Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Platelet-rich plasma combined with core decompression and allogeneic fibula rod support for the treatment of osteonecrosis of the femoral head (Ficat stage II): a short-term follow-up
*Corresponding author: Biao-fang Wei
Address: Department of Orthopaedic Surgery, Lin Yi People's
Hospital, No.27 East Jiefang Road, Linyi, 276000
E-mail: 317516288@qq.com
Received: 20 October 2017 Accepted: 25 December 2017
DOI: 10.31491/CSRC.2017.12.006
Abstract
Background: The objective of this study was to investigate the potency of platelet-rich plasma (PRP) combined with core decompression and allogeneic fibula rod support for the treatment of osteonecrosis of the femoral head (ONFH, Ficat stage II).
Methods: This was a retrospective study of clinical data of patients who were admitted to our hospital for treatment of ONFH from July 2014 to June 2015. In PRP group, patients (9 cases) were treated with platelet-rich plasma combined with core decompression and allogeneic fibula rod support. In control group, patients (7 cases) were treated with core decompression and allogeneic fibula rod support only. The time from onset of symptoms to surgery was 3–6 months (mean: 4.5 months). Harris hip scores before and after surgery were recorded (> 90, excellent; 75–90, good; 60–74, poor; and < 60, bad). An imaging study was performed 1 and 3 months after surgery.
Results: In the PRP group, the Harris scores increased from 67.82 ± 9.61 to 88.45 ± 6.02. In the control group, the Harris scores increased from 69.74 ± 8.26 to 87.36 ± 6.17. There were statistically significant differences in pretherapy and post-treatment scores (P < 0.05). Three months postsurgery, the shapes of the femoral heads in both groups were good, with no deformation or collapse. The location of the top of the allograft fibula was perfect (no fibula out). Additionally, the fibula rods began to combine with the surrounding bone after 3 months postsurgery, without infection.
Conclusion: PRP can ease hip pain and improve joint function
Keywords
PRP, ONFH, core decompression, allogeneic fibula rod
Introduction
Osteonecrosis of the femoral head (ONFH) is a painful
disorder of the hip that affects patients with various
risk factors, such as glucocorticoid use, alcohol abuse,
trauma, and autoimmune diseases (1-3). The progress
of joint destruction in ONFH can lead to hip arthritis,
which seriously affects the patient’s health. In the
absence of appropriate surgical treatment, the femoral
head will collapse within 2–3 years in the vast majority
of patients (4). Steinberg (5) reported that collapse of the
femoral head occurred in 92% of nonsurgical cases
(48 hips) within 3 years after a definite diagnosis of
ONFH. As ONFH occurs mostly in young and middle-aged patients, Garrigues et al. (6) believe that all possible
treatment measures should be taken to protect the
femoral head. At present, the main methods to protect
the femoral head in ONFH are core decompression,
with or without a bone graft, in addition to bone flap
transplantation with blood vessels and blood vessel
bundle transplantation. However, these treatments
have not achieved satisfactory clinical results.
Platelet-rich plasma (PRP), an autologous enriched
source of various growth factors, is widely used during
trauma and orthopedic surgery. PRP contains a variety
of cytokines, such as platelet-derived growth factor
(PDGF), vascular endothelial growth factor, platelet-derived angiogenesis factor, transforming growth
factor (TGF), insulin-like growth factor, and platelet-derived epidermal growth factor. PRP can accelerate
the healing of hard and soft tissues after maxillofacial,
plastic, dermatologic, dental, and orthopedic surgery
(7-9). However, evidence from preclinical and clinical research suggests that PRP has no significant
therapeutic effects on ONFH. The aim of the present
study was to investigate whether PRP promoted
healing of ONFH in a clinical setting.
Materials and Methods
Patients
This was a retrospective study of clinical data of patients who were admitted to our hospital for treatment of ONFH from July 2014 to June 2015. The patients were assigned to a PRP group or a control group. In the PRP group, there were seven males and two females aged 28–45 years (mean: 36.5 years). The group included six cases of alcoholic avascular necrosis and three cases of glucocorticoid-induced avascular necrosis. The Harris score was 60–86 points (mean: 73 points) prior to surgery. Patients were treated with platelet-rich plasma combined with core decompression and allogeneic fibula rod support. In the control group, there were five males and two females aged 26–44 years (mean: 35 years). The group contained four cases of alcoholic avascular necrosis, two cases of glucocorticoid-induced avascular necrosis, and one case of traumatic ONFH. The Harris score was 62–86 points (mean: 74 points) prior to surgery. Patients were treated with core decompression and allogeneic fibula rod support only. In all patients, ONFH was confirmed by symptoms, a physical examination, patient history, and results of imaging studies, including X-ray and magnetic resonance (MR) examinations. The time from onset of symptoms to surgery was 3–6 months (mean: 4.5 months). There were no statistically significant differences in the common data between the two groups (P > 0.05). The clinical experiments were approved by the ethics committee of Linyi People’s Hospital. All the patients volunteered to take part in the study, and all signed informed consent forms.
Preparation of PRP
After successful induction of general anesthesia, whole blood (30 mL) was collected from the marginal auricular vein using an 18-gauge catheter. The blood was then injected into a sterile centrifuge tube containing 1.5 mL of sodium citrate. The mixture was centrifuged at 1500×g for 20 min to separate the plasma from red blood cells. A straw was then used to suck out the supernatant. The plasma was centrifuged again at 1500×g for 10 min (4 °C), and the precipitated platelets (i.e., PRP) were collected (10).
Surgical procedure
A longitudinal incision (4–6 cm) was made in the big tuberosity distal along the lateral femoral hip to expose the far side of the greater trochanter of the femur, which was used as the entry point. Using C-arm X-ray machine monitoring, a bone tunnel (diameter of 12 mm) was drilled in the central necrosis area (5–7 mm under the articular cartilage). All dead bone tissue was then removed along the bone tunnel, and autologous PRP was implanted. Selecting suitable length allogeneic fibula rod support and fixed. After confirming the depth and location of allogeneic fibula rod, the incision was sutured. The surgical protocol is shown in Figure 1.
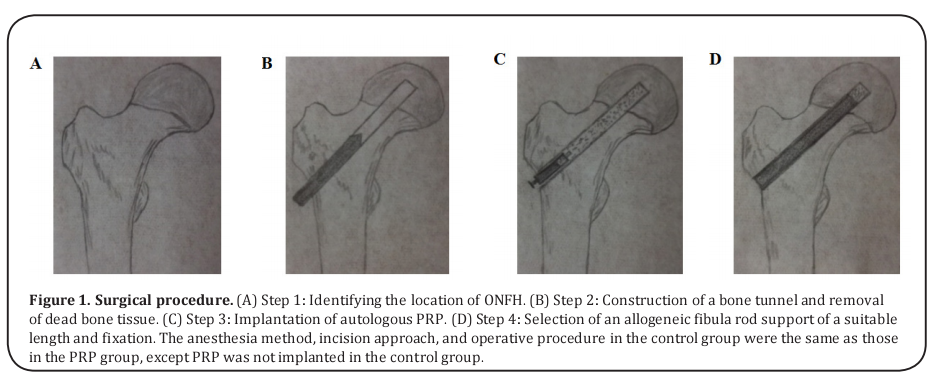
Postoperative management
All the patients were treated with anti-inflammatory agents after the surgery. After awakening from anesthesia, active flex and outreach of joint function rehabilitation exercise was carried out. Postsurgery, the patients were supported by crutches for 3 months to avoid femur neck fracture caused by falling. Each patient was advised to avoid excessive body weight and intense activity for 1 year.
Postoperative follow-up and evaluation of the curative effect
As an indicator of the curative effect, the Harris hip score was used (> 90, excellent; 75–90, good; 60–74, poor; and < 60, bad) (11). Imaging studies were performed 1 and 3 months after the surgery to evaluate changes in bone morphology.
Statistical analysis
All data are expressed as means ± standard deviation (SD). The statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups were detected using a one-way analysis of variance, followed by Scheffe’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Comparison of Harris hip scores in the two groups
In the PRP group, the Harris scores increased from 67.82 ± 9.61 to 88.45 ± 6.02 (P < 0.05, Table 1). Seven (77.8%) hips were classed as excellent, and one (22.2%) hip was classed as good. No hips were classed as poor or bad. In the control group, the Harris scores increased from 69.74 ± 8.26 to 87.36 ± 6.17 (P < 0.05, Table 1). Six (85.7%) hips were classed as excellent, and 1 (14.2%) hip was classed as good. No hips were classed as poor or bad. There were statistically significant differences in pretreatment and post-treatment scores (P < 0.05, Table 1). There were no statistically significant differences in the postoperative scores of the two groups (P > 0.05, Table 1).
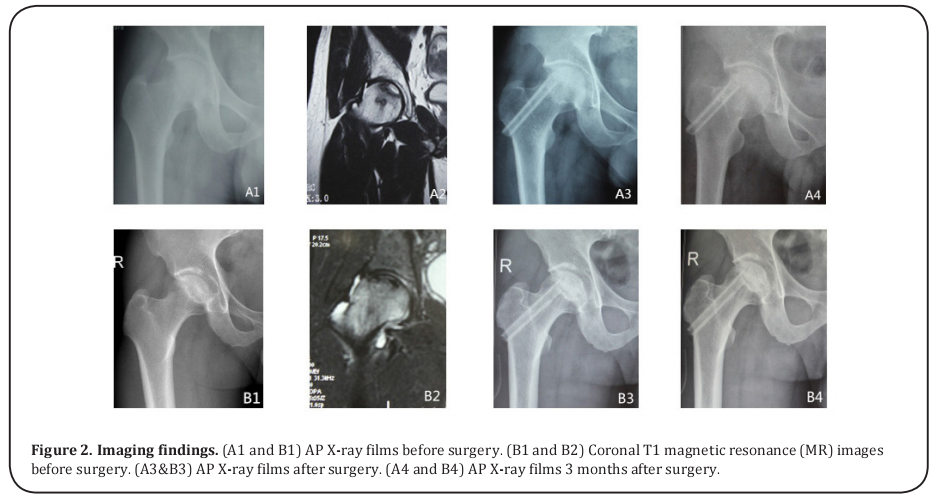
Imaging findings
Three months after the operation, the shapes of the femoral heads in the two groups were good, with no deformation and collapse. The location of the top of the allograft fibula was perfect (no fibula out), and it was located in the weight-bearing area and 5–7 mm (mean 6 mm) under the articular cartilage. The fibula rods began to combine with the surrounding bone after 3 months postsurgery, without infection. There were no statistically significant differences in the imaging findings of the two groups (Fig. 2).
PD-L1 and clinicopathological factors
Four studies [14,22-24] presented the data on PD-L1 and clinicopathological factors including gender (male vs female) and metastasis (present vs absent). As shown in Figure 4, the pooled data were: OR=0.87, 95%CI=0.56- 1.37, p=0.556 for gender (male vs female), and OR=8.51, 95%CI=4.3-16.86, p <0.001 for metastasis (present vs absent).
Discussion
The purpose of this study was to evaluate the
therapeutic effect of PRP in ONFH (Ficat stage II). In
the initial stage of ONFH, core decompression, together
with allogeneic fibula rod support, is considered an
effective surgical intervention. However, However, this
treatment retains the femoral head in only 60–80% of
cases in the short and medium term (12). Femoral head
core decompression involves drilling a tunnel to reduce
the pressure on the femoral head, thereby alleviating
pain. A combination of core decompression with other
treatments, such as a bone graft and implants, with
growth factors, can promote local osteogenesis and
avoid channel collapse. The ultimate aim is to reduce
pressure in the necrotic area, remove necrotic tissue,
and provide effective mechanical support. The clinical
application of PRP in the treatment of early-stage ONFH
offers a new strategy to combat disease progression.
The platelet (PLT) concentration in PRP is several
times higher than that in whole blood, although
findings differ on this issue. Marx et al. (13) reported
that the concentration of PLT in PRP was four times
higher than that in whole blood. In contrast, Okuda et
al. (14) concluded that the concentration of PLT in PRP
was 2.83 times higher than that in whole blood. Others
reported that the concentration of PLT in PRP was 2.05
(15), 5.05 (artificial count) (15), 5.29 (automated account)
(16), and even 9–11 times higher as compared with that
in whole blood (17).
PRP-induced bone regeneration is mainly due to growth factors, which are released in response to activation of PRP (18-20). Growth factors, especially PDGF and TGF-β play a vital role in the process of bone regeneration and repair (21). In addition to growth factors in PLTs, macrophages and endothelial cells, which synthesize and secrete PDGF, promote wound healing (22). PDGF promotes mitosis and related angiogenesis(23) and improves the activity of macrophages and other growth factors. TGF-β induce mitosis, osteoblast differentiation, osteoid formation, and bone regeneration (24) . nsulin-like growth factor (IGF) accelerates bone formation by increasing the number of osteoblasts (25). To shed light on the therapeutic effect of growth factors, Lynch et al. (26) used different concentrations of PDGF, IGF, epidermal growth factor (EGF), and Fibroblast growth factor (FGF) mixtures to repair wounds and found that a 2:1 ratio of PDGF and IGF was the best combination. They suggested that a variety of growth factors constitute a growth factor net and have a synergistic effect. For example, combined PDGF and TGF-β had a strong biological chemotactic effect on human osteoblasts (27). TGF-β not only regulated growth and differentiation of bone cells and cartilage cells but also regulated the expression of other growth factors in bone and cartilage tissue (28). PDGF and TGF-β promoted the expression of vascular endothelial growth factor (29). In combination with other growth factors, IGF promoted differentiation and maturation of a variety of cells (30). Okuda et al. (14) reported that PRP stimulated osteoblast DNA synthesis and cell division. We contend that the combined effect of a variety of the high concentration of growth factors in PRP can stimulate osteogenesis and accelerate bone repair.
In the current study, we analyzed retrospectively the clinical efficacy of 16 recent cases (16 hips) of ONFH. According to the postoperative 3-month follow-up, none of the cases in either group showed disease progression, such as femoral head collapse, osteoarthritis requiring a total hip replacement, infections, or femoral neck fracture. In all patients, hip pain was significantly reduced or had disappeared. There is no consensus in the literature on the potential role of PRP in promoting bone regeneration and repair. Whitman et al. (31)were the first to successfully use used PRP and bone transplantation to treat a bone defect in the field of maxillofacial surgery. Marx et al. (13) used PRP and a bone graft to treat a bone defect. They reported that the callus growth rate in the combined transplantation group was 1.62–2.16 times higher than that in a pure bone transplantation group. However, in a rabbit skull defect model Ahgaloo et al. (32) found that PRP did not promote bone regeneration after an autologous bone graft. Choi et al. (33) reported that PRP did not promote healing of bone defects 6 weeks later. Furthermore, in a study of PRP with an autologous bone composite, biological materials, and organic bone matrix, Kim et al. (34) used and reported that PRP did not promote bone regeneration effectively.
The present study has several limitations. First, the follow-up time was relatively short. The study was performed only in the first 3 months after the surgery during the initial stage of rehabilitation. Second, the number of cases in the study was relatively small. Finally, the study was a retrospective case series.
Conclusions
In this comparison of a PRP group and controls in a clinical setting, the use of PRP was not associated with any obvious advantages. PRP eased hip pain and improved joint function. Long-term follow-up studies are needed to clarify the potential role of PRP in promoting repairing of ONFH.
Acknowledgement
This work was supported by the Science and Technology Development Planning of Shan Dong Province(2014GSF119022)
References
1. Okazaki, S., Nagoya, S., Tateda, K., Katada, R., Mizuo, K., Watanabe, S., Yamashita, T., and Matsumoto, H. (2013) Experimental rat model for alcohol-induced osteonecrosis of the femoral head. International journal of experimental pathology 94, 312-319
2. Wu, Z., Ji, C., Li, H., Qiu, G., Gao, C., and Weng, X. (2013) Elevated level of membrane microparticles in the disease of steroid-induced vascular osteonecrosis. The Journal of craniofacial surgery 24, 1252-1256
3. Fukushima, W., Fujioka, M., Kubo, T., Tamakoshi, A., Nagai, M., and Hirota, Y. (2010) Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clinical orthopaedics and related research 468, 2715-2724
4. Chen, C. C., Lin, C. L., Chen, W. C., Shih, H. N., Ueng, S. W., and Lee, M. S. (2009) Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. The Journal of bone and joint surgery. American volume 91, 2390-2394
5. Steinberg, M. E. (1988) Management of avascular necrosis of the femoral head--an overview. Instructional course lectures 37, 41-50
6. Garrigues, G. E., Aldridge, J. M., 3rd, Friend, J. K., and Urbaniak, J. R. (2009) Free Vascularized Fibular Grafting for treatment of osteonecrosis of the femoral head secondary to hip dislocation. Microsurgery 29, 342-345
7. Eppley, B. L., Woodell, J. E., and Higgins, J. (2004) Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and reconstructive surgery 114, 1502-1508
8. van den Dolder, J., Mooren, R., Vloon, A. P., Stoelinga, P. J., and Jansen, J. A. (2006) Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue engineering 12, 3067-3073
9. Kon, E., Filardo, G., Delcogliano, M., Presti, M. L., Russo, A., Bondi, A., Di Martino, A., Cenacchi, A., Fornasari, P. M., and Marcacci, M. (2009) Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper's knee. Injury 40, 598-603
10. Wu, W., Chen, F., Liu, Y., Ma, Q., and Mao, T. (2007) Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 65, 1951-1957
11. Ahmad, M. A., Xypnitos, F. N., and Giannoudis, P. V. (2011) Measuring hip outcomes: common scales and checklists. Injury 42, 259-264
12. Feitosa, M. L., Fadel, L., Beltrao-Braga, P. C., Wenceslau, C. V., Kerkis, I., Kerkis, A., Birgel Junior, E. H., Martins, J. F., Martins Ddos, S., Miglino, M. A., and Ambrosio, C. E. (2010) Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head: preliminary results. Acta cirurgica brasileira 25, 416-422
13. Marx, R. E., Carlson, E. R., Eichstaedt, R. M., Schimmele, S. R., Strauss, J. E., and Georgeff, K. R. (1998) Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics 85, 638-646
14. Okuda, K., Kawase, T., Momose, M., Murata, M., Saito, Y., Suzuki, H., Wolff, L. F., and Yoshie, H. (2003) Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. Journal of periodontology 74, 849-857
15. Landesberg, R., Roy, M., and Glickman, R. S. (2000) Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 58, 297-300; discussion 300-291
16. Roldan, J. C., Jepsen, S., Miller, J., Freitag, S., Rueger, D. C., Acil, Y., and Terheyden, H. (2004) Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 34, 80-90
17. Weibrich, G., Hansen, T., Kleis, W., Buch, R., and Hitzler, W. E. (2004) Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 34, 665-671
18. Danesh-Meyer, M. J., Filstein, M. R., and Shanaman, R. (2001) Histological evaluation of sinus augmentation using platelet rich plasma (PRP): a case series. Journal of the International Academy of Periodontology 3, 48-56
19. Weibrich, G., Kleis, W. K., Hafner, G., and Hitzler, W. E. (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery 30, 97-102
20. Sanchez, A. R., Sheridan, P. J., and Kupp, L. I. (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. The International journal of oral & maxillofacial implants 18, 93-103
21. Bhanot, S., and Alex, J. C. (2002) Current applications of platelet gels in facial plastic surgery. Facial plastic surgery : FPS 18, 27-33
22. Yazawa, M., Ogata, H., Kimura, A., Nakajima, T., Mori, T., and Watanabe, N. (2004) Basic studies on the bone formation ability by platelet rich plasma in rabbits. The Journal of craniofacial surgery 15, 439-446
23. Risau, W., Drexler, H., Mironov, V., Smits, A., Siegbahn, A., Funa, K., and Heldin, C. H. (1992) Platelet-derived growth factor is angiogenic in vivo. Growth factors (Chur, Switzerland) 7, 261- 266
24. Liu, Y., Zheng, W. K., Gao, W. S., Shen, Y., and Ding, W. Y. (2013) Function of TGF-beta and p38 MAKP signaling pathway in osteoblast differentiation from rat adipose-derived stem cells. European review for medical and pharmacological sciences 17, 1611-1619
25. Kitoh, H., Kitakoji, T., Tsuchiya, H., Mitsuyama, H., Nakamura, H., Katoh, M., and Ishiguro, N. (2004) Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone 35, 892-898
26. Lynch, S. E., Colvin, R. B., and Antoniades, H. N. (1989) Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. The Journal of clinical investigation 84, 640-646
27. Colciago, A., Celotti, F., Casati, L., Giancola, R., Castano, S. M., Antonini, G., Sacchi, M. C., and Negri-Cesi, P. (2009) In Vitro Effects of PDGF Isoforms (AA, BB, AB and CC) on Migration and Proliferation of SaOS-2 Osteoblasts and on Migration of Human Osteoblasts. International journal of biomedical science : IJBS 5, 380-389
28. Barrett, J. M., Rovedo, M. A., Tajuddin, A. M., Jilling, T., Macoska, J. A., MacDonald, J., Mangold, K. A., and Kaul, K. L. (2006) Prostate cancer cells regulate growth and differentiation of bone marrow endothelial cells through TGFbeta and its receptor, TGFbetaRII. The Prostate 66, 632-650
29. Petersen, W., Pufe, T., Zantop, T., Tillmann, B., and Mentlein, R. (2003) Hypoxia and PDGF have a synergistic effect that increases the expression of the angiogenetic peptide vascular endothelial growth factor in Achilles tendon fibroblasts. Archives of orthopaedic and trauma surgery 123, 485-488
30. Grohmann, M., Foulstone, E., Welsh, G., Holly, J., Shield, J., Crowne, E., and Stewart, C. (2005) Isolation and validation of human prepubertal skeletal muscle cells: maturation and metabolic effects of IGF-I, IGFBP-3 and TNFalpha. The Journal of physiology 568, 229-242
31. Whitman, D. H., Berry, R. L., and Green, D. M. (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 55, 1294-1299
32. Aghaloo, T. L., Moy, P. K., and Freymiller, E. G. (2002) Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 60, 1176-1181
33. Choi, B. H., Im, C. J., Huh, J. Y., Suh, J. J., and Lee, S. H. (2004) Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. International journal of oral and maxillofacial surgery 33, 56-5934. Kim, S. G., Kim, W. K., Park, J. C., and Kim, H. J. (2002) A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 60, 1018-1025