Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Preoperative hematological parameters are inadequate for predicting mortality in Stanford Type A aortic dissection repair
*Corresponding author: Selim Durmaz
Mailing address: Department of Cardiovascular Surgery, Faculty of Medicine, Aydın Adnan Menderes University, Aydın
09100, Turkey.
E-mail: sdurmaz@adu.edu.tr
Received: 15 April 2021 / Accepted: 26 May 2021
DOI: 10.31491/CSRC.2021.06.075
Abstract
Background:Mortality in acute Type A aortic dissection is still high and unpredictable. We aimed to investigate the validity of preoperative hematological markers and possible risk factors in predicting in-hospital mortality in patients operated with deep hypothermic circulatory arrest method.
Methods: 78 consecutive patients who were admitted to the emergency service and operated on were retrospectively analyzed. Risk factors for in-hospital death were investigated to develop a predictive model.
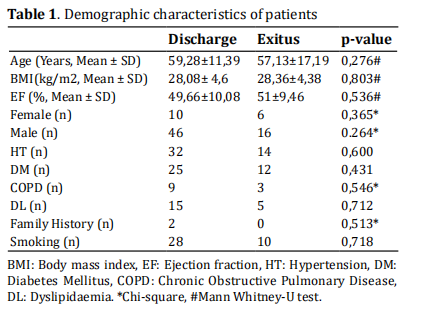
Results: There was no difference between patients in terms of the were demographic data of the patients. In
the mortality group, only preoperative creatinine levels were found to be higher (p < 0.05). Factors affecting
mortality were found as total circulatory arrest (TCA) and cross-clamp (X-clamp) times when intraoperative
data were examined (p < 0.05). ROC analysis was performed to determine the power to predict mortality and
to determine the cut-off point. In ROC analysis to predict mortality, X-Clamp time > 71 minutes, 68.2% sensitivity and 66.1% specificity, TCA > 44.5 minutes, 72.7% sensitivity and 73.2% specificity were found. In the mortality group, these values were found to be significantly higher than those who were discharged.
Conclusion: In the surgical treatment of Type A aortic dissection under deep hypothermia, hematologic biomarkers may be insufficient in estimating the risk for mortality.
Keywords
Acute; aortic dissection; biomarker; mortality
Introduction
Acute aortic dissection (AAD) is a life-threatening disease that requires rapid diagnosis and is seen in 0.3%
of patients who present to the emergency department
with chest pain [1,2]. As a result of the tear between
the intima and media layers of the aorta, restricted or
progressing along the entire aorta, it may cause symptoms of all organ systems [3,4]. Dissections starting from
the ascending aorta are classified as Type A, and dissections starting from the distal of the left subclavian
artery are classified as Type B. The current clinical
procedure is the reconstruction of Type A AAD (TAAD)
with emergency surgical intervention [5]. The mortality rate of patients who have raised mortality within
hours after diagnosis and who stay without treatment
exceeds 90% within a year. Despite satisfactory results
of surgical treatment, it still has high morbidity and
mortality rates of up to 40% [6].
Mortality in AAD is caused by a systemic inflammatory
reaction that starts with the dysfunction in multipleorgan perfusion secondary to the mechanical effect
created by blood penetrating between the aortic layers [7]. Various tests are used in the emergency room
to evaluate systemic inflammation. Therefore, the
erythrocyte distribution width (RDW), thrombocyte/
lymphocyte ratio (PLR), neutrophil/lymphocyte ratio
(NLR), and mean platelet volume (MPV) measurements were obtained from the preoperative whole
blood sample have started to be used frequently [8].
It has also been reported that it can provide predictive information about the prognosis of cardiovascular
diseases [9]. Although early diagnosis and preoperative
variables have positive effects on survival, predicting
mortality in TAAD disease is still a question that needs
an answer. It would be interesting to develop tests that
would quickly assess the severity of surgical operations to be performed on patients. However, determining the risk in surgical interventions may be dependent
on preoperative variables as well as intraoperative
variables.
Our aim in planning this retrospective clinical study
was to determine the effectiveness of preoperative
and intraoperative variables to predict mortality in patients with TAAD.
Materials and Methods
Study Population
A total of 82 consecutive patients who were diagnosed with acute TAAD in the emergency department from January 2010 to January 2018 and operated on urgently were retrospectively analyzed. Patients who underwent deep hypothermic cardiopulmonary arrest but not applied brain protection techniques were included in the study. After the study protocol was approved by Aydın Adnan Menderes University Non-Interventional Clinical Studies Ethics Committee (Ref: 53043469- 050.04.04), the data were retrospectively prepared and recorded. Patients with known hematological diseases, cancer, immunological diseases, and infections were excluded from the study. Hematological and biochemical values of 78 patients included in the study at the time of admission to the emergency department were recorded. Operational data were obtained by examining the surgery reports in files that are stored in electronic media.
Operative Technique
Deep hypothermic (18 oC) cardiopulmonary bypass was performed in all patients under general anesthesia via standard aortic, femoral, or axillary arterial cannulation and femoral or right atrial venous cannulation. Antegrade hypothermic and hyperkalemic blood cardioplegia was performed in all patients. When the deep hypothermic circulatory arrest (DHCA) was achieved, sodium pentothal was administered. The intimal tear area was found with aortotomy and was excised and made suitable for anastomosis. The anastomosis was conducted using the Dacron vascular graft instead of the removed ascending aorta. After the anastomoses were finished, the patient was started to be warmed, cardiopulmonary bypass was terminated, and the patient was transferred to intensive care.
Study groups
Study groups were divided according to the Stanford aortic dissection classification. Among the patients with TAAD, those with early postoperative mortality (within the first 30 days) were classified as Exitus, and the patients who were discharged were classified as Discharge. Regardless of hunger, preoperative blood samples taken in the emergency room were taken. Using Mindray BC 6800 (Mindray, China), full blood counts were collected, including total white blood cells, neutrophils, lymphocytes, and platelets. The ratio of thrombocytes (103 /uL) to lymphocytes (103 /uL) collected from blood samples was calculated as PLR, and the ratio of neutrophils (103 /uL) to lymphocytes (103/ uL) was calculated as NLR.
Statistical analysis
For statistical analysis, the data were analyzed using the Statistical Package for the Social Sciences (SPSS) program (IBM SPSS Statistics for Windows, Version 18.0. Armonk, NY, USA). The study of normality was carried out using either the Kolmogorov-Smirnov or Shapiro-Wilk measures. Chi-square test was used for categorical variables and Mann Whitney u test was used for continuous variables. Receiver operating curve (ROC) analysis was performed to determine the factors that may affect mortality. The significance level was accepted as p < 0.05 for all statistical evaluations.
Results
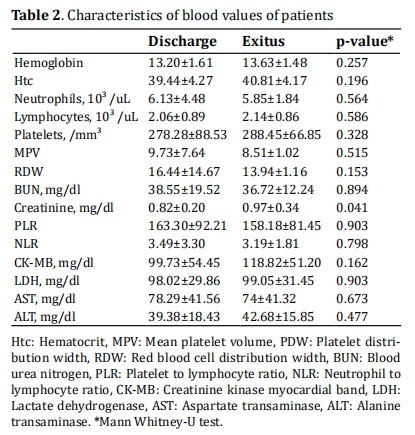
The demographic data of the patients are shown in Table 1. No difference was observed between discharged patients and patients who died. When the data were
examined in terms of preoperative blood values, there
was no difference in blood parameters such as Haemoglobin (Hb), PLR, NLR, RDW, MPV, ALT, AST, LDH, and
CK-MB. Only preoperative creatinine levels were found
to be higher in the mortality group (p < 0.05) (Table 2).
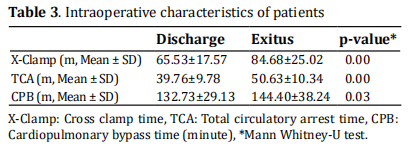
The display of intraoperative data is given in Table 3.
When these data are examined, the effect of cardiopulmonary bypass time on mortality has not been
found. It was found that the factors affecting mortality
were total circulatory arrest (TCA) and Cross clamp
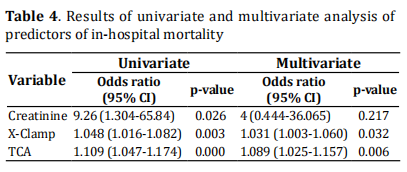
(X-clamp) times (p < 0.05). In univariate analysis, creatinine (odds ratio [OR]: 9.26; 95% CI: 1.304-65.84,
p=0.026), X-clamp (OR: 1.048; 95% CI: 1.016-1.082,
p=0.003) and TCA (OR: 1.109; 95% CI: 1.047-1.174,
p = 0.000) were associated with in-hospital mortality
after TAAD surgery. In the multivariate logistic regression model with the backward elimination method, X-clamp (OR: 1.031; 95% CI: 1.003-1.060, p < 0.032),
and TCA (OR: 1.089; 95% CI: 1.025-1.157, p = 0.006)
remained as independent predictors of in-hospital
mortality after TAAD surgery (Table 4).
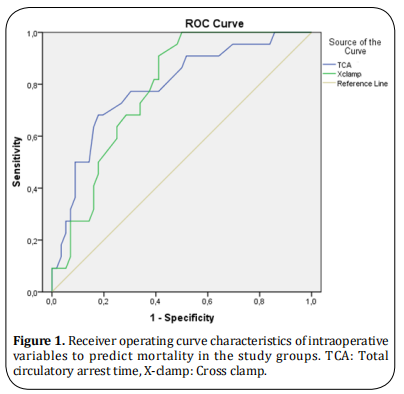
The receiver operating characteristics curve was used
to determine the sensitivity and specificity of TCA and
X-clamp time and the optimal cut-off value for predicting mortality (Figure 1). In ROC analysis, X-Clamp time
> 71 m. predicted mortality with 68.2% sensitivity and
66.1% specificity (AUC: 0.788, p < 0.05), TCA >44.5 m.
predicted mortality with 72.7% sensitivity and 73.2%
specificity (AUC: 0.778, p < 0.05). It was found that
these times were significantly higher in the mortality
group than those who were discharged.
Discussion
This study examined the relationship between mortality and preoperative hematological markers in patients
who underwent surgery for TAAD. The results we
found in our study determined that preoperative hematological markers failed to predict mortality. However, it showed that intraoperative variables, X-clamp
time and TCA times, are more important in predicting
mortality.
TAAD is a vascular clinical pathology that occurs with
high morbidity and mortality, and its incidence is increasing with prolongation of life span and prevalence
of hypertension. Patients and doctors are still concerned about the high risk of surgery. In previous studies, the efficacy of hematological markers, a simple and
inexpensive method, predicting thrombosis, inflammation, and mortality was investigated. Most of the studies conducted to show mortality are related to Type B
AAD [10]. The time from diagnosis to surgery may allow
such assessments in patients diagnosed with Type B
AAD. However, this option can often be overlooked due
to the need for rapid surgery decisions in patients diagnosed with TAAD.
Several recent studies have found an association between increased hematological inflammatory markers
and mortality [11,12]. Sbarouni et al. stated that PLR and
NLR could be used for diagnostic purposes in a group
of patients with TAAD [13]. In another study stating that
preoperative hematological markers have a predictive effect on mortality, Erdolu et al. reported that CRP and NLR were predictors of mortality [14]. In our study,
we could not find the predictive value of these markers. However, when these studies and our studies are
examined, it is controversial that it predicts mortality
despite its diagnostic benefit. One reason why it could
not be detected as a mortality indicator in our study
may be that the preoperative inflammation level may
not be high enough to cause mortality. Considering the
inflammation caused by intraoperative reasons, it may
be insufficient to determine mortality. In addition, surgical technique differences may influence in-hospital
mortality. The use of the deep hypothermic circulatory
arrest technique in our study may have had a severe
effect on intraoperative inflammation.
Many tests have been used to predict mortality. However, the reliability of these tests was limited due to
a major surgical operation affecting mortality. It was
stated that the factors affecting mortality were age,
preoperative renal dysfunction, and prolongation of
CPB duration [15–18]. In the international registry of
acute aortic dissection (IRAD) study, Rampoldi et al. [19],
it was found that prolongation of intraoperative TCA
duration was associated with mortality. In our study,
it was found to be associated with prolonged X-clamp
and TCA durations. Graft anastomosis time, which is
one of the factors that may affect the prolongation of
TCA, may be related to intact aortic tissue. In addition,
in cases where the dissection line includes the aortic
arch, mortality may increase in this patient group as
the duration of the anastomosis may increase, resulting in longer X-clamp and TCA times.
Many techniques are used to reduce mortality in the challenging surgical treatment of TAADs. Although
DHCA is one of these techniques, it has been shown
in recent years that reducing body temperature to
moderate hypothermia level with techniques in which
antegrade cerebral perfusion is combined reduces
mortality. The time to DHCA formation and re-normothermic state may have contributed to mortality by
prolonging CPB time and total operative time. Wen et
al. also found that non-TAAD survivors had a much longer X-clamp time than survivors [15]. In this study, they
stated that improving intraoperative management and
shortening the X-clamp time would contribute to improving in-hospital mortality. In addition to shortening
the intraoperative time, avoiding a deep hypothermic
state may contribute to the reduction of the systemic
inflammatory response in the patient and thus to better results.
The main technical difficulty of TAAD operations is that
they require a long time and operations are performed
under cardiopulmonary bypass. Similarly, we found a
generally known relationship between long operative
time and in-hospital mortality. However, these periods
differ in various studies. X-clamp time Nissinen et al. is
150 min, Kawahito et al. 240 min, Wu et al. found that
it was associated with the risk of mortality at 160 min
[20-22]. In our study, the X-clamp time was found to be 71
minutes according to Roc curve analysis (OR: 1.031;
95% CI: 1.003-1.060, p < 0.032). Unlike our study, we
see that antegrade cerebral perfusion techniques and
moderate hypothermia techniques are used in these
studies. In TAAD, shortening the intraoperative time
and using cerebral protection techniques contribute to
safe operation time. Increasing the safe surgical time in
operations to be performed under deep hypothermia
is an important way to improve in-hospital mortality.
The data used in this study, which was planned as a
retrospective cohort study, have limitations. The time
from the symptoms of the patients to the hospital admission may affect the level of inflammation. The time
that passes due to the distance of the patients from
our center may have an impact on mortality. It will be
useful to evaluate the results under these conditions.
Causes such as late diagnosis, tamponade due to aortic
rupture, and multiple organ failure, which are among
the data of preoperative conditions that may affect
mortality, could not be presented in our study.
In conclusion, TAAD continues to be an emergency
cardiovascular pathology with high mortality and challenging treatment. Although studies have been carried
out for years to predict mortality, and an effective option has not yet been put into use. There is a need for
studies involving larger patient groups in which the
mortality of patients undergoing surgery is evaluated
in addition to preoperative variables, perioperative
surgical, and hematologic variables.
Declarations
Authors’ contributions
Selim Durmaz: Conceived the project, carried out to acquisition and analysis of data, contributed to drafting
and approval of the final manuscript.
Ömer Faruk Rahman: Conceived the project, carried
out to acquisition and analysis of data, contributed to
drafting and approval of the final manuscript.
Conflicts of interest
All authors declared that there are no conflicts of interest.
References
1. Ohle, R., Um, J., Anjum, O., Bleeker, H., Luo, L., Wells, G., &
Perry, J. J. (2018). High risk clinical features for acute aortic dissection: a case–control study. Academic Emergency
Medicine, 25(4), 378-387.
2. Manea, M. M., Dragos, D., Antonescu, F., Sirbu, A. G., Tiron,
A. T., Dobri, A. M., & Tuta, S. (2019). Aortic Dissection:
An Easily Missed Diagnosis when Pain Doesn’t Hold the
Stage. The American journal of case reports, 20, 1788.
3. Mussa, F. F., Horton, J. D., Moridzadeh, R., Nicholson, J.,
Trimarchi, S., & Eagle, K. A. (2016). Acute aortic dissection and intramural hematoma: a systematic review. Jama, 316(7), 754-763.
4. Minegishi, S., Watanabe, H., Horita, N., Shibata, Y., Kaneko,
T., & Ishigami, T. (2016). The current evidence on diagnosis and treatment of acute aortic syndrome. Journal of
thoracic disease, 8(12), E1617.
5. Baliyan, V., Parakh, A., Prabhakar, A. M., & Hedgire, S.
(2018). Acute aortic syndromes and aortic emergencies. Cardiovascular diagnosis and therapy, 8(Suppl 1),
S82.
6. Bashir, M., Harky, A., Fok, M., Shaw, M., Hickey, G. L., Grant,
S. W., ... & Oo, A. (2017). Acute type A aortic dissection
in the United Kingdom: surgeon volume-outcome relation. The Journal of thoracic and cardiovascular surgery, 154(2), 398-406.
7. Ramanath, V. S., Oh, J. K., Sundt III, T. M., & Eagle, K. A.
(2009, May). Acute aortic syndromes and thoracic aortic
aneurysm. In Mayo Clinic Proceedings (Vol. 84, No. 5, pp.
465-481). Elsevier.
8. Bedel, C., & Selvi, F. (2019). Association of platelet to lymphocyte and neutrophil to lymphocyte ratios with in-hospital mortality in patients with type A acute aortic dissection. Brazilian journal of cardiovascular surgery, 34(6),
694-698.
9. Monteiro Júnior, J. G. D. M., & de Oliveira Cipriano Torres, D. (2019). Hematological parameters as prognostic
biomarkers in patients with cardiovascular diseases. Current Cardiology Reviews, 15(4), 274-282.
10. Zhang, J., Cheng, B., Yang, M., Pan, J., Feng, J., & Cheng, Z.
(2019). Predicting in-hospital death in patients with type
B acute aortic dissection. Medicine, 98(32).
11. Liu, H., Li, D., Jia, Y., & Zeng, R. (2020). Predictive Value of
White Blood Cells, Neutrophils, Platelets, Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios in Patients
with Acute Aortic Dissection. Brazilian Journal of Cardiovascular Surgery, 35(6):1031-1033.
12. Li, D. Z., Chen, Q. J., Sun, H. P., Zeng, R., Zeng, Z., Gao, X. M.,
... & Yang, Y. N. (2016). Mean platelet volume to platelet
count ratio predicts in-hospital complications and long term mortality in type A acute aortic dissection. Blood
Coagulation & Fibrinolysis, 27(6), 653-659.
13. Sbarouni, E., Georgiadou, P., Kosmas, E., Analitis, A., &
Voudris, V. (2018). Platelet to lymphocyte ratio in acute
aortic dissection. Journal of clinical laboratory analysis, 32(7), e22447.
14. Erdolu, B., & As, A. K. (2020). C-Reactive Protein and Neutrophil to Lymphocyte Ratio Values in Predicting Inhospital Death in Patients with Stanford Type A Acute Aortic
Dissection. The Heart Surgery Forum, 23(4), 488-492.
15. Wen, M., Han, Y., Ye, J., Cai, G., Zeng, W., Liu, X., ... & Zeng, H.
(2019). Peri-operative risk factors for in-hospital mortality in acute type A aortic dissection. Journal of thoracic
disease, 11(9), 3887.
16. Cabasa, A., & Pochettino, A. (2016). Surgical management
and outcomes of type A dissection—the Mayo Clinic experience. Annals of cardiothoracic surgery, 5(4), 296.
17. Harky, A., Singh, V. P., Khan, D., Sajid, M. M., Kermali, M., &
Othman, A. (2020). Factors Affecting Outcomes in Acute
Type A Aortic Dissection: A Systematic Review. Heart,
Lung and Circulation, 29(11), 1668-1681.
18. Zhou, W., Wang, G., Liu, Y., Tao, Y., Du, Z., Tang, Y., ... &
Xu, Z. (2019). Outcomes and risk factors of postoperative hepatic dysfunction in patients undergoing acute
type A aortic dissection surgery. Journal of thoracic disease, 11(8), 3225.
19. Rampoldi, V., Trimarchi, S., Eagle, K. A., Nienaber, C. A., Oh,
J. K., Bossone, E., ... & International Registry of Acute Aortic Dissection (IRAD) Investigators. (2007). Simple risk
models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic
Dissection score. The Annals of Thoracic Surgery, 83(1),
55-61.
20. Nissinen, J., Biancari, F., Wistbacka, J. O., Peltola, T., Loponen, P., Tarkiainen, P., ... & Tarkka, M. (2009). Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion, 24(5), 297-305.
21. Kawahito, K., Adachi, H., Yamaguchi, A., & Ino, T. (2001).
Preoperative risk factors for hospital mortality in acute
type A aortic dissection. The Annals of thoracic surgery, 71(4), 1239-1243.
22. Wu, Y., Jiang, R., Xu, P., Wang, G., Wang, J., & Yang, S. (2018).
Perioperative Results and Risk Factors for In-Hospital
Mortality In Patients With Stanford Type A Aortic Dissection Undergoing Sun’s Procedure-A Single Center Study.
The heart surgery forum, 21(6), 432-437.