Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
LncRNA NEAT1 is involved in temozolomide resistance by regu- lating MGMT in glioblastoma multiforme
*Corresponding author: Jian Shen, M.D.
Mailing address: Department of Neurosurgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, 79 Qingchun Rd., Hangzhou 310003, People’s Republic of China.
E-mail: 1314006@zju.edu.cn
Received: 20 January 2018 Accepted: 20 March 2018
DOI: 10.31491/CSRC.2018.3.011
Abstract
Introduction: As one of the most aggressive and lethal tumors, glioblastoma multiforme (GBM) is commonly treated by surgical resection combined with radiotherapy and chemotherapy. Temozolomide (TMZ) is the preferred chemotherapy medicine against GBM. However, recurrent GBM patients exhibit TMZ resistance. As reported in previous studies, NEAT1 is over-expressed in glioma cells; thus, we explored the relationship between NEAT1 and TMZ resistance in GBM.
Methods: The expression levels of NEAT1 and O-6-methylguanine-DNA methyltransferase (MGMT) in GBM tissues and cells were determined by quantitative real-time PCR. Si-RNA and the over-expression vector were transfected into GBM cells to modulate the level of related molecules. Western blot analysis was used to investigate the protein expression of MGMT. The cell viability and IC-50 were analyzed by CCK-8, and apoptosis was detected by flow cytometry assay.
Result: The expression level of NEAT1 in the TMZ-sensitive GBM tissues and cells was lower than that in the TMZ-resistant GBM tissues and cells. Furthermore, due to the down-regulation of NEAT1 in TMZ-resistant GBM cells transfected with Si-RNA, the viability and IC-50 value of the GBM cell lines were decreased, and the knockdown of NEAT1 significantly enhanced TMZ-induced cell apoptosis in GBM cells. We also determined that the mRNA and protein level of MGMT were up-regulated in TMZ-resistant GBM cells. Interference with MGMT expression led to a decrease in viability and IC-50 value in the GBM cells, and the knockdown of NEAT1 suppressed the transcription and translation levels of MGMT. However, the over-expression of MGMT enhanced TMZ resistance in NEAT1-silenced U87 and U251 cells.
Conclusion: NEAT1 participates in the TMZ resistance of GBM cells by regulating MGMT.
Keywords
lncRNA NEAT1; glioblastoma multiforme; Temozolomide; O-6-methylguanine-DNA methyltransferase.
Introduction
Among all central nervous system cancers, glioma is
the primary tumor and the most common [1,2]. Glioblastoma multiforme (GBM) is one of the most malignant types of glioma, with a poor patient survival rate
[3]. After a confirmed diagnosis, the therapy for GBM
includes radiotherapy, chemotherapy, and surgical
resection. Temozolomide (TMZ), which damages DNA
strands via the methylation of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter, is the
most effective chemotherapy in clinical application for
providing hope to GBM patients [4]. However, in longterm therapy, some GBM patients exhibit hardly any
sensitivity to TMZ, and recrudescent GBM patients also develop TMZ resistance, suppressing the efficacy of
TMZ chemotherapy [5]. It has been reported that MGMT
is over-expressed in glioblastomas, enhancing resistance to TMZ [6]. Nevertheless, several studies indicate
that the up-regulation of MGMT cannot completely
prevent TMZ resistance in GBM [7,8]. Hence, it is important to explore the underlying mechanisms of chemoresistance to TMZ.
Long non-coding RNA (lncRNA), a single-strand RNA
with about 200 nucleotides, has been found to be associated with many physiological and pathological cell
processes, such as embryonic development, cell differentiation, proliferation, and tumorigenesis [9]. On the
other hand, lncRNAs perform the duties of molecular
mediators, including cellular signaling, acting as molecular decoys, and contributing to scaffold modeling [10].
Therefore, lncRNAs play a pivotal role in the growth of
tumors. Without exception, lncRNA nuclear-enriched
abundant transcript 1 (NEAT1) has been defined as
an oncogene in many human cancers, altering the epigenetic landscape of target gene promoters to drive cell growth in prostate cancer [11], contributing to the
poor survival rate of breast cancer patients [12], and advancing the progression of non-small-cell lung cancer
[13]. As reported in previous studies, NEAT1 is over-expressed in glioma cells, promoting glioma tumorigenesis and impacting the glioma prognosis [14,15]. However,
the influence of lncRNA NEAT1 on TMZ resistance in
GBM has been unclear.
In this study, we first defined the expression level of NEAT1 in TMZ-resistant GBM cells and the impact of the down-regulation of NEAT1 on the TMZ resistance of these cells. In addition, we explored the potential relationship between NEAT1 and MGMT in the TMZ resistance of these cells. Our study offers a novel angle on the remission of TMZ resistance in GBM.
Materials and Methods
Patients and specimens
There were 112 GBM patients enrolled in our study. Of these, 40 were diagnosed with initial glioblastoma multiforme, had not undergone chemotherapy or radiotherapy, and were sensitive for TMZ. The glioblastoma multiforme of the other 72 patients, who were resistant to TMZ, was recrudescent. All patients were treated with surgical resection at our hospital. The tissues obtained were stored with liquid nitrogen after the written approval of the Institutional Research Ethics Board and the informed consent of all enrolled patients. This study was supported by Ethics committee of The First Affiliated Hospital, Zhejiang University. The expression of NEAT1 in these GBM tissues was determined by quantitative reverse transcription real-time PCR.
Cell lines
Human glioblastoma multiforme cell lines U87 and U251, which were purchased from the Cell Culture Center of the Chinese Academy of Medical Sciences (Beijing, China), were cultured in DEME medium containing 10% FBS, 100 U/mL of penicillin, and 100 μM of streptomycin at 37°C in a humid environment with 5% CO2. The method of obtaining the TMZ-resistant cell lines U87-R and U251-R has been described previously [16]. Briefly, after being maintained with 10 μM of TMZ for two weeks, the GBM cell lines U87 and U251 were sequentially exposed to TMZ, the concentration of which was enhanced about twofold every two stages until it reached the maximum concentration, which could lead to the death of all resistant cells. The TMZ-resistant cell lines U87-R and U251-R were induced to be established at 200 μM of TMZ.
Cell transfection
Si-NEAT1, used to suppress NEAT1 expression; Si-MGMT, used to depress MGMT expression; and MGMT-pcDNA vector, used to over-express MGMT, were purchased from Ribobio (Guangzhou, China). The non-targeting control Si-RNA (Mock) and the empty vector (pcDNA) served as negative controls. After being cultured for 24 hours, the GBM cell lines were performed for transfection by Opti-MEM I and Lipofectamine 3000 (Invitrogen, CA, USA) using the standard system of the manufacturer’s protocol. To obtain stable cell lines, G418 (Invitrogen, CA, USA) was added to the selection medium.
RNA isolation and qRT-PCR
TRIzol reagent (Invitrogen, San Diego, CA, USA) was used to extract the total RNA following the manufacturer’s protocol. After the extracted RNA was reversely transcribed to cDNA, the resultant was used with the miRNA qPCR Quantitation Kit (Genepharma, Shanghai, China) to perform quantitative real-time PCR in the ABI PRISM 7300 RT-PCR system (Applied Biosystems, Foster, CA, USA). The 2-ΔΔCt method was used to carry the related level of RNA expression.
Cell viability with TMZ and IC-50 value
The GBM cells were seeded in 96-well plates with 100 μM of TMZ and cultured for 24h, 48h, 72h, and 96h. At the time of harvest, 10 μL of CCK-8 (Roche Biochemicals, Mannheim, Germany) were added to every well. After 4 hours, the cell viability was indicated by the absorbance value, measured at 450 nm. IC-50, the half-maximal inhibitory concentration, represented the concentration of TMZ required for 50% inhibition of the GBM cells. The IC-50 value was detected by the CCK-8 reagent and calculated by the Graph Pad Prism software (Graph Pad Software, La Jolla, CA, USA).
Cell apoptosis
The GBM cells were stained using a V-FITC/PI double staining kit (Key GEN, Shanghai, China) according to the manufacturer’s protocol. The apoptosis of the GBM cells was surveyed by flow cytometry (FACScan, BD Biosciences).
Western blotting assay
The western blotting assay process has been described previously [17]. The antibodies against MGMT and GADPH, regarded as loading controls, were purchased from NeoMarkers (Fremont, CA, USA). The protein bands were imaged by a Fluor S Multi-Imager (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data were analyzed using the SPSS 20.0 software (SPSS, Chicago, IL, USA) and the Graph Pad Prism software (Graph Pad Software, La Jolla, CA, USA) and expressed as mean ± SD. Student’s test, the chi-square test, or one-way ANOVA were used to calculate the significance of the differences. A P value of less than 0.01 was considered statistically significant.
Results
NEAT1 was over-expressed in the recrudescent GBM tissues with TMZ resistance.
For analyzing the effect of NEAT1 expression on TMZ resistance, 40 patients who were initially diagnosed with GBM and detected as sensitive to TMZ and 74 recrudescent GBM cases with TMZ resistance were gathered. The expression of NEAT1 in the tumor tissues of the two groups was defined, and NEAT1 expression was evidently up-regulated in the recrudescent patients (Figure 1).
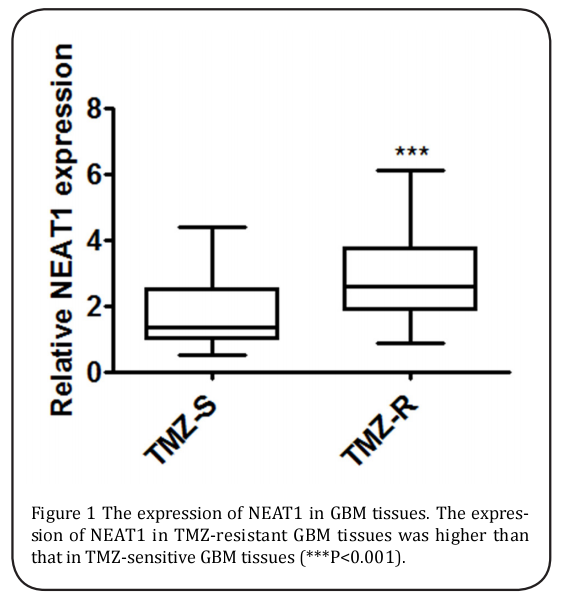
NEAT1 over-expression was associated with TMZ resistance in the GBM cells.
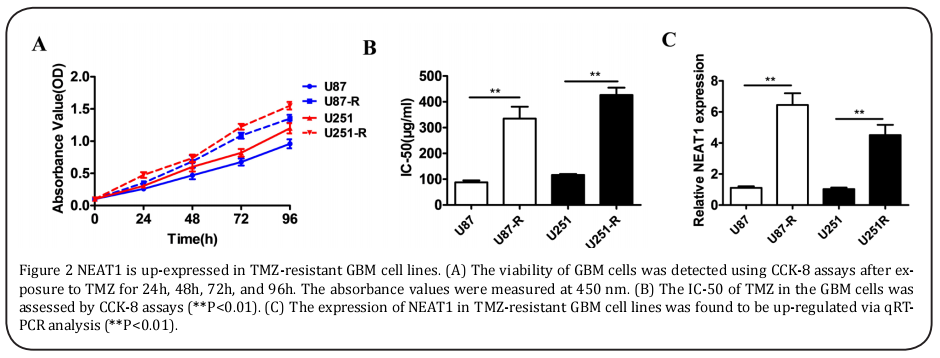
As the data above show a higher level of NEAT1 expression in the TMZ-resistant cases, the TMZ-resistant cell lines U87-R and U251-R, which were continuously exposed in the senior concentration of TMZ, were obtained. According to the CCK-8 assays, the TMZ-resistant cell lines U87-R and U251-R indicated obvious growth superiority with 100 μM of TMZ (Figure 2A). Because of their enhanced resistance, the U87-R and U251-R cells revealed the higher IC-50 values (≥three fold) compared to the U87 and U251 cells (Figure 2B). Similarly, the expression of NEAT1 was enhanced (>four fold) in the U87-R and U251-R cells (Figure 2C).
The knockdown of NEAT1 inhibited TMZ resistance and promoted cell apoptosis in the GBM cells.
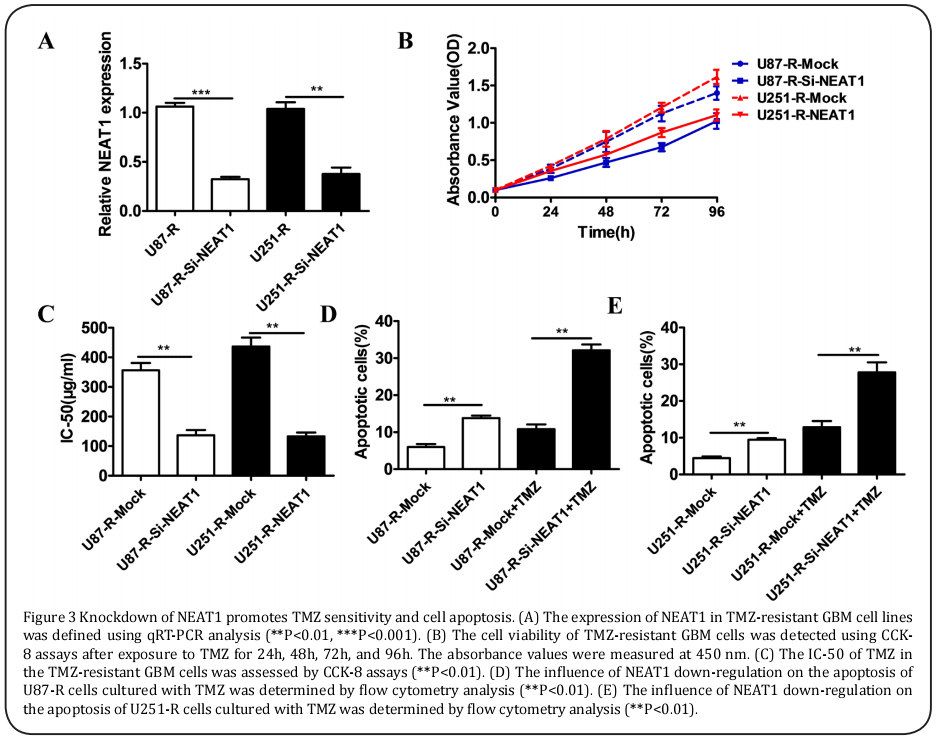
To further explore the role of NEAT1 in GBM cells, the U87-R and U251-R cells were transfected with Si-RNA, which interfered with NEAT1 regulation. As shown in Figure 3A, compared with those in the controls, the levels of NEAT1 expression were markedly lower in the U87-R-Si-NEAT1 and U251-R-Si-NEAT1 cells. In accordance with the CCK-8 assays, the cell viability with 100 μM of TMZ and the IC-50 value of the U87-R and U251-R cells transfected with Si-NEAT1 were visibly reduced compared to those of the control cells (Figure 3B and 3C). Meanwhile, the down-regulation of NEAT1 resulted in a remarkable increase in apoptotic cells in the U87-R and U215-R cells cultured with 100μM of TMZ (Figure 3D and 3E).
The down-regulation of NEAT1 depressed the expression of MGMT
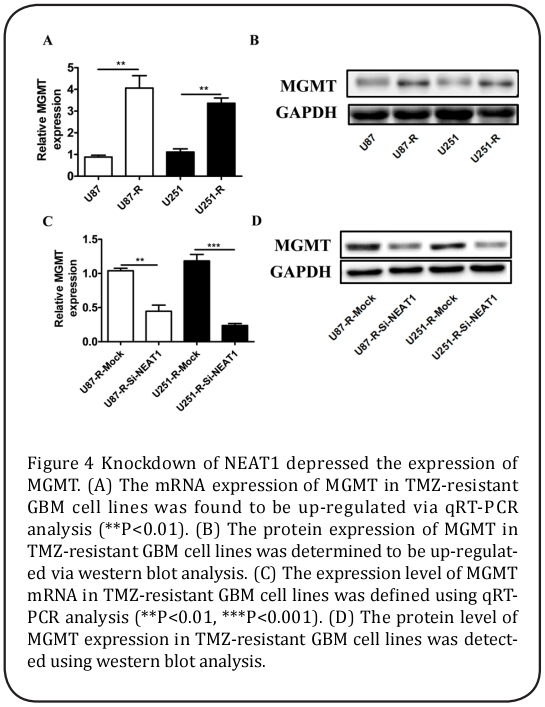
Because a large number of studies have reported that MGMT is related to the TMZ resistance of GBM cells, qRT-PCR and western blot assays were performed. The results showed that the mRNA and protein expression levels of MGMT in the U87-R and U215-R cells were significantly enhanced compared to those in the U87 and U251 cells (Figure 4A and 4B), while the levels of MGMT mRNA and protein expression in the U87-R-SiNEAT1 and U251-R-Si-NEAT1 cells were evidently suppressed by NEAT1 deletion (Figure 4C and 4D).
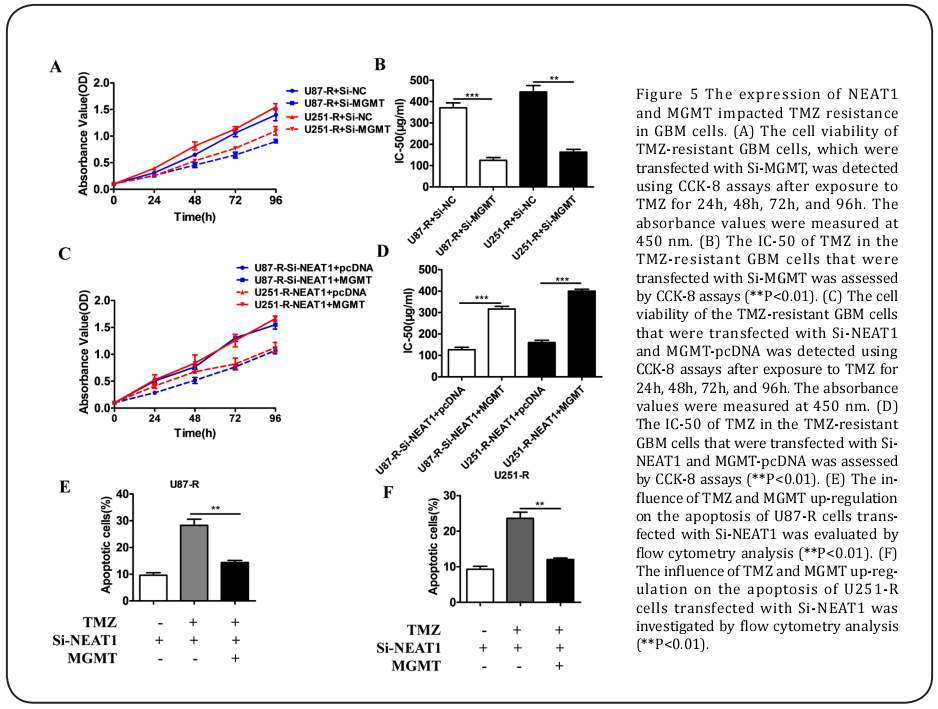
NEAT1 knockdown and the over-expression of MGMT impacted TMZ resistance in the GBM celles
The CCK-8 assays illustrated that because of the down-regulation of MGMT in the U87-R and U251-R cells, the IC-50 value and cell viability with 100 μM of TMZ in the U87-R-Si-MGMT and U251-R-Si-MGMT cells were inhibited compared to those of the controls (Figure 5A and 5B). Also, the IC-50 and cell viability with 100 μM of TMZ in the U87-R-Si-NEAT1+MGMT and U251-R-Si-NEAT1+MGMT cells were obviously increased with the over-expression of MGMT (Figure 5C and 5D). In addition, the down-regulation of NEAT1 combined with TMZ could significantly advance the apoptosis of the U87-R and U251-R cells, while the up-regulation of MGMT in the U87-R-Si-NEAT1+MGMT and U251-R-Si-NEAT1+MGMT cells combined with TMZ lowered cell apoptosis in comparison with that of the U87-R-Si-NEAT1 and U251-R-Si-NEAT1 cells with TMZ (Figure 5E and 5F).
Discussion
As one of the most aggressive and lethal tumors, GBM is commonly treated by surgical resection combined with radiotherapy and chemotherapy [18,19]. TMZ is the preferred chemotherapy medicine against GBM, for which there are very few efficient drug treatments [20]. Another unhopeful situation is that recurrent GBM patients exhibit TMZ resistance following first-time chemo-treatments with TMZ [21]. Many studies have found that lncRNAs are involved in various biological processes in cancer cells, such as cell cycle control, transcriptional and translational regulation, apoptosis, cell invasion, and cell migration[22-25].
Large amounts of evidence reveal that the chemoresistance of tumors is associated with lncRNAs. For instance, the lncRNA HOTTIP contributes to gemcitabine resistance in pancreatic cancer [26], the lncRNA UCA1 enhances cisplatin/gemcitabine resistance in bladder cancer cells [27], and the lncRNA PVT1 promotes multidrug resistance in gastric cancer [28]. Although it has been reported that the lncRNA NEAT1 is over-expressed in GBM tissues compared with healthy brain tissues [14], the function of NEAT1 in TMZ resistance has not been previously studied. Comparing lncRNA NEAT1 between recrudescent GBM tissues and initial GBM tissues, we found that NEAT1 expression was increased in the recrudescent tissues. Meanwhile, the expression level of NEAT1 in TMZ-sensitive cells (U87 and U251) was lower than that in TMZ-resistant cells (U87-R and U251-R), suggesting that NEAT1 is associated with TMZ resistance in GBM cells. The different expression levels of NEAT1 in GBM provide potential prognosis values.
Furthermore, the up-regulation or down-regulation of lncRNAs could change the progression of tumors. The knockdown of TUG1 by SiRNA has obviously depressed cell proliferation and promoted cell apoptosis in colon cancer cells [29], while the over-expression of the lncRNA GAS5 may inhibit cell growth and enhance gefitinib sensitivity in lung adenocarcinoma cells [30]. The knockdown of CARLo-5 in gastric cancer cell lines has markedly suppressed cell proliferation by inducing G0/ G1 cell-cycle arrest and apoptosis [31]. Similarly, due to the down-regulation of NEAT1 in TMZ-resistant GBM cells transfected with Si-RNA, the viability and IC-50 value of the GBM cell lines were decreased. This result shows that interfering with NEAT1 expression has the benefit of depressing TMZ resistance in GBM cells. Further, the knockdown of NEAT1 significantly enhanced TMZ-induced cell apoptosis in GBM cells. In other words, the TMZ sensitivity of GBM cells could be significantly improved. This result suggests a novel target for enhancing the efficacy of TMZ chemotherapy.
Previous studies have shown that the dysregulation of MGMT is the primary cause of TMZ resistance in GBM cells[17,32,33]. Similarly, we determined that the mRNA and protein levels of MGMT were up-regulated in TMZ-resistant GBM cells. Interfering with MGMT expression led to decreases in the viability and IC-50 value of the GBM cells, equal to those resulting from NEAT1 silencing. Moreover, the knockdown of NEAT1 suppressed the transcription and translation levels of MGMT. However, the over-expression of MGMT in NEAT1-silenced GBM U87 and U251 cells enhanced these cells’ TMZ resistance.
In summary, not only was the expression of NEAT1 enhanced in TMZ-resistant GBM cells, but NEAT1 was involved in the TMZ resistance of GBM cells by regulating MGMT.
Acknowledgements
This work was supported by grants of Zhejiang Provincial Natural Science Foundation (no. LY16H090004), National Natural Science Foundation of China (no. 81501065), Zhejiang Provincial Traditional Chinese Medicine Science and Technology Plan (no. 2016ZA115). Zhejiang Provincial Medical and Health Science and Technology Plan (no.2015KYA091).
References
1. Siegel, R., Ma, J., Zou, Z., and Jemal, A. (2014) Cancer statistics, 2014. CA: A Cancer Journal for Clinicians 64, 9–29
2. Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E.,
and Forman, D. (2011) Global cancer statistics. CA:
A Cancer Journal for Clinicians 61, 33-64, 31
3. Johnson, D. R., and O'Neill, B. P. (2012) Glioblastoma survival in the United States before and during
the temozolomide era. Journal of Neuro-Oncology
107, 359-364
4. Jiang, G., Li, L. T., Xin, Y., Zhang, L., Liu, Y. Q., and
Zheng, J. N. (2012) Strategies to improve the killing of tumors using temozolomide: targeting the
DNA repair protein MGMT. Current Medicinal
Chemistry 19, 3886-3892
5. Caldera, V., Mellai, M., Annovazzi, L., Monzeglio,
O., Piazzi, A., and Schiffer, D. (2012) MGMT hypermethylation and MDR system in glioblastoma
cancer stem cells. Cancer Genomics & Proteomics
9, 171-178
6. Taylor, J. W., and Schiff, D. (2015) Treatment Considerations for MGMT-Unmethylated Glioblastoma.
Current Neurology and Neuroscience Reports 15,
507-507
7. Yu, Z., Zhao, G., Xie, G., Zhao, L., Chen, Y., Yu, H.,
Zhang, Z., Cai, L., and Li, Y. (2015) Metformin and
temozolomide act synergistically to inhibit growth
of glioma cells and glioma stem cells in vitro and in
vivo. Oncotarget 6, 32930-32943
8. Zhang, J., Stevens, M. F., and Bradshaw, T. D. (2012)
Temozolomide: mechanisms of action, repair and
resistance. Current Molecular Pharmacology 5,
102-114
9. Wilusz, J. E. (2015) Long noncoding RNAs: Re-writing dogmas of RNA processing and stability. Biochimica et biophysica acta 1859, 128-138
10. Zhang, X., Sun, S., Pu, J. K., Tsang, A. C., Lee, D., Man,
V. O., Lui, W. M., Wong, S. T., and Leung, G. K. (2012)
Long non-coding RNA expression profiles predict
clinical phenotypes in glioma. Neurobiology of Disease 48, 1-8
11. Chakravarty, D., Sboner, A., Nair, S. S., Giannopoulou, E., Li, R., Hennig, S., Mosquera, J. M., Pauwels,
J., Park, K., and Kossai, M. (2014) The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical
modulator of prostate cancer. Nature Communications 5, 5383-5383
12. Choudhry, H., Albukhari, A., Morotti, M., Hider, S.,
Moralli, D., Smythies, J., Green, C. M., Camps, C.,
Buffa, F., and Ratcliffe, P. (2014) Tumor hypoxia induces nuclear paraspeckle formation through HIF-
2 dependent transcriptional activation of NEAT1
leading to cancer cell survival. Journal of Applied
Psychology 68, 102-114
13. Sun, C., Li, S., Zhang, F., Xi, Y., Wang, L., Bi, Y., and Li,
D. (2016) Long non-coding RNA NEAT1 promotes
non-small cell lung cancer progression through
regulation of miR-377-3p-E2F3 pathway. Oncotarget
14. Zhen, L., Yun-Hui, L., Hong-Yu, D., Jun, M., and YiLong, Y. (2016) Long noncoding RNA NEAT1 promotes glioma pathogenesis by regulating miR-
449b-5p/c-Met axis. Tumor Biology 37, 673-683
15. He, C., Jiang, B., Ma, J., and Li, Q. (2016) Aberrant
NEAT1 expression is associated with clinical outcome in high grade glioma patients. APMIS 124
16. Jiang, P., Ping, W., Sun, X., Yuan, Z., Zhan, R., Ma, X.,
and Li, W. (2016) Knockdown of long noncoding
RNA H19 sensitizes human glioma cells to temozolomide therapy. Oncotargets & Therapy 9, 3501-
3509
17. Kitange, G. J., Carlson, B. L., Schroeder, M. A., Grogan, P. T., Lamont, J. D., Decker, P. A., Wu, W., James,
C. D., and Sarkaria, J. N. (2009) Induction of MGMT
expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-Oncology
11, 281-291
18. Hegi, M. E., Janzer, R. C., Lambiv, W. L., Gorlia, T.,
Kouwenhoven, M. C. M., Hartmann, C., Deimling,
A. V., Martinet, D., Schmutz, N. B., and Diserens, A.
C. (2012) Presence of an oligodendroglioma-like
component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup
and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta
Neuropathologica 123, 841-852
19. Wang, Y., and Jiang, T. (2013) Understanding high
grade glioma: molecular mechanism, therapy and
comprehensive management. Cancer Letters 331,
139-146
20. Stupp, R., Mason, W. P., Van, d. B., Martin J., Weller,
M., Fisher, B., Taphoorn, M. J. B., Belanger, K.,
Brandes, A. A., Marosi, C., and Bogdahn, U. (2005)
Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. Dkgest of the World
Latest Medical Information 352, 987-996
21. Strowd, R. E., Blackwood, R., Brown, M., Harmon,
M., Lovato, J., Yalcinkaya, T., and Lesser, G. (2013)
Impact of temozolomide on gonadal function in
patients with primary malignant brain tumors.
Journal of Oncology Pharmacy Practice Official
Publication of the International Society of Oncology Pharmacy Practitioners 19, 321-327
22. Wang, Y., Wang, Y., Li, J., Zhang, Y., Yin, H., and Han, B.
(2015) CRNDE, a long-noncoding RNA, promotes
glioma cell growth and invasion through mTOR
signaling. Cancer letters 367, 122-128
23. Xu, W. H., Zhang, J. B., Dang, Z., Li, X., Zhou, T., Liu, J.,
Wang, D. S., Song, W. J., and Dou, K. F. (2014) Long
non-coding RNA URHC regulates cell proliferation
and apoptosis via ZAK through the ERK/MAPK
signaling pathway in hepatocellular carcinoma. International Journal of Biological Sciences 10, 664-
676
24. Li, Z., Shen, J., Chan, M., and Wu, W. (2016) TUG1: a
pivotal oncogenic long non-coding RNA of human
cancers. Cell Proliferation 49, 471-475
25. Cheng, Y., Jutooru, I., Chadalapaka, G., Corton, J.
C., and Safe, S. (2015) The long non-coding RNA
HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget 6, 10840-
10852
26. Li, Z. (2015) The long non-coding RNA HOTTIP
promotes progression and gemcitabine resistance
by regulating HOXA13 in pancreatic cancer. Journal of Translational Medicine 13, 1-16
27. Pan, J., Li, X., Wu, W., Xue, M., Hou, H., Zhai, W., and
Chen, W. (2016) Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through
CREB modulating miR-196a-5p in bladder cancer
cells. Cancer Letters 382, 64-76
28. Zhang, X. W., Bu, P., Liu, L., Zhang, X. Z., and Li, J.
(2015) Overexpression of long non-coding RNA
PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochemical & Biophysical Research Communications 462, 227-232
29. Zhai, H. Y., Sui, M. H., Yu, X., Qu, Z., Hu, J. C., Sun, H.
Q., Zheng, H. T., Zhou, K., and Jiang, L. X. (2016)
Overexpression of Long Non-Coding RNA TUG1
Promotes Colon Cancer Progression. Medical Science Monitor International Medical Journal of Experimental & Clinical Research 22, 3281-3287
30. Dong, S., Qu, X., Li, W., Zhong, X., Li, P., Yang, S.,
Chen, X., Shao, M., and Zhang, L. (2015) The long
non-coding RNA, GAS5, enhances gefitinib-induced cell death in innate EGFR tyrosine kinase
inhibitor-resistant lung adenocarcinoma cells with
wide-type EGFR via downregulation of the IGF-1R
expression. Journal of Hematology & Oncology 8,
1-13
31. Zhang, Y., Ma, M., Liu, W., Ding, W., and Yu, H. (2014)
Enhanced expression of long noncoding RNA CARLo-5 is associated with the development of gastric
cancer. International Journal of Clinical & Experimental Pathology 7, 8471-8479
32. Persano, L., Pistollato, F., Rampazzo, E., Della Puppa, A., Abbadi, S., Frasson, C., Volpin, F., Indraccolo,
S., Scienza, R., and Basso, G. (2012) BMP2 sensitizes glioblastoma stem-like cells to Temozolomide
by affecting HIF-1α stability and MGMT expression. Cell Death & Disease 3, e412