Open Access | Research Article
This work is licensed under a
Creative Commons Attribution-ShareAlike 4.0
International License.
Rate and clinical predictors of malignancy in thyroid nodules with indeterminate cytology
* Correspondence author: Feron Getachew
Mailing address: Department of surgery, Tikur Anbessa
Specialized
Hospital, College of Health Sciences, Addis Ababa University,
Addis Ababa, Ethiopia.
E-mail: getferon@gmail.com
Received: 08 October 2021 / Accepted: 20 December 2021
DOI:
10.31491/CSRC.2021.12.085
Abstract
Background: Fine needle aspiration cytology (FNAC) cannot differentiate between benign and
malignant
conditions in cytologically indeterminate thyroid lesions. Therefore, a minimum of diagnostic lobectomy is
required for definitive diagnosis. The objective of this study is to identify the rate of malignancy and
clinical
features that may possibly predict malignancy in patients with these lesions, in Ethiopian hospitals.
Methods: This was a retrospective review of the medical records of patients who underwent surgery
for cytologically
indeterminate thyroid lesions in three referral hospitals between September 2015 and September
2020.
Results: Of 85 patients with indeterminate cytology findings, 56 (63.5%) were follicular, and 29
(34.1%) were
reported to be hurthle cell neoplasms. Follicular lesions of undetermined significance (FLUS) and suspicious
for follicular neoplasm were each reported in single cases (1.7%). Malignant disease was diagnosed in 19
(22.4%) of patients. A follicular variant of papillary cancer was detected in 7 (11.5%) patients. Hard nodule
consistency was reported in 9 of 11 malignant lesions and 5 of 66 benign lesions. In multivariate binary
logistic
regression, hard nodule consistency was found to be associated with malignancy (P = 0.012, AOR = 7.28 (1.5,
34.54) 95% CI ). The ill-defined surface of a nodule was found to be associated with malignancy though the
association
was not statistically significant (P = 0.088, AOR = 0.162 (0.020, 1.313) 95% CI. Ultrasound evaluation
of thyroid nodule was performed only in 41 (47.7%) of patients.
Conclusion: The rate of malignancy in thyroid nodules with indeterminate cytology was 22.4%. The
risk of
malignancy was higher in patients with hard thyroid nodule consistency and ill-defined surface. Despite the
established benefits of ultrasound for the evaluation of thyroid nodules, the current practice of its use in
our
setup is suboptimal.
Keywords
Follicular; hurthle cell; indeterminate cytology; predictors of malignancy
Introduction
FNAC of the thyroid can generally differentiate between benign and malignant lesions except when the findings are suggestive of atypia of undetermined Research Article significance (AUS), follicular lesion of undetermined significance (FLUS), suspicious of follicular neoplasm, follicular neoplasm, or hurthle cell neoplasms [1, 2]. Follicular thyroid lesions are common cytological findings during the evaluation of thyroid nodules. Differentiating follicular thyroid cancer & Hurthle cell cancer from thyroid adenoma cannot be made by FNAC alone; rather, it requires histological evidence of vascular and capsular invasion [2]. In 70-80% of cases, nodules with cytological diagnosis of follicular neoplasms turn out to be benign [2]. Many patients with benign thyroid disease are thus subjected to potentially avoidable surgery (diagnostic lobectomy) and the associated cost. Likewise, the diagnostic confusion exposes patients with malignant disease that may potentially benefit from a single initial total or near-total thyroidectomy to undergo two surgeries. i.e., initial diagnostic Lobectomy and repeat surgery (completion thyroidectomy). Therefore, identifying factors that predict malignancy preoperatively may avoid unnecessary surgery, along with its cost and complications. Accordingly, there has been growing interest among researchers to predict malignancy preoperatively using different parameters such as clinical, ultrasound, cytological, and molecular techniques [3-6]. The intraoperative frozen section has been used in an attempt to define the adequate extent of surgery intraoperatively, but its routine use is quite limited [7]. Despite these attempts, most of the results are inconsistent and sometimes contradictory. In developing nations, such as our country, the decision-making process is primarily clinical in part due to the unavailability of advanced diagnostic modalities. Therefore, we seek to identify if there are clinical predictors of malignancy that can guide the choice and extent of therapy.
Materials and methods
This was a retrospective cross-sectional review of
charts in patients who underwent thyroid surgery
between September 2015 and September 2020 for
cytologically indeterminate thyroid nodules. All patients
operated with the FNAC diagnosis of FLUS, AUS,
hurthle cell neoplasm, suspicious for follicular neoplasm
or follicular neoplasm were selected from the
operation room logbooks of Tikur Anbessa, Yekatit 12,
and Zewditu memorial hospitals which are located in
Addis Ababa, Ethiopia. Cases with recurrence or with
inconclusive or lost biopsy results were excluded from
the study.
FNAC and biopsy were reported by different pathologists
from AAU, TASH, or other institutions. Almost all
FNA procedures were performed without ultrasound
guidance. The Bethesda system was used to classify
the FNA results. Definitive diagnosis of malignancy was
determined based on the postoperative histopathological
diagnosis. Demographic, clinical, and laboratory
data as well as pathology reports were reviewed from
individual patient charts. Sociodemographic data, mass
characteristics including size, surface, consistency, and
type of nodule as well as signs & symptoms such as
rapid tumor growth, change of voice, dysphagia, airway
obstruction, and duration of illness were analyzed for association with the presence of malignancy. SPSS
version 24 was used for data analysis.
Categorical data were presented as percentages and
frequencies of occurrence. Continuous variables were
described as means and standard deviations Associations
between categorical variables were checked with
chi-square test. Univariately associated variables were
subjected to multivariate analysis.
The degree of association was calculated using binary
logistic regression, with a statistically significant cutoff
(P < 0.05).
Ethical approval was obtained from the research and
ethics committee at the Department of Surgery, Addis
Ababa University.
Results
Demographic data
The number of patients operated on in the three hospitals
with the diagnosis of follicular or hurthle cell thyroid
neoplasm in 5 years was 115. Patients that fulfill
the inclusion criteria were 85. FNA was taken without
ultrasound guidance, in almost all of the patients. The
follicular neoplasm was diagnosed in 56 (65.9%) and
hurthle cell neoplasm in 29 (34.1%)
The mean age of presentation was 35.64 + 12.823 (age
range 18-78) years. Among all patients, 70.6% were
younger than 40 while 5.9 % were older than 60. Seventy-
four (87.1%) were female and 11 (12.9%) were
male. The male to female ratio is 1 : 6.72. The vast majority
of patients (77, 90.6%) were from Addis Ababa.
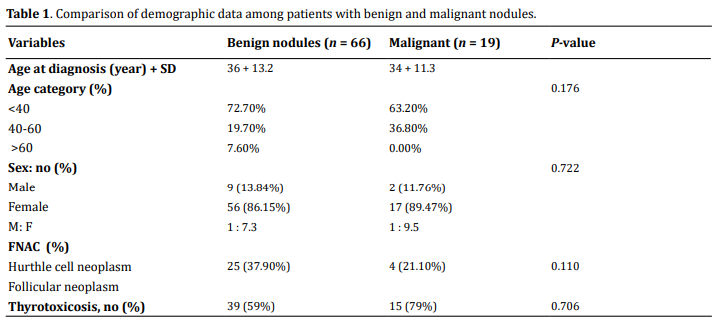
The comparison between benign and malignant lesions
is provided in Table 1 below.
Clinical presentation
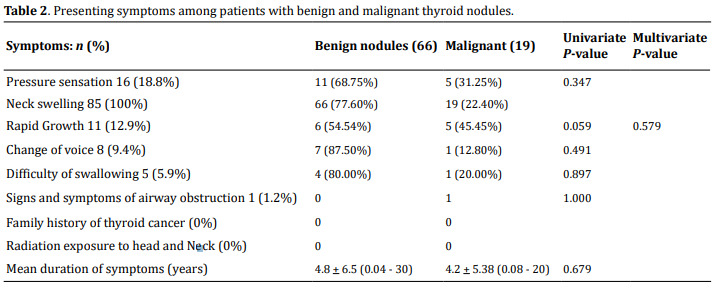
The mean duration of symptoms for all the patients was 4.68 + 6.28 (range, 15 days to 30 years). All patients had complaints of anterior neck swelling. None of the patients had a history of exposure to radiation or a family history of thyroid cancer. Other presenting complaints were as follows: pressure sensation 20 (23.5%), history of rapid growth 11 (12.9%), hoarseness of voice in 8 (9.4%) difficulty of swallowing 5 (5.9%), a symptom of airway obstruction 1 (1.2%). The comparison between the presentation of benign and malignant lesions is provided below in Table 2.
Physical examination findings
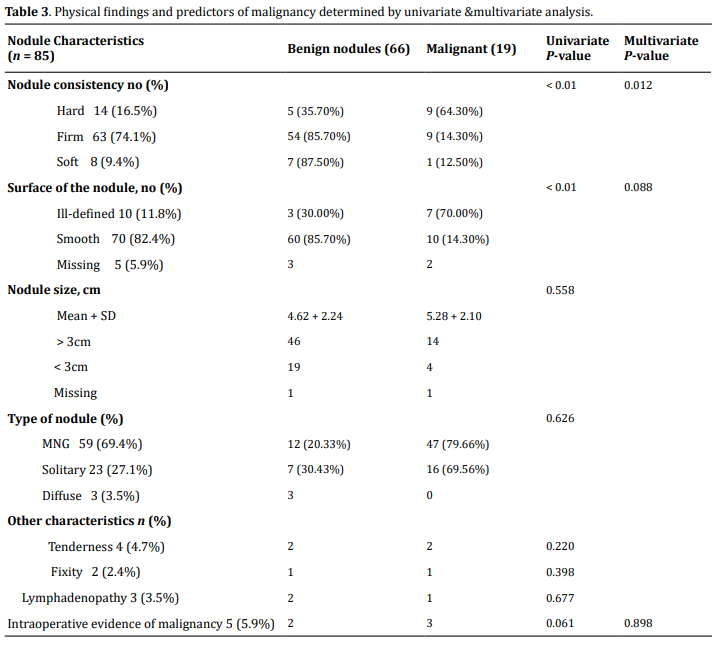
The mean size of the tumor was 4.77 + 2.21 cm, ranging from 1 cm to 15 cm. Based on size category, most patients (60, 70.6%) have size > 3.0 cm. Multinodular goiter is the commonest type of swelling (59, 69.4%) followed by solitary nodule (23, 27.1%), and diffuse (3, 3.5%) swelling. Firm consistency was the commonest (63, 74.1%) followed by hard (14, 16.5%), and soft (8, 9.4%) consistency. Most swellings (70, 82.4%) have smooth nodule surface whereas 10 (11.8%) of the nodules had irregular/ill-defined surfaces. The surface was not described in 5 (5.9%) patients' charts. Lymphadenopathy is seen in 3 (3.5%) patients. Based on preoperative serum TSH level measurement, 73 (85.9%) of the patients were found to be euthyroid. Hypothyroidism is documented in 1 (1.2%) whereas thyrotoxicosis in 11 (12.9%). Physical findings in relation to the risk of malignancy are illustrated in Table 3.
Imaging
Ultrasound of the thyroid, as an investigation modality for nodule evaluation, was used only in 39 (45.8%) patients. The rest of the patients underwent surgery based on clinical assessment, evaluation of thyroid function, and FNAC alone.
Definitive diagnosis
Definitive diagnosis of malignancy was made in 19
(22.4%) cases by postoperative histopathology evaluation.
The remaining 66 (77.6%) were benign cases.
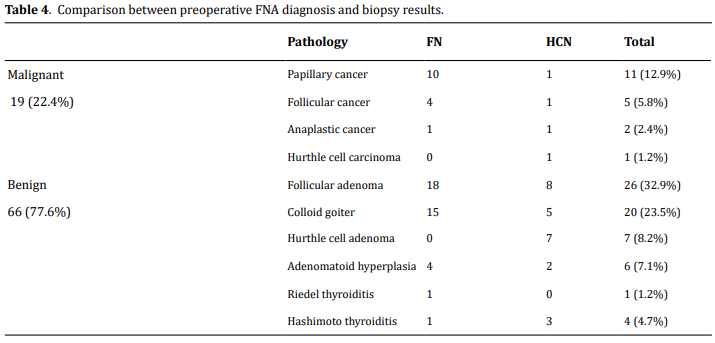
Among the malignant lesions, papillary carcinoma is
the leading malignancy (11, 12.9%) followed by follicular
carcinoma (5, 5.8%); anaplastic cancer (2, 2.4 %);
hurthle cell cancer (1, 1.2%). One of the five follicular
carcinomas was a minimally invasive encapsulated
variant.
Hurthle cell neoplasm accounts for 8 cases (12.7%);
hurthle cell adenoma 7 (8.2%) and hurthle cell carcinoma
1 (1.2%,). Benign conditions consisted of follicular
adenoma (30, 35.2%), colloid goiter (24, 28.2%), Hashimoto thyroiditis (4, 4.7%), and Riedel thyroiditis
(1, 1.2%). Comparison between cytologically indeterminate
diagnosis and postoperative definitive diagnosis
with biopsy is depicted in Table 4.
Predictors of malignancy
On univariate analysis, rapid growth (P = 0.049), hard
consistency of a nodule, and irregular surface of a
nodule showed association (each with P
< 0.01). Other variables didn't show any association with malignancy both in univariate, and bivariate
analysis.
On multivariate analysis we have found that hard consistency
is associated with thyroid malignancy with a
P-value of 0.012, AOR = 7.28 (1.5, 34.54) 95% CI and
irregular surface of the nodule found to have marginal
association; P = 0.088, AOR = 0.162 (0.020, 1.313) 95%
CI (Table 3)
Discussion
FNA cytology is generally a reliable diagnostic test in
differentiating between benign and malignant thyroid
lesions except in cytologically indeterminate lesions.
The Bethesda System is the most widely used and
standardized tool for the communication of thyroid
cytopathology [1]. Flu, AUS, follicular neoplasms, and
hurthle cell neoplasms are considered to be cytologically
indeterminate. In this group of lesions, an accurate
distinction between benign and malignant disease
cannot be made as cytology analyzes only individual
cell characteristics without basement membrane, and
the difference depends on the presence and absence
of architectural features of capsular and vascular invasion.
A diagnosis of follicular neoplasm accounted
for 63.5%
of the indeterminate cytology whereas hurthle cell
neoplasm was 34.1%. Single cases of FLUS and Suspicious
of Follicular Neoplasm were also found. Our
study found an overdiagnosis of hurthle cell neoplasm
(34.1%) on preoperative cytology compared to less
than 12% in other studies [8, 9]. HCNs are considered
variants of follicular neoplasms by many authors [10].
However, WHO classification considers Hurthle cell tumors
as a separate entity due to their peculiar genetic
profile, biological profile, and clinical features [11]. The
risk of malignancy is thought to be higher in these lesions
compared to follicular neoplasms [12]. But this is
challenged by others [13, 14].
Hurthle cells can be found in different reactive, inflammatory,
and neoplastic processes of the thyroid. For a
lesion to be diagnosed as HCN, it should be encapsulated
and predominantly consist of hurthle cells. The
amount of hurthle cells required for the diagnosis of
HCN has been defined variably in the literature. Most
authors state 75% [8, 15, 16] or 50% [17, 18] as a defining
value. In our setup, pathologists use variable cutoff
values. In the present study, only 8 out of 29 cytologically
diagnosed hurthle cell neoplasms were truly HCN
on definitive pathology diagnosis and only 1 of 29 cytologically
diagnosed HCN neoplasms turned out to be
HCC. Therefore, in our institutions, cytologic diagnosis
of HCN is not reliable and it is not associated with increased
risk of malignancy. Although, the number of
definitively diagnosed HCNs is too small in order to
make any meaningful statistical correlation.
Common biopsy findings after thyroidectomy for cytologically
indeterminate thyroid lesions include Follicular
adenoma, adenomatoid hyperplasia, follicular
carcinoma, follicular variant of papillary cancer, and
classical papillary cancer [8, 9, 15]. The incidence of malignancy
in cytologically indeterminate nodules ranges
from 14% to 48.5% [8, 9, 13, 15]. The incidence of malignancy
in the present study was 22.4%; which is comparable
with most of the reports.
Follicular variants of papillary cancer, classical papillary
cancer, and follicular thyroid cancers are malignancies
that are commonly found in such lesions [8, 9, 19].
Rarely, others such as medullary and anaplastic cancers
have been reported [9, 20]. Likewise, in the present
study, the follicular variant of papillary cancer was the
leading type, which accounted for 7 out of 19 malignant
nodules followed by follicular thyroid cancer [5, 19].
The total number of papillary cancer cases including
the classical variant was 11.
Different clinical features have been described as
predictors of malignancy in thyroid nodules. These
include the history of hoarseness of voice, history of
the rapid growth of the nodule, fixation to surrounding
structures, hard consistency of the nodule, and the illdefined
surface of the nodule [21, 22]. Furthermore, old
age, male sex, solitary nodule, and larger size of the
nodule are thought to be associated with increased
cancer risk of malignancy [9, 23]. In contrast, others reported
that old age, male sex, larger nodule size, and
solitary nodule are not predictive of malignancy [4, 24].
Large nodule size has been defined variably among different
researchers including > 3cm [9], > 4cm [4]. In the
present study, we arbitrarily used the former. Only the
hard consistency of a nodule on physical examination
was found to be associated with malignancy. Of the 15
nodules that were hard on palpation 9 (64.3%) were malignant. High rates of malignancy in hard nodules
were reported in other studies where the study group
was not limited to indeterminate cytology [25, 26].
The ill-defined surface of a nodule, usually
assessed
by
ultrasound, is suggested to be associated with malignancy
[27, 28]. In the present study, the ill-defined surface
of a nodule (irregularity) was documented based on
physical examination finding only. However, there was
a marginal correlation with malignancy, though it was
not statistically significant. Even though there was no
association with the larger size, it can be noted that the
mean size was slightly larger in malignant nodules.
Sonographic features (such as microcalcification,
hypoechoic
pattern, irregular borders), high serum thyroglobulin
concentration, genetic markers, as well as
molecular markers such as BRAF, galectin-3, RAS, RET/
PTC, and cytokeratin are associated with a high risk of
malignancy [4, 15, 29, 30]. In our series, only 45.8% of patients
had an ultrasound done for evaluation of thyroid
nodule. Most of these didn't document a complete description
of a nodule. None of the patients had serum
Thyroglobulin determined as it is not readily available
in our country. The same thing is true for genetic and
molecular means. Therefore, we couldn't analyze these
parameters as predictors of malignancy.
Though its reliability is questionable, the frozen section
is sometimes used to identify malignancy intraoperatively
and hence define the extent of surgery [7,
24]. We attempted to evaluate intraoperative clinical
evidence of malignancy as frozen section evaluation
is not available in our setup. Intraoperative features
of malignancy were present in a total of 5 patients,
among which 3 had malignancy. One of the 5 patients
had extensive fixity of the nodule to the surrounding
structures; hence malignancy was considered intraoperatively
though nothing more than debulking could
be done. Eventually, a post-operative biopsy revealed
Riedel thyroiditis.
Certain risk factors for the development of thyroid
cancer include being exposed to ionizing radiation at a
young age, having a first-degree relative with thyroid
cancer, and chronic TSH stimulation in endemic goitrous
areas [31, 32]. None of our patients had these risk
factors.
We attempted to find out if the patients are from an endemic
area. Unfortunately, it was not possible to determine
that because many patients registered only their
tentative address during the treatment period (not the
permanent address of their residence). Accordingly,
about 90% of the patients appeared to be from Addis
Ababa, which is iodine sufficient. Therefore, the meaningful
association could not be assessed. Likewise, we
had only one patient with high serum TSH who was
already on treatment.
Conclusion
The rate of malignancy in thyroid nodules with indeterminate cytology was 22.4%. Hard nodule consistency on physical examination was found to be associated with an increased risk of thyroid malignancy. The irregular surface of a nodule is marginally associated with malignancy. However other clinical parameters such as older age, large nodule size, and solitary nodule didn't show any association. FNAC is found to be inaccurate in differentiating between hurthle cell and follicular neoplasms. The routine use of ultrasound for the evaluation of thyroid nodules with cytological diagnosis of follicular or hurthle cell neoplasms in Ethiopian hospitals is low.
Operational definition
Consistency of swelling:b described as firm, soft, or
hard from physical examination findings of the most
senior examiner.
The size of the nodule:b It was described in centimters
measured along its largest dimension.
Duration of symptoms:b It described in years. When
a patient's complaint was less than a year, it is stated
infractions.
Type of swelling:b described as solitary, multinodular,
or diffuse goiter from physical examination by the
most senior examiner.
Rapid growth:b subjective complaint of the patient
claiming that there is a recent fast growth of thyroid
swelling.
Intraoperative evidence of malignancy: Intraopertive
features include infiltration or fixity to surrounding
structures /gross extrathyroidal extension, tumor
thrombus in middle thyroid and/or jugular veins,
lymph node involvement, and fragile mass.
Declarations
Availability of data and materials
A soft copy of all data used for this article are available at the corresponding author, it can be made acquired at a reasonable request.
Financial support and sponsorship
To conduct this research, the corresponding author received funding from the College of Health Sciences, Addis Ababa University.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study complied with the Declaration of Helsinki and was approved by the Research & Ethical committee of the department of surgery College of health science, Addis Ababa University.
References
1. Theoharis, C. G., Schofield, K. M., Hammers, L.,
Udelsman, R., & Chhieng, D. C. (2009). The Bethesda
thyroid fine-needle aspiration classification
system: year 1 at an academic institution. Thyroid,
19(11), 1215-1223.
2. Cibas, E. S., & Ali, S. Z. (2017). The 2017 Bethesda
system for reporting thyroid cytopathology. Thyroid,
27(11), 1341-1346.
3. Davis, N. L., Gordon, M., Germann, E., Robins, R.
E., & McGregor, G. I. (1991). Clinical parameters
predictive of malignancy of thyroid follicular neoplasms.
The American journal of surgery, 161(5),
567-569.
4. Gulcelik, N. E., Gulcelik, M. A., & Kuru, B. (2008).
Risk of malignancy in patients with follicular neoplasm:
predictive value of clinical and ultrasonographic
features. Archives of Otolaryngology-Head
& Neck Surgery, 134(12), 1312-1315.
5. Melck, A. L., & Yip, L. (2012). Predicting malignancy
in thyroid nodules: Molecular advances. Head &
neck, 34(9), 1355-1361.
6. Hawasli, A., Rizzo, P., Khoury, H., & McCaffrey, J.
L. (2002). Can fine-needle aspiration biopsy of
thyroid nodule help in determining the extent of
surgery in follicular and Hurthle cell neoplasm at a
community teaching institution? Am Surg, 68(10),
907.
7. Najah, H., & Tresallet, C. (2019). Role of frozen section
in the surgical management of indeterminate
thyroid nodules. Gland surgery, 8(Suppl 2), S112.
8. McHenry, C. R., Thomas, S. R., Slusarczyk, S. J., &
Khiyami, A. (1999). Follicular or Hu ̈rthle cell neoplasm
of the thyroid: Can clinical factors be used
to predict carcinoma and determine extent of thyroidectomy?
Surgery, 126(4), 798-804.
9. Baloch, Z. W., Fleisher, S., LiVolsi, V. A., & Gupta, P. K.
(2002). Diagnosis of “follicular neoplasm”: a gray
zone in thyroid fine-needle aspiration cytology. Diagnostic
cytopathology, 26(1), 41-44.
10. Ahmadi, S., & Stang, M. (2016). Hürthle cell carcinoma:
current perspectives. OncoTargets and
therapy, 9, 6873.
11. Klöppel, G., Couvelard, A., Hruban, R., Klimstra, D.,
Komminoth, P., Osamura, R., ... & Rindi, G. (2017).
WHO classification of tumours of endocrine organs.
Lyon, France: World Health Organization.
12. Zdon, M. J., Fredland, A. J., & Zaret, P. H. (2001).
Follicular neoplasms of the thyroid: predictors of
malignancy? Am Surg, 67(9), 880.
13. Öz, B., Doğan, S., Emek, E., Akyüz, M., Akcan, A.,
Sözüer, E., ... & Arslan, E. (2018). Predictive Factors
of Malignancy in Cytology of Indeterminate Follicular
and Hürthle Cell Neoplasms of the Thyroid
Gland. International Surgery, 103(1-2), 9-14.
14. Ren, Y., Kyriazidis, N., Faquin, W. C., Soylu, S., Kamani,
D., Saade, R., ... & Stathatos, N. (2020). The
presence of Hürthle cells does not increase the
risk of malignancy in most Bethesda categories in
thyroid fine-needle aspirates. Thyroid, 30(3), 425-
431.
15. Lee, K. H., Shin, J. H., Ko, E. S., Hahn, S. Y., Kim, J. S.,
Kim, J.-H., & Oh, Y. L. (2013). Predictive factors of
malignancy in patients with cytologically suspicious
for Hurthle cell neoplasm of thyroid nodules.
International Journal of Surgery, 11(9), 898-902.
16. Kroeker, T., Prisman, E., Shah, M., MacMillan, C., &
Freeman, J. (2014). Hurthle cell lesions-a retrospective
review of final surgical pathology. Thyroid
Disord Ther, 3(155), 2.
17. Melck, A., Bugis, S., Baliski, C., Irvine, R., Anderson,
D., Wilkins, G., ... & Wiseman, S. (2006). Hemithyroidectomy:
the preferred initial surgical approach
for management of Hurthle cell neoplasm. The
American journal of surgery, 191(5), 593-597.
18. Hudak, K., Mazeh, H., Sippel, R. S., & Chen, H. (2012).
Hürthle cell metaplasia on fine-needle aspiration biopsy is not by itself an indication for thyroid
surgery. The American journal of surgery, 203(3),
287-291.
19. Lee, S. H., Baek, J. S., Lee, J. Y., Lim, J. A., Cho, S. Y.,
Lee, T. H., ... & Kim, M. J. (2013). Predictive factors
of malignancy in thyroid nodules with a cytological
diagnosis of follicular neoplasm. Endocrine pathology,
24(4), 177-183.
20. Doddi, S., Chohda, E., Maghsoudi, S., Sheehan, L.,
Sinha, A., Chandak, P., & Sinha, P. (2015). The final
outcome of indeterminate cytology of thyroid nodules
in a District General Hospital. Il Giornale di
chirurgia, 36(3), 122.
21. Mittal, M., Ganakumar, V., Shukla, R., & Kumar, G. M.
(2020). Thyroid Nodule: Approach and Management.
22. Schlinkert, R. T., Van Heerden, J. A., Goellner, J. R.,
Gharib, H., Smith, S. L., Rosales, R. F., & Weaver, A.
L., editors. Factors that predict malignant thyroid
lesions when fine-needle aspiration is “suspicious
for follicular neoplasm”. Mayo Clinic Proceedings;
1997 : Elsevier.
23. Trimboli, P., Condorelli, E., Catania, A., & Sorrenti, S.
(2009). Clinical and ultrasound parameters in the
approach to thyroid nodules cytologically classified
as indeterminate neoplasm. Diagnostic cytopathology,
37(10), 783-785.
24. You, S. H., Jung, C. K., Chae, B. J., Song, B. J., Jung, S.
S., & Bae, J. S. (2012). Predictive Factors of Malignancy
in Thyroid Nodules Diagnosed as Follicular
Neoplasm or Hürthle Cell Neoplasm on FNA. Korean
Journal of Endocrine Surgery, 12(4), 231-238.
25. Christensen, S., Bondeson, L., Ericsson, U., & Lindholm,
K. (1984). Prediction of malignancy in the
solitary thyroid nodule by physical examination,
thyroid scan, fine-needle biopsy and serum thyroglobulin. A prospective study of 100 surgically
treated patients. Acta chirurgica scandinavica,
150(6), 433-439.
26. Dedivitis, R. A., do Couto Netto, S. D., de Castro, M.
A. F., Pfuetzenreiter Jr, E. G., Nardi, C. E. M., & de
Barbara, E. C. D. (2010). Predictive Value for Malignancy
of the Thyroid Nodule Macroscopically.
Arquivos Internacionais de Otorrinolaringologia,
14(02), 225-230.
27. Moon, W.-J., Jung, S. L., Lee, J. H., Na, D. G., Baek,
J.-H., Lee, Y. H., ... & Lee, D. H. (2008). Benign and
malignant thyroid nodules: US differentiation—
multicenter retrospective study. Radiology, 247(3),
762-770.
28. Takashima, S., Fukuda, H., Nomura, N., Kishimoto,
H., Kim, T., & Kobayashi, T. (1995). Thyroid nodules:
re-evaluation with ultrasound. Journal of
clinical ultrasound, 23(3), 179-184.
29. Bartolazzi, A., Orlandi, F., Saggiorato, E., Volante,
M., Arecco, F., Rossetto, R., ... & Bussolati, G. (2008).
Galectin-3-expression analysis in the surgical selection
of follicular thyroid nodules with indeterminate
fine-needle aspiration cytology: a prospective
multicentre study. The lancet oncology, 9(6),
543-549.
30. Nikiforov, Y. E., Steward, D. L., Robinson-Smith, T. M.,
Haugen, B. R., Klopper, J. P., Zhu, Z., ... & Nikiforova,
M. N. (2009). Molecular testing for mutations in
improving the fine-needle aspiration diagnosis of
thyroid nodules. The Journal of Clinical Endocrinology
& Metabolism, 94(6), 2092-2098.
31. Feldt-Rasmussen, U. (2001). Iodine and cancer.
Thyroid, 11(5), 483-486.
32. Liu, Y., Su, L., & Xiao, H. (2017). Review of factors
related to the thyroid cancer epidemic. International
journal of endocrinology, 2017.