera was fxed to the top of the box to record their movement and behavior for off-line analysis. Behavioral changes (i.e., latency, rearing, ambulation, and immobility time) were recorded.
Rotarod test
The rotarod test was performed using a rotary rod apparatus as previously described [18]. The rats were placed in the apparatus (diameter: 3 cm, height: 90 cm), which was operated at a constant speed of 10 rpm. Before the experiment, the rats were pre-trained for 3 days. After the open feld test, the animals were evaluated for a period of 5 min, and the time spent by each rat on the rod was recorded.
Cell culture
A dopaminergic neuronal cell line (MN9D cells) was purchased from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Dulbecco’s Modifed Eagle Medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin (Invitrogen, Waltham, MA, USA), and 100-μg/mL streptomycin (Invitrogen) and cultured in an incubator with a humidifed atmosphere of 95% air and 5% CO2 at 37 °C.
Cell treatment
MN9D cells were treated with rotenone to establish the in vitro PD model. The cells were exposed to different con centrations of rotenone (10, 20, 50, and 100 nM) for 24 h. For cell transfection, they were transfected with miR-24 inhibitor, miR-24 mimic, si-DJ-1, pcDNA-DJ-1, or their negative control using Lipofectamine 2000 (Invitrogen) for 24 h prior to 100 nM rotenone exposure.
MTT assay
MN9D cell viability was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were seeded into 96-well plates at a density of 1 × 104 cells/well and cultured for 24 h. They were then transfected with miR-24 inhibitor and/or si-DJ-1 for 24 h, followed by exposure to 100 nM rotenone for another 24 h. Next, the old medium was removed, and the MTT solution (0.5 mg/mL, 10 µL) was added into each well. Four hours later, the supernatants were removed, and 150 μL of DMSO was added into each well to dissolve the resultant formazan crystals. The optical density value was read at 490 nm using a microplate reader. Results are expressed as the percentage of MTT reduction, assuming the absorbance of the control cells to be 100%.
Quantitative real-time polymerase chain reaction
Total RNA was extracted from tissues or cells using TRIzol reagent (Invitrogen). Then, complementary DNA (cDNA) was synthesized using a PrimeScript One Step RT-PCR Kit (Takara Biotechnology, Dalian, China) following the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with an ABI PRISM 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The relative expression of miR-24, miR-30e, miR-29b-3p, miR-652, miR-1274, and DJ-1 mRNA was calculated using the 2− ΔΔCt method.
Western blot
Total protein was extracted from tissues and cells using radioimmunoprecipitation assay (RIPA) buffer. Then, an equal amount of total protein (10 μg) was separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (MilliporeSigma, Burlington, MA, USA). The membranes were frst blocked with 5% skim milk at room temperature for 1 h and then incubated with the primary antibodies overnight at 4 °C. After being washed three times with tris-buffered saline with tween (TBST), the membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 1 h. The immune-blot signals were developed using an enhanced chemiluminescence method. The anti-DJ-1 antibody, anti-LC3-I antibody, anti-LC3-II antibody, anti-Beclin 1 antibody, and anti-β-actin antibody (Cell Signaling Technology, Danvers, MA, USA) were used as the primary antibodies at 1:1000 dilutions.
Dual-luciferase reporter assay
DJ-1 three prime untranslated region (3’UTR) fragments containing wild type (WT) or mutant (Mut) miR- 24 binding sites were amplifed and cloned by PCR. The PCR products were inserted into a pMIR-REPORT-DJ- 1-3’UTR plasmid (Invitrogen). MN9D cells were seeded into a 24-well plate at a density of 1 × 105 cells/mL and cultured for 24 h. They were then transfected with a WT or Mut pMIR-DJ-1-3’UTR plasmid, along with miR-24 mimic or inhibitor using Lipofectamine 2000 (Invitrogen). Forty-eight hours later, the relative luciferase activity was analyzed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
All data were presented as means ± standard deviations and processed with GraphPad Prism 7.0. The difference between the two groups was analyzed using a Student’s t-test. The difference among more than two groups was analyzed by a one-way analysis of variance (ANOVA), followed by the Newman-Keuls post hoc test. A P-value of < 0.05 was considered statistically signifcant.
Results
MiR-24 expression increased in the brain tissues of PD rats
To investigate the relationship between miR-24 and PD, rats were divided into the Sham group and PD group. From the results of Tyrosine hydroxylase (TH) staining, compared with the Sham group, the number of TH-positive neurons in the PD group was signifcantly reduced. In addition, rats in the PD group had a signifcant deterioration in motor performance and coordination ( Figure 1A ). Then, we conducted a screening experiment for miRNAs. From the qRT-PCR results, we found that miR-24 expression was upregulated while miR-30e expression was downregulated in the PD group ( Figure 1B ). The expressions of miR-29b-3p, miR-652, and miR-1274 had no signifcant change in the brain tissues of PD rats compared with the Sham group ( Figure 1B ). The mechanism of miR-30e in PD has been reported [11]. but the mechanism of miR-24 in PD has not been reported. For this reason, we selected miR-24 for further study. We also measured the expressions of DJ-1, LC3-I, LC3-II, and Beclin 1 in the brain tissues of these rats. Compared with the Sham group, DJ-1 expression, the LC3-II/LC3-I ratio, and the protein level of Beclin 1 were downregulated in the brain tissues of PD rats ( Figure 1C ).
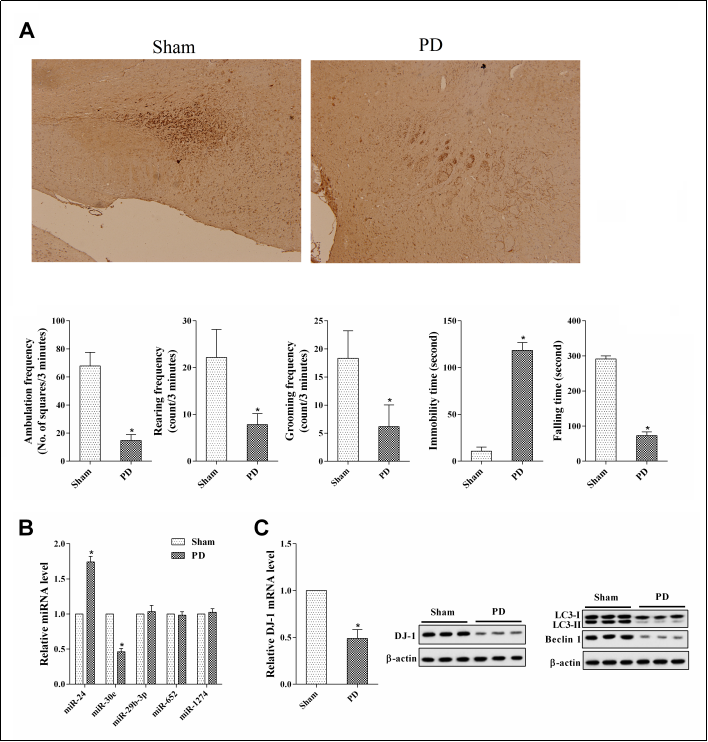
Figure 1. MiR-24 expression in the brain tissues of Parkinson’s disease (PD) rats. Rats were divided into a Sham group and a PD group of 6 rats each. (A) Tyrosine hydroxylase (TH) staining, Open feld test, and rotarod test. *P < 0.05, vs Sham. (B) The expressions of miR-24, miR-30e, miR-29b-3p, miR-652 and miR-1274 in the brain tissues of these rats. (C) The expressions of DJ-1, LC3-I, LC3-II, and Beclin 1 in the brain tissues of these rats. *P < 0.05, vs Sham.
Interfering with miR-24 alleviated rotenone-induced dopaminergic neuronal cell injury
To investigate whether miR-24 played an important role in rotenone-induced dopaminergic neuron injury, we frst investigated its expression in MN9D cells treated with different concentrations of rotenone (10, 20, 50, and 100 nM). As shown in Figure 2A, miR-24 expression was dose-dependently up-regulated after rotenone treatment, while DJ-1 expression was dose-dependently downregulated. Thus, we chose 100 nM of rotenone for further experiments. We divided the MN9D cells into Control, Rotenone, Rotenone + NC, and Rotenone + miR-24 inhibitor groups. The results showed that the miR-24 inhibitor offset the up-regulation of miR-24 expression and the down-regulation of DJ-1 expression induced by rotenone ( Figure 2B, C ). The results of the MTT assay showed that interfering with miR-24 changed the inhibition effect of rotenone on MN9D cell viability ( Figure 2D ).
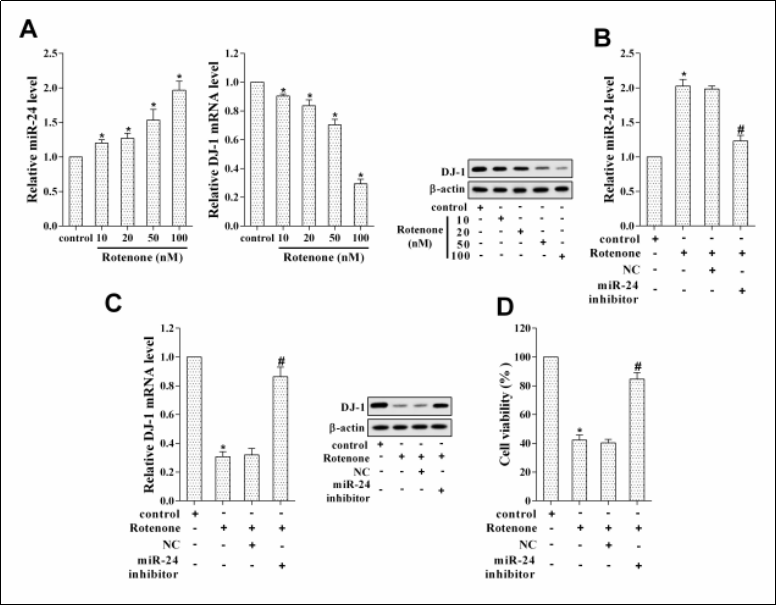
Figure 2. Interfering with miR-24 alleviated rotenone-induced dopaminergic neuronal cell injury. MN9D cells were treated with different concentrations of rotenone (10, 20, 50, and 100 nM). (A) The expressions of miR-24 and DJ-1. MN9D cells were divided into Control, Rotenone, Rotenone + negative control (NC), and Rotenone + miR-24 inhibitor groups. *P < 0.05, vs control. (B) MiR-24 expression. *P < 0.05, vs control. #P < 0.05, vs Rotenone + NC. (C) DJ-1 expression. *P < 0.05, vs control. #P < 0.05, vs Rotenone + NC. (D) MN9D cell viability. *P < 0.05, vs control. #P < 0.05, vs Rotenone + NC.
MiR-24 affected autophagy by targeting DJ-1 and rotenone affected dopaminergic neuronal cell injury through miR-24/DJ-1
To explore the mechanism of miR-24, we tried to investigate its target gene. Based on bioinformatics analysis with TargetScan, we found that DJ-1 was one of the putative target genes of miR-24 ( Figure 3A ). From the results of dual-luciferase reporter assay, miR-24 could negatively regulate the luciferase activity of DJ-1 ( Figure 3B ). To test whether miR-24 regulated DJ-1 expression, we measured DJ-1 expression upon the transfection of miR-24 inhibitor or mimic. The results of the qRT-PCR analysis suggested that the mRNA and protein levels of DJ-1 were significantly up-regulated after the transfection of miR- 24 inhibitor, while the transfection of miR-24 mimic produced the opposite effect ( Figure 3C ). Besides, to investigate the role of miR-24/DJ-1 in autophagy, we initially measured the protein levels of LC3-I, LC3-II, and Beclin 1 in MN9D cells treated with rotenone, miR-24 inhibitor, si-DJ-1, and RAPA (autophagy agonist). The results showed that miR-24 inhibitor offset the down-regulation of LC3-II/LC3-I ratio and Beclin 1 protein levels induced by rotenone, while si-DJ-1 could reverse the effect of miR-24 inhibitor ( Figure 3D ). As expected, RAPA, in turn, reversed the effect of si-DJ-1 ( Figure 3D ). Then, we detected the protein levels of LC3-I, LC3-II, and Beclin 1 in MN9D cells treated with miR-24 mimic, pcDNA-DJ-1, and 3-MA (autophagy inhibitor). As shown in Figure 3E, the overexpression of miR-24 could down-regulate the LC3-II/LC3-I ratio and Beclin 1 protein level, while the transfection of pcDNA-DJ-1 changed this trend. As expected, 3-MA, in turn, reversed the effect of pcDNADJ-1. In addition, in order to investigate the role of miR- 24/DJ-1 in rotenone-induced dopaminergic neuron injury, MN9D cells were treated with rotenone, miR-24 inhibitor, and si-DJ-1. MTT assay results revealed that rotenone reduced MN9D cell activity, while the transfection of miR- 24 inhibitor reversed the effect of rotenone ( Figure 3F ).After the transfection of si-DJ-1, the activity of MN9D cells was reduced again ( Figure 3F ).
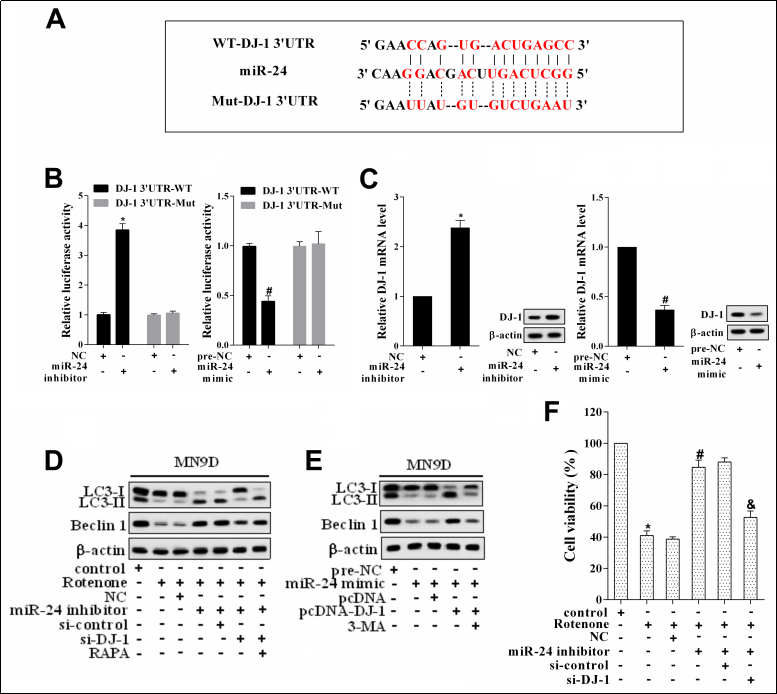
Figure 3. The role of miR-24 and DJ-1 in autophagy. (A) The binding sites between miR-24 and DJ-1 (TargetScan). (B) The relative luciferase activity. *P < 0.05, vs NC. #P < 0.05, vs pre-NC. (C) DJ-1 expression in MN9D cells transfected with miR-24 inhibitor or mimic. *P < 0.05, vs NC. #P < 0.05, vs pre-NC. (D) MN9D cells were divided into control, Rotenone, Rotenone + NC, Rotenone + miR-24 inhibitor, Rotenone + miR-24 inhibitor + si-control, Rotenone + miR-24 inhibitor + si-DJ-1, and Rotenone + miR-24 inhibitor + si-DJ-1 + RAPA group. The protein levels of LC3-I, LC3-II, and Beclin 1 were measured by western blot. (E) MN9D cells were divided into pre-NC, miR-24 mimic, miR-24 mimic + pcDNA, miR-24 mimic + pcDNA-DJ-1, and miR-24 mimic + pcDNA-DJ-1 + 3-MA group. The protein levels of LC3-I, LC3-II, and Beclin 1 were measured by western blot. (F) MN9D cell activity. *P < 0.05, vs control. #P < 0.05, vs Rotenone+NC. &P < 0.05, vs Rotenone+miR-24 inhibitor+si-control.
Interfering with miR-24 relieved nerve injury in PD rats
To verify the mechanism of miR-24 on nerve injury, we compared the NC and miR-24 inhibitor groups. Compared with the NC group, the rats in the miR-24 inhibitor group exhibited a signifcant deterioration in motor performance and coordination ( Figure 4A ). In addition, miR-24 expression was downregulated and DJ-1 expression was upregulated in the brain tissues of rats in the miR-24 inhibitor group ( Figure 4B, C ). The LC3-II/LC3-I ratio and the protein expression of Beclin 1 were increased in the brain tissues of rats in the miR-24 inhibitor group ( Figure 4D ).
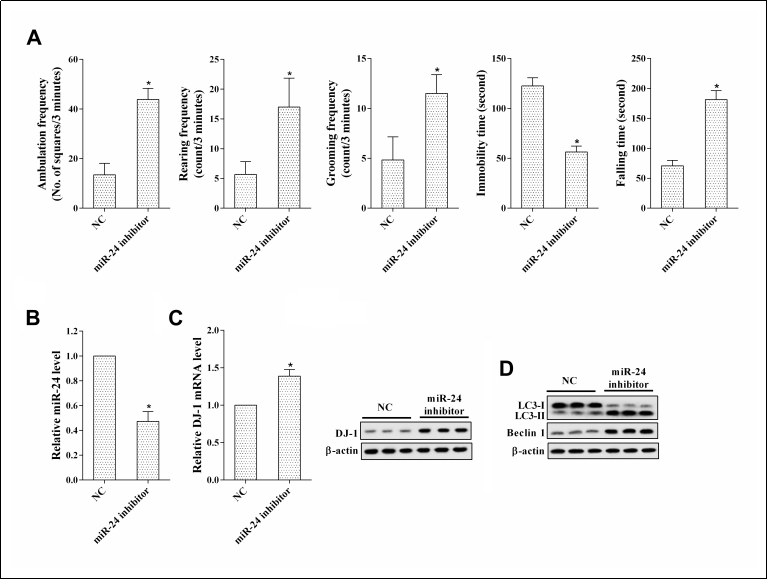
Figure 4. Interfering with miR-24 relieved nerve injury in PD rats. PD rats were divided into an NC group and a miR-24 inhibitor group of 6 rats each. (A) Open feld test and rotarod test. *P < 0.05, vs NC. (B) MiR-24 expression. *P < 0.05, vs NC. (C) DJ-1 expression. *P < 0.05, vs NC. (D) The protein levels of LC3-I, LC3-II, and Beclin 1.
Discussion
This study reveals a signifcant increase in miR-24 expression in the brain tissues of PD rats and rotenone-induced
dopaminergic neuronal cells. These results indicated that
miR-24 might be related to the occurrence of PD. This is
the frst study on the role of miR-24 in PD.
MiRNAs have been reported to regulate various biological
processes, including cell proliferation, apoptosis, and metastasis. Recently, miRNAs have been reported to regulate
the development of PD. Kim et al. [19] showed that miR-
126 contributed to PD by downregulating IGF-1/PI3K/
AKT signaling. Li et al. [20] reported that overexpression
of miR-221 promoted the viability and proliferation of
nerve cells in PD. In addition, miRNAs have been shown
to be involved in autophagy in PD. For instance, upregulation of miR-124 was found to regulate impaired autophagy processes in PD by suppressing Bim expression [21], and miR-7 was found to promote autophagy in PD by reducing α-synuclein levels [22]. Our study indicated
that interfering with miR-24 alleviated rotenone-induced
dopaminergic neuronal cell injury, and the effect of miR-
24 might be related to autophagy.
Autophagy is crucial in cell differentiation, proliferation,
death, and response to environmental stress. It plays an
important role in preventing certain diseases, such as cancer and neurodegenerative diseases, resisting infection by
pathogenic microorganisms, and delaying aging, prolonging life span [23]. DJ-1 is an important protective protein,
which plays a neuroprotective role in many reactions. For
example, overexpression of DJ-1 was found to increase
cell viability and decrease cell sensitivity to oxidative
stress through the ERK1/2 signaling pathway [24]. In PD,
it has been reported that DJ-1 reduces the neurotoxicity of rotenone on dopaminergic neurons by enhancing
autophagy [25]. This is in line with the findings of our
study. Moreover, our results showed that the role of DJ-1
in enhancing autophagy was related to miR-24. As miR-
24 negatively regulated DJ-1 expression, interfering with
it enhanced autophagy by increasing DJ-1 expression and
then relieved rotenone-induced dopaminergic neuronal
cell injury. The PD rat model confrmed these fndings.
In conclusion, our in vitro and in vivo experiments revealed the role of miR-24/DJ-1 in autophagy in PD and
demonstrated that interfering with miR-24 alleviated rotenone-induced dopaminergic neuron injury via enhancing
autophagy by upregulating DJ-1, which had the potential
to be a biomarker for the prevention and treatment of PD.
Declaration
Funding
This study was supported by the Natural science foundation of Inner Mongolia autonomous region (no. 2017MS0301).
Conflicts of interest
The authors declare that they have no conflict of interest.
References
1. Hirsch L, Jette N, Frolkis A, et al. The incidence of Parkinson's disease: a systematic review and meta-analysis. Neuroepidemiology, 2016, 46(4): 292-300.
2. Lashuel H A, Overk C R, Oueslati A, et al. The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nature Reviews Neuroscience, 2013, 14(1): 38.
3. Karabiyik C, Lee M J, Rubinsztein D C. Autophagy impairment in Parkinson’s disease. Essays in biochemistry, 2017, 61(6): 711-720.
4. Moors T E, Hoozemans J J M, Ingrassia A, et al. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Molecular neurodegeneration, 2017, 12(1): 11.
5. Saito, Y. DJ-1 as a Biomarker of Parkinson's Disease. Adv Exp Med Biol, 2017, 1037: 149-171.
6. Choi D J, Eun J H, Kim B G, et al. AP arkinson's disease gene, DJ-1, repairs brain injury through S ox9 stabilization and astrogliosis. Glia, 2018, 66(2): 445-458.
7. De Miranda B R, Rocha E M, Bai Q, et al. Astrocytespecific DJ-1 overexpression protects against rotenoneinduced neurotoxicity in a rat model of Parkinson's disease. Neurobiology of disease, 2018, 115: 101-114.
8. Xu C Y, Kang W Y, Chen Y M, et al. DJ-1 inhibits alphaSynuclein aggregation by regulating chaperone-mediated autophagy. Frontiers in aging neuroscience, 2017, 9: 308.
9. Zhang L, Lu Y, Zhang L. MiR-449b inhibits the migration and invasion of colorectal cancer cells through the negative regulation of MMP2. Clinical Surgery Research Communications, 2018, 2(3): 27-33.
10. Ma W, Li Y, Wang C, et al. Serum miR-221 serves as a biomarker for Parkinson's disease. Cell biochemistry and function, 2016, 34(7): 511-515.
11. Li D, Yang H, Ma J, et al. MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Human cell, 2018, 31(2): 106-115.
12. Wen Z, Zhang J, Tang P, et al. Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson's disease. Molecular medicine reports, 2018, 17(1): 131-137.
13. Kang H, Rho J G, Kim C, et al. The miR-24-3p/p130Cas: a novel axis regulating the migration and invasion of cancer cells. Scientific reports, 2017, 7: 44847.
14. Vallelunga A, Ragusa M, Di Mauro S, et al. Identification of circulating microRNAs for the differential diagnosis of Parkinson's disease and Multiple System Atrophy. Frontiers in cellular neuroscience, 2014, 8: 156.
15. Tong X, Wang X, Wang C, et al. Elevated levels of serum MiR-152 and miR-24 in uterine sarcoma: potential for inducing autophagy via SIRT1 and deacetylated LC3. British journal of biomedical science, 2018, 75(1): 7-12.
16. Barnum C J, Tansey M G. Neuroinflammation and nonmotor symptoms: the dark passenger of Parkinson’s disease?. Current neurology and neuroscience reports, 2012, 12(4): 350-358.
17. Abdelkader N F, Safar M M, Salem H A. Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of Parkinson’s disease: modulation of mitochondrial perturbations. Molecular neurobiology, 2016, 53(2): 810-817.
18. Gao H, Yang W, Qi Z, et al. DJ-1 protects dopaminergic neurons against rotenone-induced apoptosis by enhancing ERK-dependent mitophagy. Journal of molecular biology, 2012, 423(2): 232-248.
19. Kim W, Lee Y, McKenna N D, et al. miR-126 contributes to Parkinson's disease by dysregulating the insulin-like growth factor/phosphoinositide 3-kinase signaling. Neurobiology of aging, 2014, 35(7): 1712-1721.
20. Li L, Xu J, Wu M, et al. Protective role of microRNA-221 in Parkinson's disease. Bratislavske lekarske listy, 2018, 119(1): 22-27.
21. Wang H, Ye Y, Zhu Z, et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of P arkinson's Disease by Targeting to B im. Brain pathology, 2016, 26(2): 167-176.
22. Choi D C, Yoo M, Kabaria S, et al. MicroRNA-7 facilitates the degradation of alpha-synuclein and its aggregates by promoting autophagy. Neuroscience letters, 2018, 678: 118-123.
23. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell, 2008, 132(1): 27-42.
24. Gu L, Cui T, Fan C, et al. Involvement of ERK1/2 signaling pathway in DJ-1-induced neuroprotection against oxidative stress. Biochemical and biophysical research communications, 2009, 383(4): 469-474.
25. Hui-Feng L , Wei-Wei Y , Hua G , et al. DJ-1 alleviates rotenone-induced cell injuries via enhancing autophaty in MN9D cells. Basic & Clinical Medicine, 2013, 33(6):648- 654.