Open Access | Research article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
MiR-449b Inhibits the Migration and Invasion of Colorectal Cancer Cells through the Negative Regulation of MMP2
*Corresponding author: Lian-feng Zhang
Mailing address: Department of Gastroenterology, The First
Affiliated Hospital of Zhengzhou University. No. 1 Jianshe East
road, Zhengzhou 450052, Henan Province, China.
E-mail: Zhanglianfeng110@163.com
Received: 25 July 2018 Accepted: 25 September 2018
DOI: 10.31491/CSRC.2018.9.020
Abstract
Background: An increasing number of miRNAs were confirmed to be involved in the initiation and progression of colorectal cancer (CRC) by acting as cancer suppressor genes and oncogenes. The purpose of this study was to uncover the role of miR-449b in CRC and its underlying mechanisms.
Methods: The expression profile of miR-449b in human CRC tissues and cell lines was determined using quantitative real-time polymerase chain reaction (qRT-PCR) analysis. The effect of miR-449b on CRC was assessed by CRC cell migration and invasion as determined using a transwell assay. A luciferase reporter assay was also carried out to explore the interaction between MMP2 3′UTR and miR-449b.
Results: The current study revealed that the relative expression of miR-449b was decreased significantly in human CRC tissues and cell lines; meanwhile, miR-449b in metastatic CRC tissues was significantly lower than that in non-metastatic CRC tissues. We also observed that the forced miR-449b in CRC cells significantly restrained cell migration and invasion in vitro. In contrast to miR-449b, our study found that both the MMP- 2 mRNA and protein levels were increased in CRC cells. MiR-449b negatively regulated MMP2 in CRC cells, and the result was corroborated by luciferase reporter assay. Most importantly, overexpression of MMP2 significantly reversed the miR-449b-mediated inhibition on the invasion and migration of CRC cells.
Conclusion: Our data provided encouraging evidence that miR-449b possessed a strong inhibitory effect on CRC cell migration and invasion by negative regulation of MMP2.
Keywords
Colorectal cancer; MiR-449b; MMP2; Migration; Invasion
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors and has a high morbidity and mortality worldwide [1]. At present, radical surgical resection is the predominant clinical treatment for CRC [2]. Metastasis is the major reason for a poor prognosis and death in all cancer patients, including those with CRC [3]. Clinically, most CRC patients already have micrometastases before radical surgery takes place, which is the direct cause of metastasis and recurrence following CRC surgery. There is currently a lack of effective diagnostic modalities for early metastasis, so an investigation into the mechanism of CRC metastasis has great significance on its diagnosis and treatment.
Emerging evidence has suggested that the differential expression of microRNAs (miRNAs) shows significant associations with the proliferation, adhesion, invasion, and migration of tumor cells [4]. miRNAs are a class of noncoding small RNA with a length of about 19–25 nucleotides. miRNAs instruct the functional RNA-induced silencing complex (RISC) to trigger mRNA degradation and/or translation inhibition through direct binding to the 3-untranslated coding regions (UTR) of the target mRNA. miRNA is closely related to the progression of tumors. About 50% of miRNAs are located on the fragile sites associated with tumor development in the genome and are more prone to deletion, amplification, and translocation. An increasing number of miRNAs are confirmed to be involved in the initiation and progression of human tumors by acting as cancer suppressor genes and oncogenes.
Existing studies have shown that multiple miRNAs were implicated in many steps of CRC, including tumor growth, angiogenesis, and metastasis [5]. For instance, miR-133b was found to be significantly decreased in human CRC tissue samples (n=54); the induction of miR-133b in CRC cells clearly inhibited cell proliferation, apoptosis, and microtubule formation in vitro [6,7]. A recent study confirmed that miR-133b could suppress CRC metastasis by targeting the HOXA9/ZEB1 pathway and was also an independent and significant factor for the overall survival of CRC patients [8]. miR- 449b has been identified as a tumor suppressor in human cancers [9,10]. More importantly, miR-449b was found to inhibit the proliferation of colon cancer stem cells, suggesting its vital role in CRC progression [11]. To date, the functional role of miR-449b in the development of CRC is not yet known.
According to previous reports, matrix metalloproteinase 2 (MMP2), a 72-kDa type IV collagenase, plays a critical role in regulating the invasion and metastasis of a variety of human cancers. MMP2 is also closely associated with tumorigenesis and metastasis through various functions. A bioinformatics analysis (microrna. org) revealed that MMP2 might be a novel downstream target of miR-449b. Herein, we inferred that miR-449b could be an important regulator associated with CRC metastasis by interacting with MMP2. In the present study, the expression profile of miR-449b in human CRC tissues was determined, and miR-449b was found to inhibit the migration and invasion of CRC cells by negatively regulating MMP2 in vitro.
Materials and Methods
Human CRC tissue samples
The patients underwent radical resection at The First Affiliated Hospital of Zhengzhou University; radiotherapy and chemotherapy were not administered before the resection. The obtained fresh tissue samples were stored at -80°C until pathological analysis. This study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University, and each patient provided written informed consent to participate in this study.
Quantitative real-time polymerase chain reaction analysis
The relative expression of miR-449b and MMP2 mRNA in human CRC tissues and cell lines was analyzed using quantitative real-time polymerase chain reaction (qRTPCR) with U6 or β-actin as references, respectively. The total RNA and miRNA were isolated using a Trizol reagent (Invitrogen) and a mirVANA RNA Isolation Kit (Ambion). The purity and concentration values were detected using a NanoDrop spectrophotometer (Thermo Scientific). Following miR-449b quantitation, the total miRNA was reverse-transcribed according to the manufacturer’s instructions provided with the TaqMan microRNA reverse transcription kit (Applied Biosystems) to form cDNA. To detect MMP2 mRNA, the total RNA was also reverse-transcribed into cDNA using a cDNA Synthesis Kit (Fermentas) according to the manufacturer’s protocol. The qRT-PCR procedure followed the manufacturer’s instructions for the SYBR Premix Ex Taq Kit (TaKaRa) with gene-specific primers. The relative expression of miR-449b and MMP2 mRNA was assessed using 2-△△Ct analysis.
Cell lines and cell culture
Human colon cancer cell lines HCT-116 and SW480
were purchased from the Institute of Biochemistry
and Cell Biology at the Chinese Academy of Sciences
(Shanghai, China), and normal colon epithelial FHC
cells were obtained from the American Type Culture
Collection (ATCC, USA). The CRC cells were cultured in
RPMI-1640 media (HyClone) supplemented with 10%
fetal bovine serum (FBS, Gibco) and 1% penicillin/
streptomycin (HyClone) at 37°C with 5% CO
Western blot analysis
The levels of MMP2 protein expression in the HCT- 116, SW480, and FHC cells were analyzed by western blot using β-actin as a reference. The total protein was extracted from these cells using a RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific) and quantified with a BCA protein assay kit (Pierce). The total protein (25 μg) of each sample was heated at 100°C followed by SDS-PAGE electrophoresis. The separated proteins were transferred onto PVDF membranes (Amersham Biosciences). These PVDF membranes were blocked with 5% skim milk for 1 h and then probed with the primary antibodies against MMP2 (1:1000, Abcam) and β-actin (1:2000, Abcam) overnight. After incubation of the horseradish peroxidase-conjugated secondary antibodies, an ECL detection kit (Invitrogen) was used to detect the target proteins.
Cell transfection
SW480 and HCT-116 CRC cells were transfected with a miR-449b mimic to modulate the intracellular levels of miR-449b. These SW480 and HCT-116 cells (5×105 cells/well) were cultured in six-well plates overnight. The miR-449b mimic (60 nM) or mimic control was purchased from Genepharm Company (Shanghai, China) and transfected into these cells using Lipofectamine 2000 (Invitrogen) according to the manufacturers’ instructions. After 48 h of transfection, the in vitro transfection efficiency was verified using qRT-PCR analysis, as previously described.
Luciferase reporter assay
The luciferase reporter vector carrying MMP2-3′UTR (WT) or the mutated MMP2-3′UTR (Mutant) was constructed. The 293T cells (2×105 cells/well) were cultured in 24-well plates overnight. The recombinant vector was co-transfected into the cells combined with the miR-449b mimic, inhibitor, or their negative control using Lipofectamine 2000 (Invitrogen). After 48 h of transfection, the cells were lysed, and the intracellular relative activity of luciferase was detected using a luciferase reporter assay (Promega).
Cell migration and invasion analysis by transwell assay
A transwell system (Corning) with an 8-μm aperture microporous membrane coated with or without matrigel (Sigma) was used to analyze the CRC cell invasion and migration, respectively. The complete medium (500 μL) was added to the lower chamber. CRC cells (2×105 cells in 200 μL serum-free media) were added to the upper chamber and cultured at 37°C with 5% CO2 for 48 h. The cells that crossed the membrane were fixed in neutral formalin and stained with 0.1% crystal violet (Sigma-Aldrich). The migrated or invaded cells were counted on five random fields under an inverted microscope
Statistical analysis
All data came from three independent repeated experiments and were expressed as the mean ± standard deviation. Student’s t-test was used to compare the differences between the two groups. A paired t-test was used to analyze the significant differences in the relative expression of miR-449b between metastatic CRC (CRC-M) and normal CRC (CRC-N). A P-value <0.05 was considered to have statistical significance. Western blot analysis was performed four times with similar results, and a representative image was presented in this article.
Results
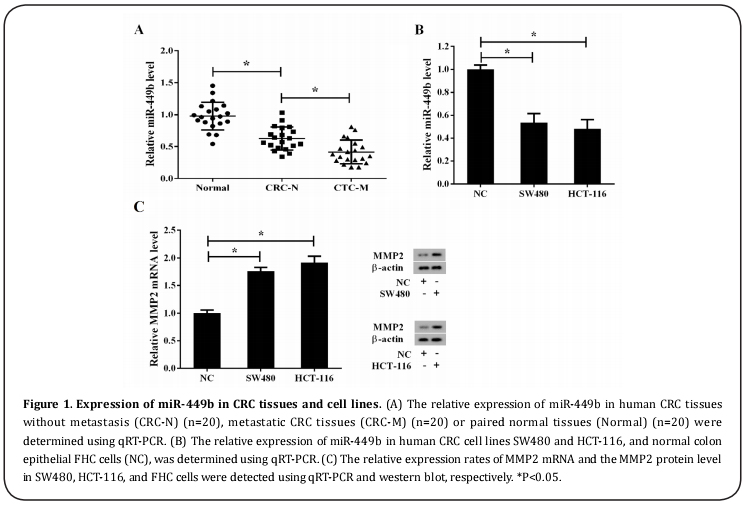
Expression of miR-449b in CRC tissues and cell lines
The relative expression of miR-449b in human CRC tissues without metastasis (CRC-N) (n=20), as determined by qRT-PCR, was significantly lower than that in the paired normal tissues (Normal) (n=20) (Figure 1A). Moreover, compared with CRC-N, the relative expression of miR-449b decreased significantly in the metastatic CRC tissues (CRC-M) (n=20) (Figure 1A). Following qRT-PCR analysis, miR-449b was also found to significantly downregulate human CRC cell lines SW480 and HCT-116, compared with that of the control cells (NC), normal colon epithelial FHC cells (Figure 1B). In contrast, MMP2 had a high expression in SW480 and HCT-116 cells at both the mRNA and protein levels (Figure 1C)
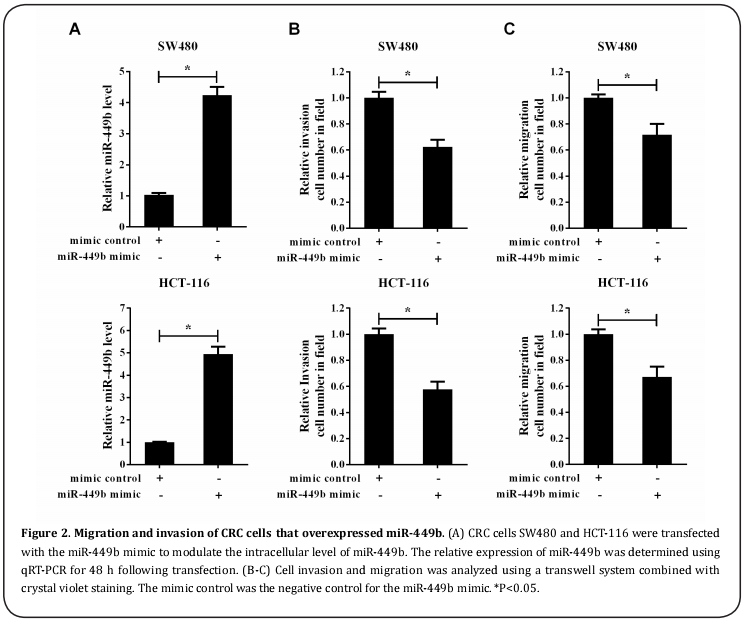
Migration and invasion of miR-449b overexpressed CRC cells
The miR-449b mimic was transfected into CRC SW480 and HCT-116 cells to upregulate the intracellular level of miR-449b and also to observe the effect of miR- 449b on cell migration and invasion. The transfection efficiency in vitro was verified using qRT-PCR analysis and indicated that the relative expression of miR-449b was dramatically induced in SW480 and HCT-116 cells due to transfection by the miR-449b mimic (Figure 2A). Subsequently, the cell invasion and migration rates were analyzed using the transwell system. As shown in Figure 2B, the invasion ability of miR-449b to overexpress SW480 and HCT-116 cells was significantly lower than that of the cells transfected with the mimic control. In addition, SW480 and HCT-116 cells that overexpressed miR-449b also significantly restrained the migration of CRC cells (Figure 2C).
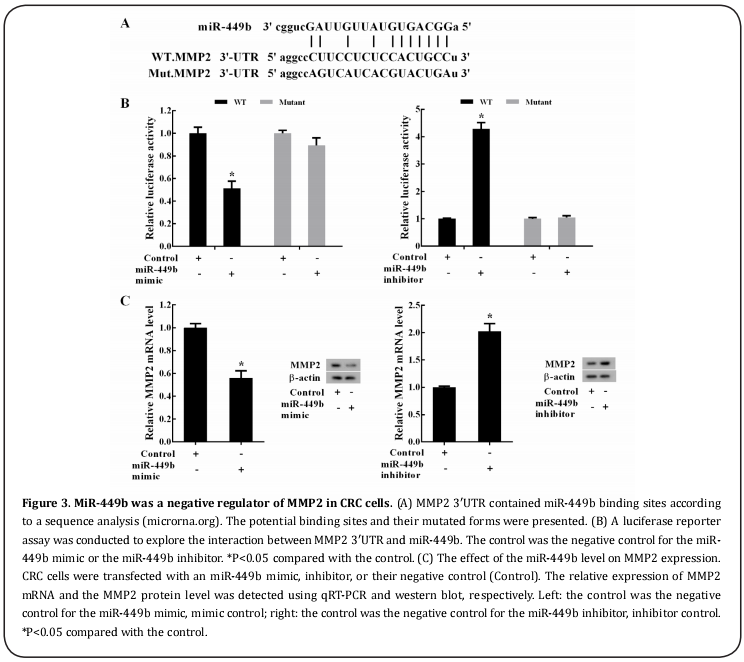
MiR-449b was a negative regulator of MMP2 in CRC cells
According to our sequence analysis (microrna.org), MMP2 3'-UTR contained miR-449b binding sites. The potential binding sites and their mutated forms are presented in Figure 3A. A luciferase reporter assay was designed to explore the interaction between MMP23′UTR and miR-449b. The results revealed that compared with the control (mimic control), transfection of the miR-449b mimic decreased the relative activity of luciferase in the cells transfected with recombinant vector carrying luciferase and MMP2-3′UTR (WT) (Figure 3B, left). However, miR-449b overexpression had no significant influence on intracellular luciferase activity in the cells transfected with recombinant vector carrying luciferase and mutated MMP2-3′UTR (Mutant) (Figure 3B, left). These results were corroborated by the luciferase reporter assay with downregulated miR- 449b (Figure 3B, right), suggesting an interaction between MMP2 3′UTR and miR-449b. To further verify this association, CRC cells were transfected with the miR-449b mimic, inhibitor, or their negative control (Control). Both the MMP2 mRNA and protein levels were significantly reduced by the miR-449b mimic but were significantly induced by the miR-449b inhibitor (Figure 3C).
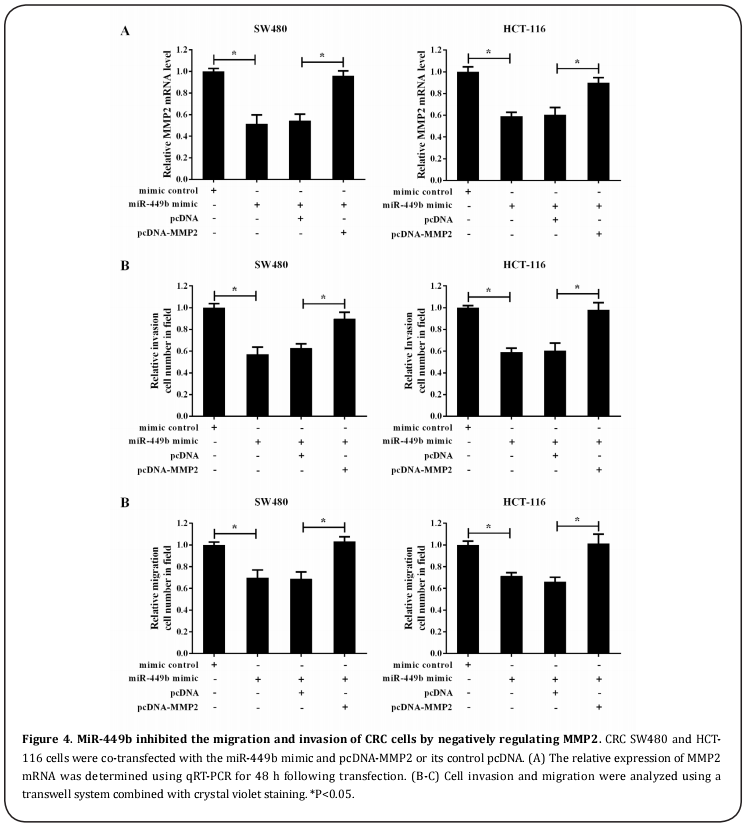
miR-449b inhibited the migration and invasion of CRC cells by negatively regulating MMP2
According to our results, miR-449b inhibited the migration and invasion of SW480 and HCT-116 cells, as well as the intracellular MMP2 expression. We further explored whether the effect on CRC cells might be mediated by MMP2. As shown in Figure 4A, co-transfection of the miR-449b mimic and pcDNA-MMP2 almost completely lifted the inhibitory effect on MMP2 expression induced by miR-449b. Importantly, co-transfection of the miR-449b mimic and pcDNA-MMP2 significantly potentiated the invasion and migration ability of SW480 and HCT-116 cells compared with the cells co-transfected with the miR-449b mimic and pcDNA; this outcome indicated that pcDNA-MMP2 also significantly reversed the miR-449b-mediated inhibition of cell invasion and migration (Figure 4B and 4C). It was concluded that miR-449b inhibited the migration and invasion of CRC cells by negatively regulating MMP2.
Discussion
Thus far, research into miR-449b’s function has mainly concentrated on its involvement in the pathogenesis of human tumors. The downregulation of miR-449b was discovered in a series of cancer cell lines induced by histone H3 Lys27 trimethylation or histone acetylation [9,12]. Moreover, miR-449b arrested the cell cycle in the G1 phase of breast cancer cells and lung carcinoma cells by targeting oncogenic CDK6 and CDC25A [9]. A previous study showed that miR-449b inhibited liver cancer cell migration in vitro and tumor growth in mice [12]. In addition, Fang et al. reported that miR- 449b reduced the proliferative ability of colon cancer stem cells SW1116, suggesting an inhibitory effect of miR-449b on CRC [11]. The current study focused on the biological roles of miR-449b in CRC metastasis and revealed that the relative expression of miR-449b was decreased significantly in human CRC tissues and cell lines; meanwhile, miR-449b in metastatic CRC tissues was significantly lower than that present in non-metastatic CRC tissues. We also observed that the forced miR-449b in CRC cells significantly restrained cell migration and invasion in vitro. The results indicated that miR-449b also served as a tumor suppressor in CRC progression.
CDK6, CDC25A, CCND1, E2F3, and SOX4 were identified as the targets for miR-449b and were negatively regulated by miR-449b by directly binding to their 3-UTRs [9-12]. MMP2, a 72-kDa type-IV collagenase, played a critical role in regulating the invasion and metastasis of a variety of human cancers, including CRC [13]. Previous studies have demonstrated that a high level of MMP-2 protein was detected in human CRC samples and was positively correlated with a poor survival outcome for CRC patients [14-16]. As a cell surface transducer, the MMP-2 protein has also been found to promote CRC metastasis [17]. Based upon our bioinformatics analysis, we presumed that miR-449b inhibited the migration and invasion of CRC cells by modulating MMP-2 protein expression. In contrast, our study found that both the MMP-2 mRNA and protein levels were increased in CRC cells, which was consistent with the result of previous studies. However, our focus was on the role of MMP2 in the mechanism by which miR-449b represses CRC metastasis. The results of a luciferase reporter assay suggested an interaction between MMP2 3′UTR and miR-449b, and the result was corroborated by the fact that the MMP2 mRNA and protein levels were significantly reduced in CRC cells that overexpressed miR- 449b but significantly induced by miR-449b silencing. Most importantly, the overexpression of MMP2 significantly reversed the miR-449b-mediated inhibition on the invasion and migration of CRC cells, indicating that miR-449b inhibited the migration and invasion of CRC cells by negatively regulating MMP2. A high expression of MMP2 in CRC tissues could promote the invasion of CRC cells into the gut wall by enhancing the degradation of the extracellular matrix.
In conclusion, our study demonstrated that miR-449b was significantly decreased in human CRC tissues and cell lines, and that miR-449b possessed a strong inhibitory effect on CRC cell migration and invasion. In addition, MMP2 was identified as a new target for miR-449b in a CRC context. This study will help to determine the pathogenesis of CRC and also provides a promising therapeutic target for CRC treatment.
References
1. Hadjipetrou, A., Anyfantakis, D., Galanakis, C. G.,
Kastanakis, M., and Kastanakis, S. (2017) Colorectal
cancer, screening and primary care: A mini literature review. World journal of gastroenterology 23, 6049-6058
2. Wan Kim, Y. (2017) Surgical treatment for colorectal
cancer in octogenarians and nonagenarians. Journal of
B.U.ON. : official journal of the Balkan Union of Oncology
22, 578-585
3. Puthiamadathil, J. M., and Weinberg, B. A. (2017) Emerging combination therapies for metastatic colorectal cancer - impact of trifluridine/tipiracil. Cancer management
and research 9, 461-469
4. Mansoori, B., Mohammadi, A., Shirjang, S., and Baradaran, B. (2017) MicroRNAs in the Diagnosis and Treatment of Cancer. Immunol Invest 46, 880-897
5. Pan, C., Yan, X., Li, H., Huang, L., Yin, M., Yang, Y., Gao,
R., Hong, L., Ma, Y., Shi, C., Qin, H., and Zhang, P. (2017)
Systematic literature review and clinical validation of
circulating microRNAs as diagnostic biomarkers for colorectal cancer. Oncotarget 8, 68317-68328
6. Kara, M., Yumrutas, O., Ozcan, O., Celik, O. I., Bozgeyik, E.,
Bozgeyik, I., and Tasdemir, S. (2015) Differential expressions of cancer-associated genes and their regulatory
miRNAs in colorectal carcinoma. Gene 567, 81-86
7. Dai, J., Wu, H., Zhang, Y., Gao, K., Hu, G., Guo, Y., Lin, C.,
and Li, X. (2016) Negative feedback between TAp63 and
Mir-133b mediates colorectal cancer suppression. Oncotarget 7, 87147-87160
8. Wang, X., Bu, J., Liu, X., Wang, W., Mai, W., Lv, B., Zou, J.,
Mo, X., Li, X., Wang, J., Niu, B., Fan, Y., and Hou, B. (2017)
miR-133b suppresses metastasis by targeting HOXA9 in
human colorectal cancer. Oncotarget 8, 63935-63948
9. Yang, X., Feng, M., Jiang, X., Wu, Z., Li, Z., Aau, M., and Yu,
Q. (2009) miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1
activity through a feedback loop by targeting CDK6 and
CDC25A. Genes & development 23, 2388-2393
10. Ye, W., Xue, J., Zhang, Q., Li, F., Zhang, W., Chen, H., Huang,
Y., and Zheng, F. (2014) MiR-449a functions as a tumor
suppressor in endometrial cancer by targeting CDC25A.
Oncology reports 32, 1193-1199
11. Fang, Y., Gu, X., Li, Z., Xiang, J., and Chen, Z. (2013) miR-
449b inhibits the proliferation of SW1116 colon cancer
stem cells through downregulation of CCND1 and E2F3
expression. Oncology reports 30, 399-406
12. Sandbothe, M., Buurman, R., Reich, N., Greiwe, L., Vajen,
B., Gurlevik, E., Schaffer, V., Eilers, M., Kuhnel, F., Vaquero,
A., Longerich, T., Roessler, S., Schirmacher, P., Manns, M.
P., Illig, T., Schlegelberger, B., and Skawran, B. (2017) The
microRNA-449 family inhibits TGF-beta-mediated liver
cancer cell migration by targeting SOX4. J Hepatol 66,
1012-1021
13. Bauvois, B. (2012) New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression.
Biochimica et biophysica acta 1825, 29-36
14. Salem, N., Kamal, I., Al-Maghrabi, J., Abuzenadah, A.,
Peer-Zada, A. A., Qari, Y., Al-Ahwal, M., Al-Qahtani, M.,
and Buhmeida, A. (2016) High expression of matrix
metalloproteinases: MMP-2 and MMP-9 predicts poor
survival outcome in colorectal carcinoma. Future oncology (London, England) 12, 323-331
15. Groblewska, M., Mroczko, B., Gryko, M., Pryczynicz, A.,
Guzinska-Ustymowicz, K., Kedra, B., Kemona, A., and
Szmitkowski, M. (2014) Serum levels and tissue expression of matrix metalloproteinase 2 (MMP-2) and tissue
inhibitor of metalloproteinases 2 (TIMP-2) in colorectal
cancer patients. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and
Medicine 35, 3793-3802
16. Kostova, E., Slaninka-Miceska, M., Labacevski, N., Jakovski, K., Trojachanec, J., Atanasovska, E., Janevski, V., Jovanovik, R., and Janevska, V. (2014) Expression of matrix
metalloproteinases 2, 7 and 9 in patients with colorectal
cancer. Vojnosanitetski pregled 71, 52-59
17. Kim, B. R., Kang, M. H., Kim, J. L., Na, Y. J., Park, S. H., Lee, S. I., Kang, S., Joung, S. Y., Lee, S. Y., Lee,
D. H., Min, B. W., and Oh, S. C. (2016) RUNX3 inhibits the metastasis and angiogenesis of colorectal cancer. Oncology reports 36,
2601-2608Surgical treatment on lower tibial fracture associated with posterior malleolus fracture. Journal of Clinical Orthopaedics 19, 192-192