Open Access | Research article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Radiotherapy Alters the Polarization of Tumor-associated Macrophage to Suppress Lung Cancer Progression via Up-regulation of LincRNA-p21
*Corresponding author: Wan-ru Geng
Mailing address: Department of Blood tumor, Affiliated Hospital
of Inner Mongolia University for Nationalities, Tongliao,
Neimenggu 028050, China.
E-mail: 2607050123@qq.com
Received: 2 Jan 2019 Accepted: 10 March 20
DOI: 10.31491/CSRC.2019.03.026
Abstract
Objective: It has been reported that high tumor-associated macrophages (TAMs) numbers in tumors were correlated with poor tumor responses to irradiation. However, few studies have attempted to demonstrate the roles of TAMs in radiotherapy for suppressing the progression of lung cancer.
Methods: TAMs were separated from C57BL/6 mice which were inoculated Lewis lung cancer cells and exposed to radiation therapy, then the expression of maker genes of polarized macrophages and lincRNA-p21 were analyzed using qPCR. Cell viability and invasion of Lewis cells cultured with TAMs exposed to radiation therapy were detected with CKK-8 assay and cell invasion assay, respectively. Ad-LincRNA-p21 and Ad-SiLincRNA-p21 were used to examine the effect of lincRNA-p21 on TAMs.
Results: Radiotherapy effectively suppressed the tumor growth in C57BL/6 mice transplanted with Lewis lung cancer cell. Radiotherapy promoted the polarized of TEMs into M1 macrophage and up-regulated the level of lincRNA-p21 in TEMs. Moreover, TEMs transfected with Ad-LincRNA-p21 presented anti-tumor effect and AdSi-LincRNA-p21 might reverse the effect of radiotherapy on tumor growth.
Conclusion: Our results suggest that radiotherapy promotes the polarization of TEMs into M1 macrophage to suppress lung cancer progression and lincRNA-p21 plays an important role in the radiotherapy treatment for lung cancer.
Keywords
radiotherapy; polarization; tumor-associated macrophage; lung cancer; lincRNA-p21
Introduction
Emerging evidence has revealed that there was a strict correlation between increased density of tumor-infiltrating macrophages and poor prognosis for patients in various malignancies, including breast, prostate, glioma and lung [1]. Tumor-associated macrophages (TAMs) are macrophages that infiltrate in tumor tissues or other tumor-enriched micro-environments (pleural or peritoneal effusions, etc.). TAMs originate from bone marrow precursors, as well as circulating and splenic monocytes [2]. Several reports indicated that TAMs have complex dual functions in their interaction with neoplastic cells and were a part of inflammatory circuits that promoted tumor progression [3]. In particular, reports of an association between high TAM counts and lung cancer invasion and metastasis supported the potential important role of TAMs in lung cancer progression [4]. Up to now, radiotherapy is the mainstay of treatments for patients with lung cancer. Moreover, it has been reported that high TAM numbers in mouse tumors were correlated with poor tumor responses to irradiation [5]. However, few studies have attempted to demonstrate the roles of TAMs in radiotherapy for the progression of lung cancer, and little is known about the gene regulation in TAMs exposed to radiotherapy in lung cancer cells.
On the basis of tissue micro-environments or inflammatory status, TAMs might be induced polarization and differentiate predominantly into two major subsets: one is classical or M1 macrophages, which generally exhibit microbicidal activity and a pro-inflammatory phenotype; another is alternative or M2 macrophages, which are able to tune inflammatory responses and an anti-inflammatory phenotype [1]. In addition, M1/2 macrophages express different maker genes, which are up-regulated in one macrophage phenotype and down-regulated in another macrophage phenotype. Specially, typical M1 markers are Nos2, TNF-α, interleukin-12 (IL-12), IL-6 and typical M2 markers are Arginase-1 (Arg-1), Ym1, FIZZ1 and IL-10 [1].
Long intergenic noncoding RNAs (lincRNAs) represent a new class of RNAs and thousands of lncRNA genes exist in mammalian genomes [6]. Dysregulation of lincRNAs has also been implicated in a variety of human diseases, including cancer. LincRNAs regulate target gene expression at both the transcriptional and post-transcriptional level [7]. Among the identified lincRNAs, lincRNA-p21 has been found to be a downstream target of p53 and to modulate the expression of numerous genes at the transcriptional level [8]. The expression of lincRNA-p21 is found to be down-regulated in several types of tumor, suggesting that lincRNA-p21 may function as a tumor suppressor [9]. LincRNA-p21 was reported to increase the sensitivity of radiotherapy for colorectal cancer by targeting the β-catenin signaling pathway [9]. However, the role of lincRNA-p21 in lung cancer still needs to be elucidated.
Here, we have investigated the effects of radiotherapy on TAMs polarization and the role of lincRNA-p21 in this regulation in lung cancer for the first time to our knowledge. We found that TEMs exposed to radiotherapy predominantly polarized to M1 macrophage and exerted the function of inhibiting cell viability and invasion of Lewis cells. In addition, radiotherapy up-regulated the expression of lincRNA-p21 in TEMs, which presented anti-tumor activity when transfected with Ad-LincRNA-p21.
Methods
Cell culture
The Lewis lung cancer lines were obtained from American Type Culture Collection (USA). The cell lines were maintained in Dulbecco’s modified Eagle’s medium, containing 10% FBS with 100 U/ml penicillin and 100 µg/ml streptomycin, and were cultured in a humidified 5% CO2 incubator at 37°C. The medium was changed every 2 days. Exponentially growing cells were used for experiments.
Animals and tumor injections
Male C57B6/L mice were purchased from Better Biotechnology Co., Ltd. (Nanjing, China). All of the animals were fed a diet of animal chow and water. Lewis mice lung cancer cell lines, cultured in vitro and existed in logarithmic phase, were prepared into the cell suspension, and then inoculated into the right hind leg of C57B6/L mice. Each C57B6/L was inoculated 1 × 106 cells. Tumor volumes were measured with calipers and calculated with the formula L × W2 × 0.5. The procedures of animal experiments were performed by Guide for the Care and Use of Laboratory Animals and were approved by the ethics committee of Affiliated Hospital of Inner Mongolia University for Nationalities.
Tumor irradiation
After eight days of Lewis mice lung cancer cell inoculation, C57B6/L mice were exposed to a high single-dose X-rays with 12 Gy/one fractions/day, and served as radiation therapy group; equal number C57B6/L mice without received X-rays were as control group. Mice were anesthetized (Nembutal) during the radiation treatment, and were immobilized in a customized harness that allowed the right hind leg to be exposed, whereas the remainder of the body was shielded by 3.5 cm of lead. Mice were irradiated in a Gammacell Cesium 137 (Atomic Energy of Canada) source operating at a rate of 125 cGy/min.
Isolation of tumor-associated macrophages
Isolation of tumor-associated macrophages was performed as described by Rui Wang et al. [4]. Tumor tissue was cut into 2 mm fragments and digested by collagenase (0.3 mg/ml type I, Worthington Biochemical Corp, NJ, USA) for 1 h at 37°C. The suspension was filtered through a 70 m stainless steel wire mesh to generate a single-cell suspension. The suspension was centrifuged and washed twice with PBS. Cells were left to adhere in serum-free RPMI 1640 for 40 min. Non-adherent cells were washed away. For TAM-conditioned medium collection, the cells remained in serumfree conditions for 24 h prior to medium collection.
Cell viability assay
Cell counting kit-8 (CCK-8) assay was performed to detect cell viability. TAMs and Lewis cells were co-cultured with equal number through seeding Lewis cells in the lower chamber of a 6-well plate and TAMs in Transwell. After 24 h incubation, TAMs cells and the media were discarded. The attached Lewis cells in each well were then quantified by the CCK-8 method. Briefly, 20 μg of CCK-8 (Sigma) in phosphate buffered saline was added to each well. After incubation at 37°C for 4 h, the supernatants were collected and measured using a microculture plate reader (BioTek) at a wavelength of 490 nm. The experiments were repeated three times.
Cell invasion assays
Cell invasion assay was performed using Transwell cell culture inserts (Invitrogen) as described in previous study [4]. The transfected cells were maintained and allowed to migrate for 24 h. The passed cells were stained with hematoxylin and eosin and photographed under the microscope. Independent experiments were repeated in triplicate.
Quantitative real-time PCR
Total RNA was isolated using TRI Reagent, purified with RNeasy MinElute Cleanup Kits (Qiagen), and quantified using a NanoDrop 1000 spectrophotometer. Complementary DNA was reverse transcribed from total RNA with SuperScript III First-Strand Synthesis Kits (Life Technologies) using oligo(dT) and purified with QIAQuick PCR Purification Kits (Qiagen). Quantitative RTPCR was performed with a Power SYBR-Green PCR Master Mix (Roche, Switzerland) and an Applied Biosystems 7500 Fast System. Relative levels of gene expression were determined with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the control. 2-ΔΔCt method was used to determine the target gene levels relative to those of GAPDH.
Preparation of bone marrow-derived macrophages (BMDMs)
One femur was removed from each of the mice described in the previous section and bone marrow cells harvested by flushing 1 ml of sterile PBS through the bone marrowcavities with a 25 5/8-gauge syringe. Cell numbers were determined, and 20,000 cells were cytospun onto a slide, and differential cell counts were performed as above. On average, 2 to 4% of the extruded bone marrow cells are monocytes.
Construction of Ad-LincRNA-p21 (or Ad-Si-LincRNA-p21) adenovirus and the infection of TAMs
The AdEasy system was used to construct the Ad-LincRNA-p21 (or Ad-Si-LincRNA-p21) adenovirus to overexpress lincRNA-p21 (or knockdown lincRNA-p21) and the control Ad-GFP adenovirus. TAMs were plated into a 6-well plate in 1000 μl of complete medium at a density of 4×105 cells per well and incubated overnight until 50-80% confluent. TAMs were infected with Ad-LincRNA-p21 (or Ad-Si-LincRNA-p21) and the control Ad-GFP adenovirus. The infection efficiency was evaluated by observation of GFP expression under a fluorescent microscope at 48 h post-infection. Cells were harvested at 48 h post-infection and used for total RNA and protein analysis.
Data analysis
Data are expressed as mean ± standard deviation. All data in the study were evaluated using SPSS Version 13.0 software (SPSS, Inc.). Student’s t-test or one-way ANOVA test were performed to analyze the significance of differences between sample means obtained from three independent experiments. The P value <0.05 was considered to be statistically significant.
Results
The effect of radiotherapy on the polarization of separated tumor-associated macrophage and the expression of lincRNA-p21
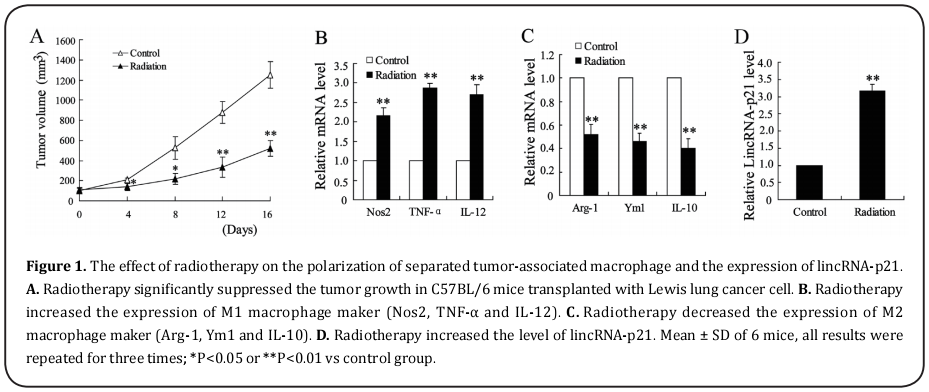
Mounting evidence indicated that radiotherapy was an effective treatment for lung cancer [10]. Here, we detected the tumor growth in C57BL/6 mice transplanted with Lewis lung cancer cell. After eight days of inoculating Lewis lung cancer cells into C57BL/6 mice, C57BL/6 mice were exposed to radiotherapy and a single dose reached 12 Gy/1f/1d. It was shown that tumor growth was significantly repressed in radiation therapy group comparing to control group (Fig. 1A). This finding suggests that radiotherapy may be a functional way to inhibit tumor growth. TAMs polarization was reported to be highly associated with tumor growth and development [11]. To examine the effect of radiotherapy on the polarization of TAMs, we separated TAMs from the above C57BL/6 mice and detected the expression of M1 macrophage makers (Nos2, TNF-α and IL-12) and M2 macrophage makers (Arg-1, Ym1 and IL-10). As shown in Fig.1B and 1C, radiotherapy significantly increased the mRNA level of M1 macrophage makers and down-regulated the mRNA level of M2 macrophage makers. These data suggested that radiotherapy contributed to the polarization of TAMs into M1 macrophage. In addition, the expression of lincRNA-p21 was measured with quantitative polymerase chain reaction (qPCR). Higher level of lincRNA-p21 was observed in TAMs from C57BL/6 mice exposed to radiotherapy, which indicated that lincRNA-p21 might involve in effect of radiotherapy on TAMs polarization (Fig.1D).
The effect of TAMs exposed to radiotherapy on cell viability and invasion of Lewis cells
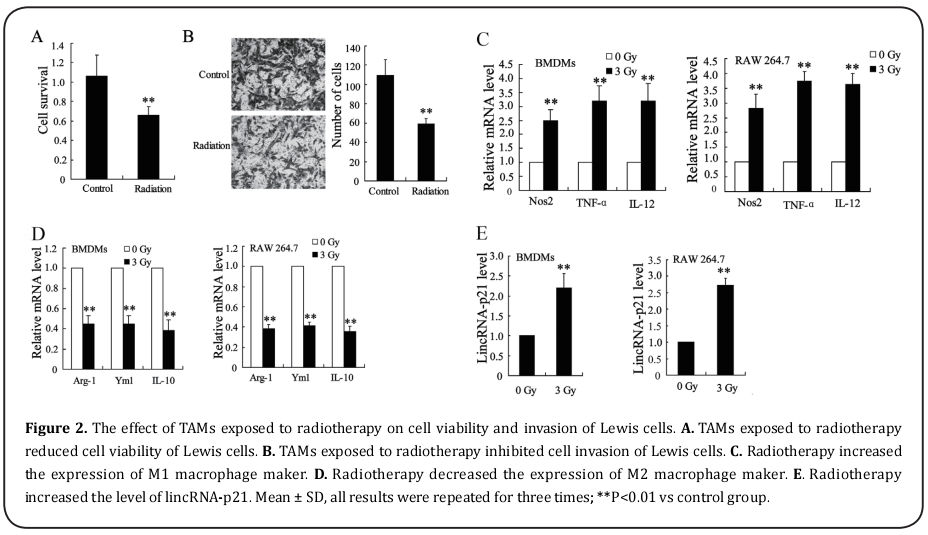
To investigate the effect of TAMs exposed to radiotherapy on cell viability and invasion of Lewis cells, Lewis cells were cultured with TAMs which was exposed to radiotherapy or not. MTT assay indicated that TAMs exposed to radiotherapy significantly lowered the cell viability of Lewis cells (Fig. 2A). In addition, it also suppressed the number of invasive Lewis cells, as shown in Fig. 2B. These data suggested that radiotherapy contributed to the effect of TAMs on reducing cell viability and inhibiting cell invasion of Lewis cells. We further validated the effect of radiotherapy on macrophages polarization in bone marrow-derived macrophages (BMDMs) and macrophages RAW 264.7. BMDMs and RAW 264.7 were exposed to 3 Gy X-ray for 24 h, and we found that the mRNA level of Nos2, TNF-α and IL-12 were both up-regulated in BMDMs and RAW 264.7 (Fig. 2C). On the other hand, the mRNA level of Arg-1, Ym1 and IL-10 were all decreased in BMDMs and RAW 264.7 (Fig. 2D). At the same time, we analyzed the expression of lincRNA-p21 and identified that the level of lincRNA-p21 was increased in BMDMs and RAW 264.7 exposed to X-ray (Fig. 2E). As the same with the results in separated TAMs, radiotherapy also facilitated the polarization of BMDMs and RAW 264.7 into M1 macrophage and up-regulated the expression of lincRNA-p21.
TAMs transfected with Ad-LincRNA-p21 presents anti-tumor activity
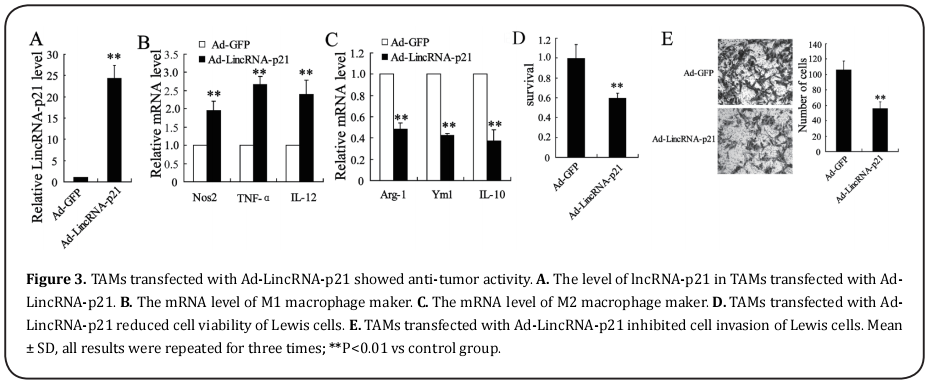
As lincRNA-p21 might play important role in radiotherapy contributing TAMs polarization, we set out to transfect the Ad-LincRNA-p21 or Ad-GFP into TAMs which did not exposed to X-ray. It was shown that lincRNA-p21 was up-regulated by Ad-LincRNA-p21 in TAMs (Fig. 3A). In addition, TAMs transfected with Ad-LincRNA-p21 expressed higher level of M1 macrophage makers comparing to TAMs transfected with Ad-GFP (Fig. 3B). Moreover, Ad-LincRNA-p21 decreased the mRNA level of M2 macrophage makers (Fig. 3C). When the above TAMs cultured with Lewis cells, it was shown that cell survival was suppressed and the number of invasive cells were suppressed by TAMs transfect with Ad-LincRNA-p21 (Fig. 3D and 3E). Taken together, these data indicated that Ad-LincRNA-p21 participated in the anti-tumor activity of TAMs for Lewis cells.
The effect of lincRNA-p21 knockdown on tumor growth of C57BL/6 mice transplanted with Lewis cell
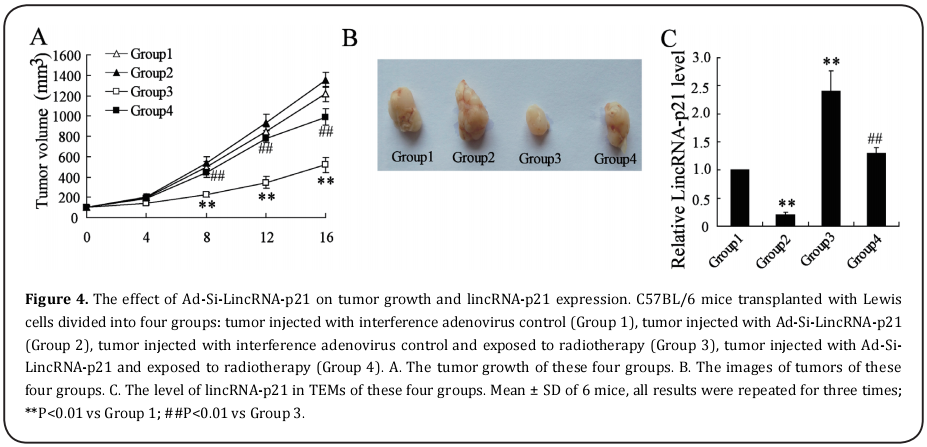
To further elucidate the effect of lincRNA-p21 on tumor growth, C57BL/6 mice transplanted with Lewis cell divided into four groups: tumor injected with interference adenovirus control (Group 1), tumor injected with Ad-Si-LincRNA-p21 (Group 2), tumor injected with interference adenovirus control and exposed to radiotherapy (Group 3), tumor injected with Ad-Si-LincRNA-p21 and exposed to radiotherapy (Group 4). The tumor growth of these four groups were examined and the results showed that Ad-Si-LincRNA-p21 injected into tumors reversed the suppressed effect of radiotherapy on tumor growth and enhanced the tumor growth of C57BL/6 mice transplanted with Lewis cell (Fig. 4A and 4B). Then, we separated the TEMs from the mice of these four Groups. As shown in Fig. 4C, the level of lincRNA-p21 was significantly reduced in mice injected with Ad-Si-LincRNA-p21 comparing to the control group
Discussion
In this study, we found that radiotherapy effectively suppressed the tumor growth in C57BL/6 mice transplanted with Lewis lung cancer cells. In addition, radiotherapy promoted the polarized of TEMs into M1 macrophage and up-regulated the level of lincRNA-p21 in TEMs. When exposed to radiotherapy, TEMs could significantly lower the cell viability and suppress cell invasion of Lewis cells. Moreover, it was also found that TEMs transfected with Ad-LincRNA-p21 presented anti-tumor effect and Ad-Si-LincRNA-p21 might reverse the effect of radiotherapy on tumor growth. Taken together, our results suggest that radiotherapy alters the polarization of TEMs to suppress lung cancer progression and lincRNA-p21 plays an important role in the radiotherapy treatment for lung cancer.
Extensive literature has reported the possibility of improving survival with radiotherapy for patients with lung cancer [12,13]. Consistent with previous study, we found that tumors growth was significantly repressed in C57BL/6 mice which transplanted with Lewis lung cancer cells and exposed to X-ray. TAMs represent one of the most abundant cell components in the tumor environment and key contributors to cancer-related inflammation [14]. Several reports suggested that increased TAMs infiltration were observed in lung and mammary tumors grown subcutaneously following irradiation and TAMs might be the main players for impeding the therapeutic efficacy of various anticancer agents, including cytotoxic chemotherapy, radiotherapy and molecular targeting therapies [5,15]. Milas et al. reported a correlation between high TAM numbers and poor tumor responses to irradiation in mouse tumors [16]. In this study, TEMs exposed to X-ray were observed to polarize into M1 macrophages and significantly suppress viability and invasion of Lewis cells. Current opinion regarding TEMs suggests that M1 macrophages exert anti-tumoral functions via several pathways, and M2 macrophages generally participate in tumorigenesis, tumor growth, invasion and dissemination, which facilitate tumor growth, angiogenesis and migration [17,18]. Tumor microenvironment might accelerate TEMs to polarize into M2 macrophages. These data indicated that radiotherapy might inhibit the lung cancer progression through making TAMs polarize to M1 macrophages and representing anti-tumoral functions. To examine the genetic mechanism of radiotherapy facilitating M1 polarized macrophages, we detected the regulation of lincRNA-p21 in this process. LincRNA-p21 is a long noncoding RNA and a transcriptional target of p53 and HIF-1α. Mounting evidence indicated that the expression level of lincRNA-p21 was significantly lower in tumor tissues. For example, the report of Haiyan Zhai et al. suggested that lincRNA-p21 might contribute to colorectal cancer disease progression [19]. Je-Hyun Yoon et al. identified a role for lincRNA as a posttranscriptional inhibitor of translation [20]. In addition, Wu et al. found lincRNA-p21 as a novel regulator of cell proliferation and apoptosis in atherosclerosis [21]. LincRNA-p21 was reported to enhance the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/β-catenin signaling pathway [9]. Here, we found the up-regulation of lincRNA-p21 in TEMs exposed to radiotherapy. Moreover, inhibition of lincRNA-p21 also resulted in tumor growth of Lewis lung cancer cell.
Conclusion
In summary, these data point to the polarization of tumor-associated macrophage and lincRNA-p21 as a key modulator of tumor growth in the radiotherapy for lung cancer. We identified that radiotherapy suppressed lung cancer progression through altering the polarization of tumor-associated macrophage and up-regulation of lincRNA-p21. This work provides novel insights into the role of lincRNA-p21 and leads us to propose that lincRNAs may serve as key regulatory hubs in radiotherapy for lung cancer.
References
1. Sica, A., Larghi, P., Mancino, A., Rubino, L., Porta, C.,
Totaro, M. G., Rimoldi, M., Biswas, S. K., Allavena, P., and
Mantovani, A. (2008) Macrophage polarization in tumour
progression. Seminars in cancer biology 18, 349-355
2. Cortez-Retamozo, V., Etzrodt, M., Newton, A., Rauch,
P. J., Chudnovskiy, A., Berger, C., Ryan, R. J., Iwamoto, Y.,
Marinelli, B., Gorbatov, R., Forghani, R., Novobrantseva, T.
I., Koteliansky, V., Figueiredo, J. L., Chen, J. W., Anderson, D.
G., Nahrendorf, M., Swirski, F. K., Weissleder, R., and Pittet,
M. J. (2012) Origins of tumor-associated macrophages
and neutrophils. Proceedings of the National Academy of
Sciences of the United States of America 109, 2491-2496
3. Jinushi, M., and Komohara, Y. (2015) Tumor-associated
macrophages as an emerging target against tumors:
Creating a new path from bench to bedside. Biochimica
et biophysica acta 1855, 123-130
4. Wang, R., Zhang, J., Chen, S., Lu, M., Luo, X., Yao, S.,
Liu, S., Qin, Y., and Chen, H. (2011) Tumor-associated
macrophages provide a suitable microenvironment for
non-small lung cancer invasion and progression. Lung
cancer (Amsterdam, Netherlands) 74, 188-196
5. De Palma, M., and Lewis, C. E. (2013) Macrophage
regulation of tumor responses to anticancer therapies.
Cancer cell 23, 277-286
6. Wang, K. C., and Chang, H. Y. (2011) Molecular mechanisms
of long noncoding RNAs. Molecular cell 43, 904-914
7. Wapinski, O., and Chang, H. Y. (2011) Long noncoding
RNAs and human disease. Trends in cell biology 21, 354-
361
8. Huarte, M., Guttman, M., Feldser, D., Garber, M., Koziol, M. J.,
Kenzelmann-Broz, D., Khalil, A. M., Zuk, O., Amit, I., Rabani,
M., Attardi, L. D., Regev, A., Lander, E. S., Jacks, T., and Rinn,
J. L. (2010) A large intergenic noncoding RNA induced by
p53 mediates global gene repression in the p53 response.
Cell 142, 409-419
9. Wang, G., Li, Z., Zhao, Q., Zhu, Y., Zhao, C., Li, X., Ma, Z.,
Li, X., and Zhang, Y. (2014) LincRNA-p21 enhances the
sensitivity of radiotherapy for human colorectal cancer
by targeting the Wnt/beta-catenin signaling pathway.
Oncology reports 31, 1839-1845
10. Chen, J. X., Chen, M., Zheng, Y. D., Wang, S. Y., and Shen,
Z. P. (2015) Up-regulation of BRAF activated non-coding
RNA is associated with radiation therapy for lung cancer.
Biomedicine & pharmacotherapy = Biomedecine &
pharmacotherapie 71, 79-83
11. Solinas, G., Germano, G., Mantovani, A., and Allavena, P.
(2009) Tumor-associated macrophages (TAM) as major
players of the cancer-related inflammation. Journal of leukocyte biology 86, 1065-1073
12. Dang, J., Li, G., Zang, S., Zhang, S., and Yao, L. (2014)
Comparison of risk and predictors for early radiation
pneumonitis in patients with locally advanced non-small
cell lung cancer treated with radiotherapy with or without
surgery. Lung cancer (Amsterdam, Netherlands) 86, 329-
333
13. Cui, Y., Chen, W., Kong, F. M., Olsen, L. A., Beatty, R. E., Maxim,
P. G., Ritter, T., Sohn, J. W., Higgins, J., Galvin, J. M., and Xiao,
Y. (2015) Contouring variations and the role of atlas in
non-small cell lung cancer radiation therapy: Analysis
of a multi-institutional preclinical trial planning study.
Practical radiation oncology 5, e67-75
14. Guo, C., Buranych, A., Sarkar, D., Fisher, P. B., and Wang, X.
Y. (2013) The role of tumor-associated macrophages in
tumor vascularization. Vascular cell 5, 20
15. Jinushi, M. (2014) Immune regulation of therapy-resistant
niches: emerging targets for improving anticancer drug
responses. Cancer metastasis reviews 33, 737-745
16. Milas, L., Wike, J., Hunter, N., Volpe, J., and Basic, I. (1987)
Macrophage content of murine sarcomas and carcinomas:
associations with tumor growth parameters and tumor
radiocurability. Cancer research 47, 1069-1075
17. Lewis, C. E., and Pollard, J. W. (2006) Distinct role of
macrophages in different tumor microenvironments.
Cancer research 66, 605-612
18. Mantovani, A., Allavena, P., and Sica, A. (2004) Tumourassociated macrophages as a prototypic type II polarised
phagocyte population: role in tumour progression.
European journal of cancer (Oxford, England : 1990) 40,
1660-1667
19. Zhai, H., Fesler, A., Schee, K., Fodstad, O., Flatmark, K., and Ju,
J. (2013) Clinical significance of long intergenic noncoding
RNA-p21 in colorectal cancer. Clinical colorectal cancer
12, 261-266
20. Yoon, J. H., Abdelmohsen, K., Srikantan, S., Yang, X.,
Martindale, J. L., De, S., Huarte, M., Zhan, M., Becker, K. G.,
and Gorospe, M. (2012) LincRNA-p21 suppresses target
mRNA translation. Molecular cell 47, 648-655
21. Wu, G., Cai, J., Han, Y., Chen, J., Huang, Z. P., Chen, C., Cai,
Y., Huang, H., Yang, Y., Liu, Y., Xu, Z., He, D., Zhang, X., Hu,
X., Pinello, L., Zhong, D., He, F., Yuan, G. C., Wang, D. Z.,
and Zeng, C. (2014) LincRNA-p21 regulates neointima
formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation 130, 1452-1465