Open Access | Original article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Artesunate Improves Drug Resistance of Lung Carcinomas Via Regulation of MiR-493-5p
*Corresponding author: Zhi-qiao Xu
Mailing address: Department of tumor center, Kaifeng central
hospital, Hejie road no.85, Kaifeng, Henan, 475000, China.
E-mail: esc7312@163.com
Received: 20 April 2018 Accepted: 26 June 2018
DOI: 10.31491/CSRC.2018.6.015
Abstract
Objective: The aim of this study was to explore the potential involvement of the miR-493-5p/BRCA1 pathway in the anti-cell drug resistance activity of artesunate in lung carcinomas.
Methods: Two drug-resistant lung carcinoma cell lines, A549 and SBC-3, resistant to cisplatin (DDP) and adriamycin (ADM), respectively, and their parental cell lines (A549 and SBC-3) were used in this study. Cell viability was determined by an MTT assay. The relative expression of miR-493-5p and BRCA1 mRNA was analyzed by quantitative real-time PCR. The protein expression of BRCA1, breast cancer resistance protein (BCRP), and (p)PI3K/AKT was determined by a Western blot.
Results: Artesunate attenuated the viability of A549/DDP and SBC-3/ADM cells and suppressed the expression of BCRP, an important multidrug resistance protein in lung carcinoma cells, accompanied by upregulation of miR-493-5p and downregulation of BRCA1. In artesunate-treated A549/DDP and SBC-3/ADM cells, miR- 493-5p silencing reversed the anti-cell drug resistance activity of artesunate and resulted in increased cell viability and BRCP expression. Simultaneously, miR-493-5p silencing contributed to BRCA1 upregulation and phosphorylation of PI3K and AKT. As shown by a luciferase reporter gene assay, BRCA1 was a target gene for miR-493-5p, and its expression was negatively regulated by miR-493-5p. miR-493-5p silencing reversed the anticell drug resistance activity of artesunate, and this finding was further supported by a murine tumorbearing model.
Conclusion: The data support the anti-cell drug resistance activity of artesunate in lung carcinoma cells and sheds light on the role of the miR-493-5p/BRCA1 pathway in this process.
Keywords
Artesunate; BRCA1; drug resistance, miR-493-5p; lung carcinoma
Introduction
Lung carcinomas are a leading cause of cancer-related
deaths worldwide [1]. According to data from the National Cancer Center of China, among all types of cancer
in China, lung cancer is responsible for the highest morbidity and mortality. The treatment of lung carcinomas
is hampered by the ineffectiveness of current therapy,
leading to a poor prognosis [2]. Multidrug resistance of
lung carcinoma cells remains a major problem that has
yet to be resolved [2].
Antitumor agents for cancers have increasingly attracted attention, particularly studies of Chinese traditional
plant compounds. One such agent is artesunate, which
has antineoplasmic activity. Artesunate belongs to drug
monomers and is derived from the leaves of Artemisia
annua L (also known as qinghao or sweet wormwood),
a plant used in traditional Chinese medicine [3] and renowned globally for its antimalaria activity [4]. Recently,
artesunate has been recognized as an anticancer agent
in various cancers, such as breast cancer [5], cervical
cancer [6], and lung cancer [7]. In addition, accumulating
evidence points to anti-tumor cell drug resistance activity of artesunate in prostate cancer [8], head and neck
cancer [9], and multiple myelomas [10]. However, to date,
there is no direct evidence of an effective role of artesunate in multidrug resistance of lung carcinoma. MicroRNAs (miRNAs) are a class of small noncoding RNAs with
a length of ~22 nucleotides. Although they have no protein-coding ability, miRNAs are attracting widespread
attention because of their regulatory role in functional
protein expression via post-transcription modification
processing. Unexpectedly, in previous studies, miRNAs
interplaying with critical proteins developed into a molecular mechanism for drug resistance of lung carcinomas [11-13]. miR-493 has been recently recognized as an
antitumor miRNA in breast cancer [14], gastric cancer [15], colon cancer [16], and lung cancer [17]. Research also
showed that miR-493 was involved in regulating chemoresistance of gastric cancer cells [18].
We previously investigated the role of artesunate in drug resistance of lung carcinoma cells and explored the potential involvement and downstream pathway of miR-493 in this process. Drug resistant-associated proteins regulated by miRNAs underlie the regulatory mechanism of drug resistance of cancer in various cancers [11]. The aim of this study was to determine the possibly regulated BRCA1, a tumor suppressor gene and drug-resistance related gene, in lung carcinomas [19], by miR-493 in artesunate-treated lung carcinoma cells.
Materials and Methods
Cell culture and treatment
Two drug-resistant lung carcinoma cell lines, A549/ DDP (resistant to cisplatin) and SBC-3/ADM (resistant to adriamycin), and their parental cell lines, A549 and SBC-3 (obtained from ATCC) were cultured in RPMI- 1640 medium, supplemented with 10% fetal calf serum and 1% penicillin–streptomycin solution. A549/ DDP and SBC-3/ADM were established by A549 and SBC-3 cells step by step and treated with increasing concentrations of DDP or ADM. The cells were maintained in a humidified atmosphere at 37° C with 5% CO2 , and the medium was replaced with new medium every 2–3 days.
To evaluate the cell cytotoxic activity of DDP or ADM, the A549/DDP and A549 cells were exposed to DPP at concentrations of 12.5, 25, 50, 75, and 100 µM, and the SBC-3/ADM and SBC-3 cells were exposed to ADM at concentrations of 0.05, 0.1, 0.5, 1.0, and 2.0 µM. The cells were treated with combination treatment (25 µM DPP and 1.0 µM ADM) and different doses of artesunate (7.5, 15, and 30 µM) or 15 µM artesunate (A549/ DDP and SBC-3/ADM cells).
MTT assay
An MTT assay was performed to evaluate the cytotoxic activity of DDP, ADM, and artesunate. The cells (1 × 104/ml) were seeded into a 96-well plate, and different doses of DPP, ADM, or artesunate were added. The cells were cultured for 48 h and added with an MTT reaction kit. After incubation for 4 h, the reaction in each well was stopped. The generated formazan crystals of samples were resolved by wells to which 150 µl/ of dimethyl sulfoxide (DMSO) was added per well. The OD value of each dissolution mixture was examined at 570 nm by a microplate reader.
Western blot analysis of the protein expression of breast cancer resistance protein (BCRP), BRCA1, PI3K/ AKT, and (p)PI3K/AKT
Cells or tumor tissues were collected for total protein extraction with RIPA lysis buffer, supplemented with protease inhibitor. Protein samples were quantified by the bicinchoninic acid method. Subsequently, an equal quantity of protein from all the protein samples was subjected to SDS-polyacrylamide gel electrophoresis. The proteins in the gel were then transferred to polyvinylidene fluoride membrane (Millipore), followed by incubation with primary antibodies against BCRP, BRCA1, PI3K/AKT, and (p)PI3K/AKT at 4° C overnight. The next day, the membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Finally, target genes were visualized using an ECL Western blotting substrate kit. The electrophoretic band of β-actin was regarded as an internal control.
Quantitative real-time PCR (qRT-PCR) determination of the relative expression of miR-493-5p and BRCA1 mRNA
Cells or tumor tissues were collected for total RNA isolation using an RNA extraction kit (Tiangen, Beijing, China), according to the manufacturer’s protocol. RNAs were subjected to reverse transcription using an miRNA First Strand Synthesis Kit (Takara, Dalian, China) or PrimeScript™ RT Master Mix (Takara, Dalian, China). For the real-time PCR reaction, the cDNA product was amplified by SYBR® Premix Ex TaqTM II (Takara, Dalian, China) using an Applied Biosystems 7300 Fast Real-Time PCR System. The U6 gene or GAPDH was used as an endogenous control.
Luciferase reporter gene assay
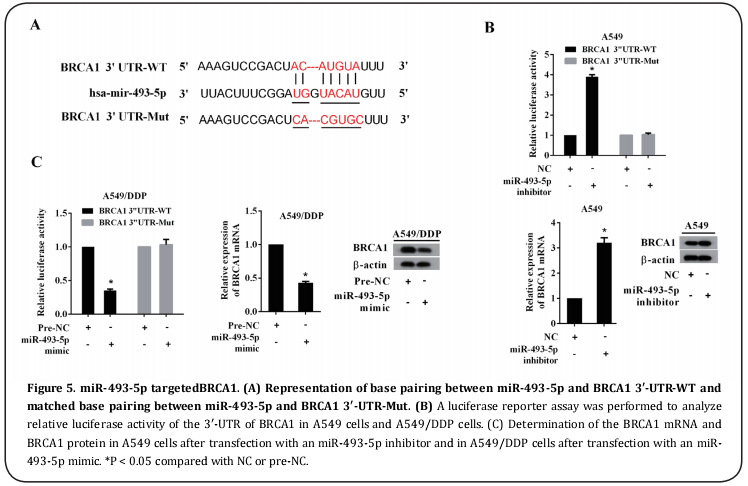
Basing on the predicted pairing bases between the BRCA1 3’-untranslated region (3’-UTR) and miR-493- 5p, the BRCA1 3′-UTR was amplified and packaged into a luciferase vector forming a BRCA1 3’-UTR-WT (wild type) recombinant. A fragment of mutant pairing bases of BRCA1 3’-UTR was constructed into a BRCA1 3’-UTRMut (mutant type) recombinant and regarded as a positive control. To determine the regulatory role of miR- 493-5p in BRCA1 3′-UTR activity, a BRCA1 3′-UTR-WT recombinant or BRCA1 3′-UTR-Mut recombinant was co-transfected with an miR-439-5p inhibitor or miR- 439-5p mimic into A549 cells or A549/DDP cells. After 48 h transfection, the cells were harvested for luciferase activity analysis.
Murine tumor-bearing model
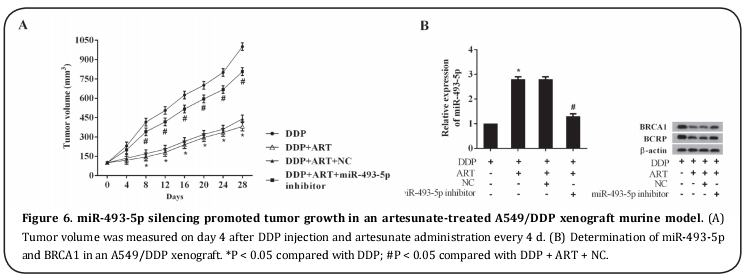
BALB/c nude mice (6 weeks) were subcutaneously injected with 2 × 106 A549/DPP cells and randomly divided into four groups according to drug administration (DDP, DDP + artesunate, DDP + artesunate + negative control (NC), DDP + artesunate + lentivirus (LV) miR-493-5p inhibitor). After the cell injection, tumor volume was measured every 4 d. Drug administration commenced when the tumor volume was 100 mm3 . DDP was delivered via an intraperitoneal injection, and artesunate was delivered via the oral route. A lentivirus constructed miR-493-5p inhibitor (LV-miR-493-5p inhibitor) was administrated via an intravenous injection through the tail. Tumor volume was measured continuously for 4 wk. The animals were then sacrificed, and tumor tissues were removed for determination of miR-493-5p and BRCA1 expression.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 software and version 15.0 SPSS software. Data were analyzed by a one-way analysis of variance or the student’s t test and represented as mean±SD. A twotailed P value less than 0.05 was considered statistically significant.
Results
Artesunate improved the drug resistance of lung carcinoma cells
First, we evaluated the degree of drug resistance of two drug-resistant lung carcinoma cells lines, A549/DDP and SBC-3/ADM. The results revealed that A549/DDP and SBC-3/ADM showed resistance to DDP and ADM and that they exhibited higher cell viability than paired nondrug-resistant cells (A549 and SBC-3) at different drug doses (Figure 1A). The drug resistance of A549/ DDP to DDP and SBC-3/ADM to ADM was attenuated by artesunate in a dose-dependent manner (Figure 1B). In addition, the protein level of BCRP, an imporant multidrug resistance protein in lung carcinoma cells [20] decreased in different doses of artesunate-treated A549/DDP and SBC-3/ADM cells, pointing to the anti-cell drug resistance activity of artesunate in lung carcinoma cells.
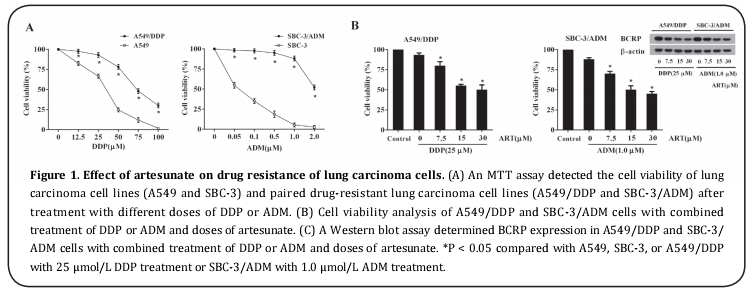
Artesunate contributed to upregulation of miR-493-5p and downregulation of BRCA1 in A549/DDP and SBC- 3/AD cells
The potential involvement of miR-493-5p and BRCA1 in the anti-cell drug resistance activity of artesunate was determined by examination of their expression levels in artesunate-treated A549/DDP and SBC-3/ADM cells. As shown in Figure 2A, the relative expression level of miR-493-5p gradually increased and that of BRCA1 mRNA decreased in accordance with an increase in the dose of artesunate. Similar to BRCA1 mRNA, the expression level of the BRCA1 protein in A549/DDP and SBC-3/ADM cells was also reduced, depending on the artesunate dose administered (Figure 2B).
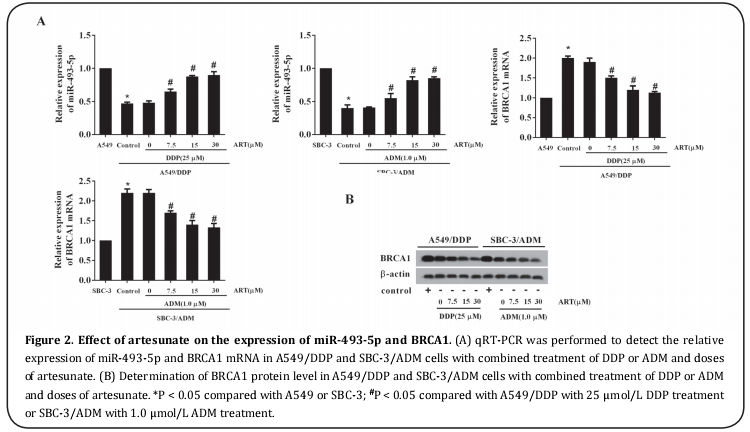
miR-493-5p silencing reversed the anti-cell drug resistance activity of artesunate
To confirm the regulatory role of miR-493-5p in the anti-cell drug resistance activity of artesunate, we explored the effect of miR-493-5p silencing on the viability of A549/DDP and SBC-3/ADM cells and found that miR-493-5p silencing reversed the anti-cell drug resistance activity of artesunate and restored cell viability (Figure 3A). Furthermore, BCRP expression was upregulated in miR-493-5p inhibitor-transfected A549/DDP and SBC-3/ADM cells (Figure 3B).
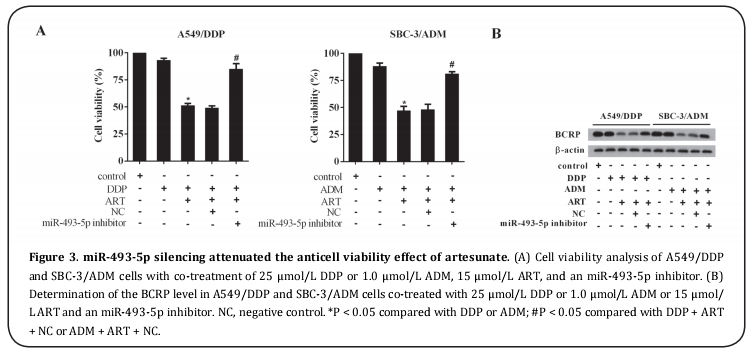
miR-493-5p silencing was negatively correlated with the upregulation of BRCA1 pathway
Next, we investigated the potentiating effect of miR- 493-5p on the BRCA1 pathway in artesunate-treated A549/DDP and SBC-3/ADM cells. miR-493-5p silencing resulted in upregulation of BRCA1 mRNA and the BRCA1 protein (Figs 4A and B). As the PI3K/AKT path-way is a downstream pathway for BRCA1 in drug resistance of NSCLC cell lines [20], we determined the phosphorylated level of PI3K and AKT in artesunate-A549/ DDP and SBC-3/ADM cells. The results revealed that artesunate inhibited the phosphorylation of PI3K and AKT. Notably, the inhibitory effect of artesunate on PI3K and AKT phosphorylation was alleviated by miR- 493-5p silencing, suggesting the responding BRCA1/ PI3K/AKT pathway to miR-493-5p in anti-cell drug resistance activity of artesunate (Figure 4B).
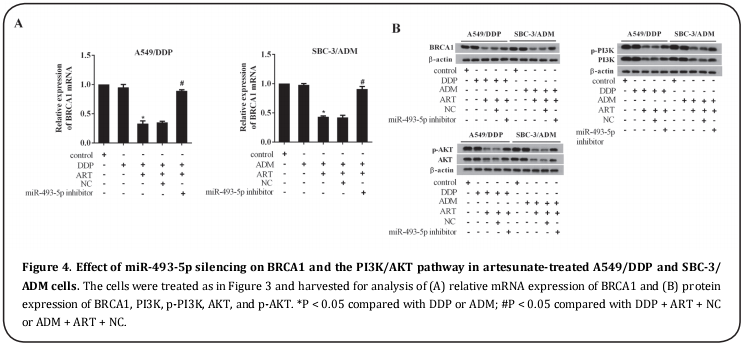
miR-493-5p directly targeted BRCA1
Figure 5A shows the base pairing between miR-493-5p and BRCA1 3′-UTR, indicating potential direct binding between them. The results of the luciferase reporter gene assay demonstrated the regulatory role of miR- 493-5p in BRCA1 3′-UTR activity in A549 and A549/ DDP cells (Figure 5B). Consequently, BRCA1 mRNA and BRCA1 protein expression were enhanced by miR-493- 5p silencing in A549 cells and inhibited by miR-493-5p overexpression (Figure 5C).
Determination of the effect of miR-493-5p silencing on tumor growth in tumor-bearing mice following artesunate administration
The effect of miR-493-5p silencing on the anti-cell drug resistance activity of artesunate was studied in an A549/DDP xenograft mice model by observing tumor growth (Figure 6A). Compared with DDP, artesunate treatment suppressed tumor volume, and the inhibitory effect of artesunate on tumor growth was substantially weakened in an LV-miR-493-5p inhibitor tumor xenograft model. In accordance with the results of the cell experiments, the analysis of miR-493-5p and BRCA1 in tumor xenografts showed that miR-493-5p was upregulated in mice with artesunate administration and downregulated in LV-miR-493-5p inhibitor tumor xenografts. The BRCA1 protein level was lower in artesunate-treated tumors and higher when miR-493-5p was silenced (Figure 6B).
Discussion
ADM- and DDP-based chemotherapies are commonly used clinically for pulmonary malignant tumors. However, resistance of cancer cells to these agents results in treatment failure [21]. Recently, Chinese traditional medicines have attracted attention as effective antitumor agents for various cancers. The antitumor activity of artesunate has been demonstrated in a wide range of cancers [22]. The present study showed that artesunate treatment resulted in an effective improvement in the cell drug resistance of lung carcinoma to ADM and DDP. Thus, artesunate can be regarded as an effective anti-cell drug resistance agent.
Evidences pointing to the tight relationship between miRNAs and carcinogenesis promote the investigation of mechanism by which miRNAs mediate multi-drug resistance of cancer cells [23]. The data in the present study suggested that miR-493, a recently identified antitumor miRNA [24,25], mediated the process of anti-cell drug resistance of lung carcinoma cells by artesunate. Previous research reported that miR-493 was downregulated in several cancers. Accordingly, upregulation of miR-493 by vector-mediated gene transfection contributed to inhibition of proliferation and metastasis of gastric cancer cells [26] and impairment of cancer cell growth and migration of lung cancer [17] and bladder cancer [25]. Our data suggested that miR-493-5p was increasingly expressed in the process of anti-cell drug resistance of lung carcinoma cells by increased concentrations of artesunate in both A549/DDP and SBC-3/ ADM cells, pointing to the involvement of miR-493 in the anti-cell drug resistance activity of artesunate. This idea was confirmed by observation of the reversal effect of miR-493-5p silencing on cell viability protection of artesunate. These data confirmed the anti-chemoresistant role of miR-493 [15].
We also observed downregulation of BRCA1, accompanied by miR-493-5p upregulation, in artesunate-treated A549/DDP and SBC-3/ADM cells. Ectopic expression of BRCA1, a gene conferring susceptibility to early-onset breast and ovarian cancer [27] and a tumor-suppressive protein [28], was recently reported in patients with metastatic non-small cell lung cancer who received cisplatin-based chemotherapy [19]. Our data also revealed upregulation of BRCA1 mRNA and the BRCA1 protein, as well as an increase in the phosphorylation of PI3K/AKT, a known downstream pathway for BRCA1 [20], in artesunate-treated A549/DDP and SBC-3/ADM cells when miR-493 was silenced. These data pointed to the possible regulatory mechanism of miR-493 in the BRCA1 pathway. Control modes of miRNA for gene expression mainly focus on the directly complementary combination of them to target genes transcript. Data from a bioinformatics analysis suggested that BRCA1 may be a potential target gene for miR-493-5p. The results of the luciferase reporter gene assay confirmed this idea. Based on these data, we conclude that BRCA1/PI3K/AKT signals serve as a downstream pathway for miR-493 and that together they form a molecular mechanism by which artesunate exhibits anti-cell drug resistance activity against lung carcinomas.
In summary, this study is the first to demonstrate the anti-cell drug resistance activity of artesunate against lung carcinoma cells and to shed light on the potential molecular mechanism underlying this activity, which involves miR-493-5p/BRCA1 and the downstream PI3K/AKT pathway. The findings provide evidence for the use of artesunate as a treatment for lung carcinomas.
References
1. Sousa, V., Reis, D., Silva, M., Alarcão, A. M., Ladeirinha, A.
F., D’Aguiar, M. J., Ferreira, T., Caramujo-Balseiro, S., and
Carvalho, L. (2016) Amplification of FGFR1 gene and expression of FGFR1 protein is found in different histological types of lung carcinoma. Virchows Archiv 469, 1-10
2. Mateen, S., Raina, K., and Agarwal, R. (2013) Chemopreventive and anti-cancer efficacy of silibinin against
growth and progression of lung cancer. Nutrition & Cancer 65 Suppl 1, 3
3. Meshnick, S. R., Taylor, T. E., and Kamchonwongpaisan, S.
(1996) Artemisinin and the antimalarial endoperoxides:
from herbal remedy to targeted chemotherapy. Microbiological reviews 60, 301-315
4. Pan, H. Z., Lin, F. B., and Zhang, Z. A. (1989) Effect of sodium artesunate on malaria infected human erythrocytes.
Proceedings of the Chinese Academy of Medical Sciences and the Peking Union Medical College = Chung-kuo i
hsueh k'o hsueh yuan, Chung-kuo hsieh ho i k'o ta hsueh
hsueh pao 4, 181-185
5. Bachmeier, B., Fichtner, I., Killian, P. H., Kronski, E., Pfeffer, U., and Efferth, T. (2011) Development of resistance
towards artesunate in MDA-MB-231 human breast cancer cells. PloS one 6, e20550
6. Thanaketpaisarn, O., Waiwut, P., Sakurai, H., and Saiki, I.
(2011) Artesunate enhances TRAIL-induced apoptosis in
human cervical carcinoma cells through inhibition of the NF-kappaB and PI3K/Akt signaling pathways. International journal of oncology 39, 279-285
7. Ma, H., Yao, Q., Zhang, A. M., Lin, S., Wang, X. X., Wu, L.,
Sun, J. G., and Chen, Z. T. (2011) The effects of artesunate
on the expression of EGFR and ABCG2 in A549 human
lung cancer cells and a xenograft model. Molecules 16,
10556-10569
8. Nunes, J. J., Pandey, S. K., Yadav, A., Goel, S., and Ateeq,
B. (2017) Targeting NF-kappa B Signaling by Artesunate
Restores Sensitivity of Castrate-Resistant Prostate Cancer Cells to Antiandrogens. Neoplasia 19, 333-345
9. Roh, J. L., Kim, E. H., Jang, H., and Shin, D. (2017) Nrf2
inhibition reverses the resistance of cisplatin-resistant
head and neck cancer cells to artesunate-induced ferroptosis. Redox biology 11, 254-262
10. Papanikolaou, X., Johnson, S., Garg, T., Tian, E., Tytarenko,
R., Zhang, Q., Stein, C., Barlogie, B., Epstein, J., and Heuck, C. (2014) Artesunate overcomes drug resistance in
multiple myeloma by inducing mitochondrial stress and
non-caspase apoptosis. Oncotarget 5, 4118-4128
11. An, X., Sarmiento, C., Tan, T., and Zhu, H. (2017) Regulation of multidrug resistance by microRNAs in anti-cancer
therapy. Acta pharmaceutica Sinica. B 7, 38-51
12. Fang, C., Chen, Y. X., Wu, N. Y., Yin, J. Y., Li, X. P., Huang, H.
S., Zhang, W., Zhou, H. H., and Liu, Z. Q. (2017) MiR-488
inhibits proliferation and cisplatin sensibility in nonsmall-cell lung cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling pathway. Scientific reports
7, 40384
13. Meng, F., Wang, F., Wang, L., Wong, S. C., Cho, W. C., and
Chan, L. W. (2016) MiR-30a-5p Overexpression May
Overcome EGFR-Inhibitor Resistance through Regulating PI3K/AKT Signaling Pathway in Non-small Cell Lung
Cancer Cell Lines. Frontiers in genetics 7, 197
14. Zhao, L., Feng, X., Song, X., Zhou, H., Zhao, Y., Cheng, L.,
and Jia, L. (2016) miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4. Oncology reports 36, 1007-1015
15. Jia, X., Li, N., Peng, C., Deng, Y., Wang, J., Deng, M., Lu, M.,
Yin, J., Zheng, G., Liu, H., and He, Z. (2016) miR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer cells. Oncotarget 7, 7044-7054
16. Sakai, H., Sato, A., Aihara, Y., Ikarashi, Y., Midorikawa, Y.,
Kracht, M., Nakagama, H., and Okamoto, K. (2014) MKK7
mediates miR-493-dependent suppression of liver metastasis of colon cancer cells. Cancer science 105, 425430
17. Gu, Y., Cheng, Y., Song, Y., Zhang, Z., Deng, M., Wang, C.,
Zheng, G., and He, Z. (2014) MicroRNA-493 suppresses
tumor growth, invasion and metastasis of lung cancer by
regulating E2F1. PloS one 9, e102602
18. Tambe, M., Pruikkonen, S., Maki-Jouppila, J., Chen, P.,
Elgaaen, B. V., Straume, A. H., Huhtinen, K., Carpen, O.,
Lonning, P. E., Davidson, B., Hautaniemi, S., and Kallio,
M. J. (2016) Novel Mad2-targeting miR-493-3p controls
mitotic fidelity and cancer cells' sensitivity to paclitaxel.
Oncotarget 7, 12267-12285
19. Papadaki, C., Sfakianaki, M., Ioannidis, G., Lagoudaki, E.,
Trypaki, M., Tryfonidis, K., Mavroudis, D., Stathopoulos,
E., Georgoulias, V., and Souglakos, J. (2012) ERCC1 and
BRAC1 mRNA expression levels in the primary tumor
could predict the effectiveness of the second-line cisplatin-based chemotherapy in pretreated patients with
metastatic non-small cell lung cancer. Journal of thoracic
oncology : official publication of the International Association for the Study of Lung Cancer 7, 663-671
20. Galetti, M., Petronini, P. G., Fumarola, C., Cretella, D., La
Monica, S., Bonelli, M., Cavazzoni, A., Saccani, F., Caffarra,
C., Andreoli, R., Mutti, A., Tiseo, M., Ardizzoni, A., and Alfieri, R. R. (2015) Effect of ABCG2/BCRP Expression on
Efflux and Uptake of Gefitinib in NSCLC Cell Lines. PloS
one 10, e0141795
21. Xin, Y., Yin, F., Qi, S., Shen, L., Xu, Y., Luo, L., Lan, L., and
Yin, Z. (2013) Parthenolide reverses doxorubicin resistance in human lung carcinoma A549 cells by attenuating NF-kappaB activation and HSP70 up-regulation. Toxicology letters 221, 73-82
22. Efferth, T. (2017) From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in cancer biology
23. Chen, Y., Gao, Y., Zhang, K., Li, C., Pan, Y., Chen, J., Wang,
R., and Chen, L. (2015) MicroRNAs as Regulators of Cisplatin Resistance in Lung Cancer. Cellular physiology
and biochemistry : international journal of experimental
cellular physiology, biochemistry, and pharmacology 37,
1869-1880
24. Lehmann, U., Streichert, T., Otto, B., Albat, C., Hasemeier, B., Christgen, H., Schipper, E., Hille, U., Kreipe, H. H.,
and Langer, F. (2010) Identification of differentially expressed microRNAs in human male breast cancer. BMC
cancer 10, 109
25. Ueno, K., Hirata, H., Majid, S., Yamamura, S., Shahryari,
V., Tabatabai, Z. L., Hinoda, Y., and Dahiya, R. (2012) Tumor suppressor microRNA-493 decreases cell motility
and migration ability in human bladder cancer cells by
downregulating RhoC and FZD4. Molecular cancer therapeutics 11, 244-253
26. Zhou, W., Zhang, C., Jiang, H., Zhang, Z., Xie, L., and He,
X. (2015) MiR-493 suppresses the proliferation and invasion of gastric cancer cells by targeting RhoC. Iranian
journal of basic medical sciences 18, 1027-1033
27. Abel, K. J., Xu, J., Yin, G. Y., Lyons, R. H., Meisler, M. H., and
Weber, B. L. (1995) Mouse Brca1: localization sequence
analysis and identification of evolutionarily conserved
domains. Human molecular genetics 4, 2265-2273
28. Turner, J. G., Dawson, J., and Sullivan, D. M. (2012) Nuclear export of proteins and drug resistance in cancer. Biochemical pharmacology 83, 1021-1032Figures legends