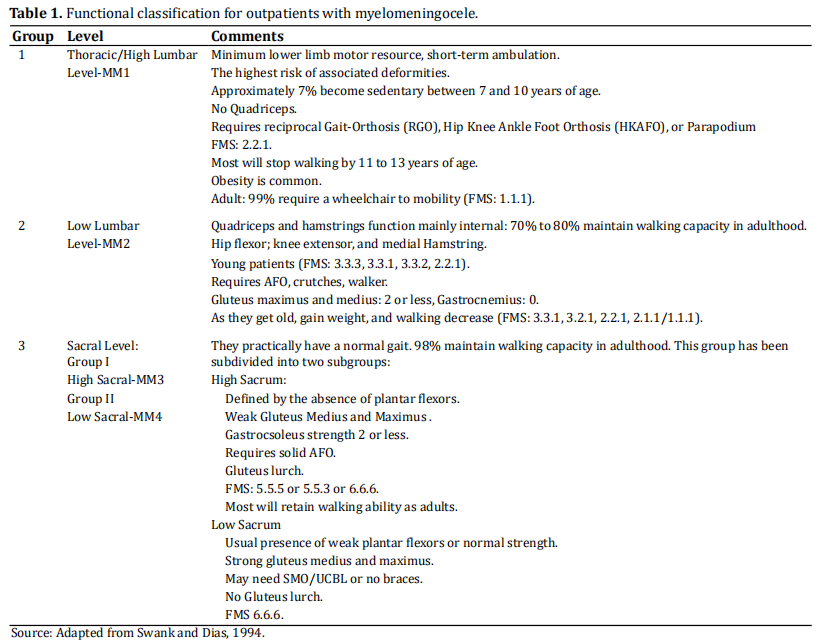
Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Spina Bifida: alternative approaches and treatment, based on evidence through gait analysis
*Corresponding author: Marcel Rupcich G
Mailing address: Department of Pediatrics, Centro Médico Docente La Trinidad, Caracas, Venezuela.
Email: marcelrupcich@gmail.com
24 October 2020 / Accepted: 12 January 2021
DOI: 10.31491/CSRC.2021.03.067
Abstract
Myelomeningocele results from failure of the neural tube to close in the developing fetus and is associated with neurological impairment (Incidence 1:1000 births). The level of the anatomic lesion generally correlates with the neurological deficit and ranges from complete paralysis to minimal or in some cases no motor involvement. Myelomeningocele or Spina bifida can lead to health problems, physical disabilities, and learning problems. Most commonly, associated with paralysis of the lower extremities and neurogenic bladder. Treatment requires multidisciplinary participation. The functional classification that concerns us in this review includes three types and were obtained through gait analysis.
Keywords
Spina bifida; instrumental; gait analysis; kinematics and kinetics; orthotics
Introduction
The term spinal dysraphism [1] refers to those conditions that result from a defective development in the
midline of the dorsal aspect of the embryo, resulting in
bone or nervous system deformities. Cutaneous manifestations can be accompanied, but they are not always
present.
Spina bifida belongs to a group of developmental disorders of the vertebral arches or the cranial vault. They are often associated with disorders of the formation of structures derived from the neural tube and meninges, and can lead to cystic formations. The causes of spina bifida appear multifactorial. Folic acid deficiency is a significant factor and there appears to be a genetic component.
Three types of spina bifida can be described: The
severity ranges from occult, in which no obvious abnormalities are seen, to protruding sacs (cystic spina
bifida), and to a completely open spine with severe
neurological disability and death.
Occult Spina bifida is a defect located in one or more
vertebral arches. It develops as a failure of the vertebral arches, remaining unfused in the third month. The
spinal cord and meninges remain within the vertebral
canal.
The meningocele is a cystic mass of the dura and arachnoid that protrudes through a defect in the vertebral
arches under the skin. The spinal cord is completely
confined to the vertebral canal, but abnormalities can
occur.
Myelomeningocele (MMC), or open spina bifida, where
the exposed elements of the spine are fully exposed.
The spectrum of clinical presentation is huge, from
lethal rachischisis to asymptomatic occult spina bifida
with a small lipoma. The diversity of presentations
suggests that causal factors exert their effects in different periods of development, in addition to genetics and
the environment that must be considered. Other associated abnormalities found in spina bifida are congenital spinal deformity, Sprengel deformity, tethered cord,
neurogenic bladder, and clubfoot. A high incidence of
allergy to latex has also been observed.
Progressive neurological deterioration can occur because hydrocephalus in association with an ArnoldChiari type II defect is common and develops in 80% of children with thoracolumbar myelomeningocele [2-5].
Instrumental Gait Analysis
The instrumental gait analysis (IGA) is the record of
the biomechanical variables of human movement related to the way we walk. Allowing to know the principles
that govern the human movement, making its analysis
more objective and able to be measurable.
Nowadays it is compound by:
1. The clinical exam using muscular force measurement through dynamometry;
2. Observational Analysis (three-dimensional video);
3. Kinematics and muscle length;
4. Kinetics;
5. Muscle activity (dynamic EMG);
6. Energy consumption;
7. Baropodography.
This type of analysis has made it possible to identify
the prerequisites for normal gait, making it easier to
recognize deviations that occur in pathological gait.
Functional Impact
Regarding independence in daily life, at present, the
most accepted classification is the one that arises from
the available motor function [6-8], so it should be clear
what the patient’s motor resources are, mainly the antigravity muscles, which are also related to the prognosis of gait and its maintenance over time.
Ambulation is also affected by age, obesity, spasticity,
orthopedic deformities, etc. [9] The greater the commitment, the greater the disability and implications such
as survival, associated deformities, and the ability to
walk.
Motor level and balance are two main factors that
compromise the ability to walk and subsequently the degree of support required.
Motor Level
Classification and motor implications
The level of neurological involvement is one of the key
determinants of a child’s ambulation.
The most accepted classification of spina bifida is based
on the neurological level of the lesion [6-8] (Table 1). Patients are divided into three groups according to the
level of injury, functional ability, and ambulation.
As reference, we used the classification proposed by
Swank Dias [6] to make communication easier and
simpler, based on the motor function level (FML) and
the functional mobility scale (FMS), which should always be used together.
FML is based on what type of assisted device the patient is using and what type of brace they are wearing,
while FMS is based on the ability to walk in three different distances (5/50/500 meters).
The classification criteria for patients, based on the
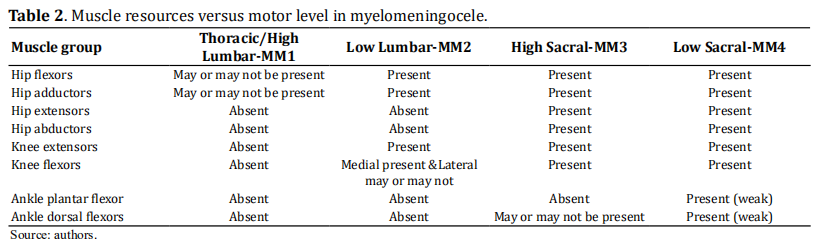
muscle resource present in the lower limbs, are summarized in Table 2.
It is important to note that the lesion tends to be asymmetric in most cases, therefore the classification leans
towards the more involved side.
Balance
Balance also affects the ability to walk and the degree
of support required and is related to the presence or
absence of shunt [10], the function of the shunt and the
number of shunt reviews.
Statistical reports from the literature of specialized
centers [8] show that of 70-80% of the lower lumbar
level and 98% of the sacral level retain their ability to
walk independently in adult life, whereas thoracic or
upper lumbar levels, 7% lose the ability to walk between 7 and 10 years [6-8].
Quality of Ambulation and Gait Analysis
Factors affecting gait quality include [11]: muscle weakness, severe scoliosis, flexion contracture of the hip,
abduction contracture of the hip, subluxation or dislocation of the hip (with or without soft tissue contracture), rotational deformities of the hip (internal or
external) [12], flexion contracture of the knee [13-15] and
tibial torsion (internal or external) [16,17]. Since a large
percentage of outpatients maintain their ability to
walk, its quality must be considered. Gait analysis can
be used to access and help quantify its quality during
ambulation [9].
Generally, a clinical assessment of strength alone rarely
reflects the asymmetry noted during gait. An instrumental gait analysis should be the standard of expert
care for children with movement abnormalities secondary to spina bifida [18,19].
The IGA has made it possible to measure the impact of
the orthoses with the use of kinematics [20] and kinetics on the loads applied to the joints of the lower limbs during movement [8,17,21,22]. With which the benefit or
not, of walking aid options is documented, and their
impact on biomechanics [23].
It allows observing the knee moments in the coronal
plane to define if there are valgus forces [24], as well as
documenting the rotation patterns of the trunk and
pelvis in the transverse plane [25] due to the combination of bone deformities and muscle weakness.
IGA also shows muscle function, especially in the
ankle, due to muscle hyperactivity, muscle shortening,
or weakness [22,26,27] resulting from growth, puberty, or
cord retention, when a given treatment is considered.
It documents postoperative changes that are used for
subsequent decision making. All this information allows the specialist to formulate treatment plans and
protocols in order to develop the maximum potential
and independence of the patient [8,17,21,22].
Evidence From Instrumental Gait Analysis
This section shows more in-depth the IGA evidence applied to the management of patients with MMC [28],
with examples based on gait kinematics and kinetics.
Kinematics and kinetics derived from functional modeling in 3-dimensional instrumental gait analysis provide subject-specific data and can detect not only static
but also functional alignment.
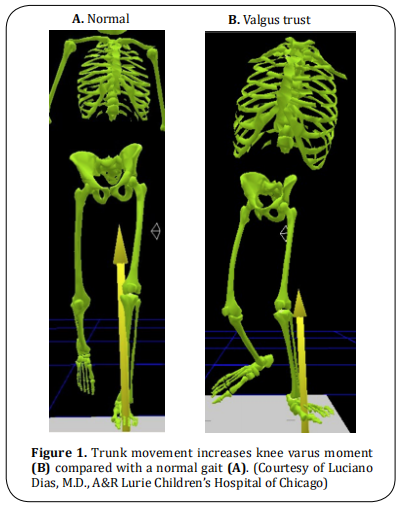
Gluteus weakness is the cause of compensation with
the lateralization of the trunk during ipsilateral support (Figure 1), also affecting the movements of the
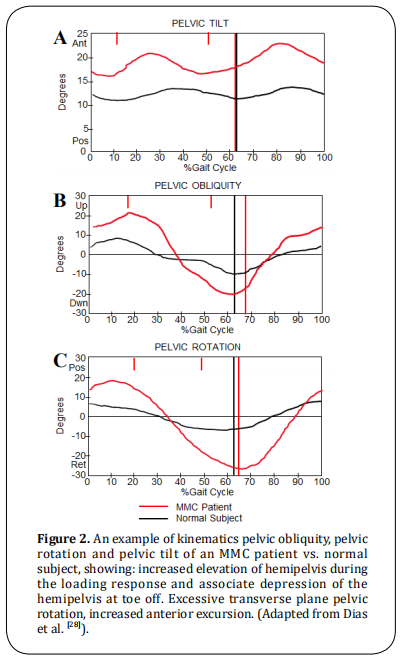
pelvis and trunk in the coronal and transverse planes [excessive pelvic obliquity and rotation (Figure 2), and
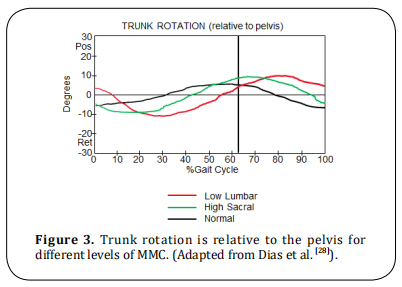
excessive trunk rotation (Figure 3), in kinematics] and
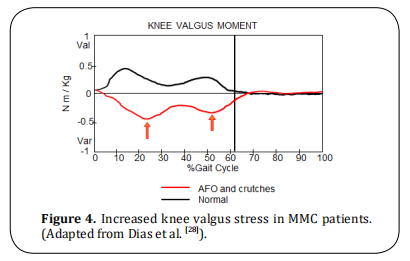
at the knee (increased valgus stress, Figure 4).
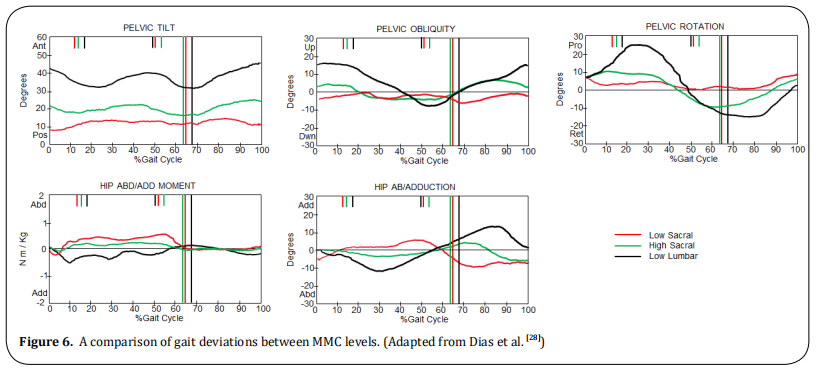
The primary factor of the pelvic obliquity (Figure 2B)
is the hip abductor weakness which induces a lateral
trunk movement over the stance limb. Generally higher
levels will show a greater pelvic oblique pattern.
The deviation in the pelvic rotation (Figure 2C) is a
compensatory motion in the presence of decreased
strength of the ankle plantar flexor (primary power
generator of walking) and weak hip extensor.
MMC patients show greater than a normal excursion
of anterior pelvic tilt progression during single-limb
stance (Figure 2A). Its primary factor obeys a decreased hip extensor strength (Gluteus Maximus).
Its primary cause is a weak plantar flexor, and or hip
extensor, or quadriceps. Prolonged muscular activity
recorded on EMG of the hamstring during the stance
phase is needed to control pelvic tilt.
Swing phase pelvic rotation and hip abduction can interrupt the pendulum action of the swing limb. Hip and
knee flexor contractures are generally more associated
with high levels, affecting the pattern and magnitude
of movement.
As can be seen in the graphs of (Figure 6), the pelvic
tilt and pelvic rotations increase with the level of the lesion.
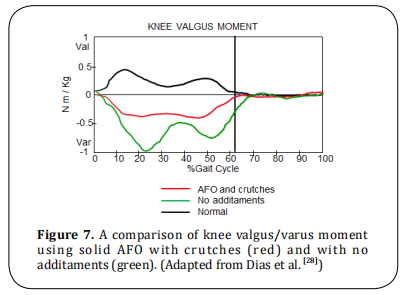
IGA also shows the impact of the use of orthosis and
additaments (Figure 7). Solid AFOs stabilize the ankle
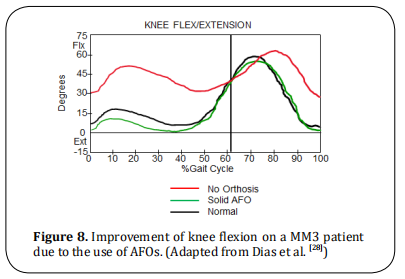
and hindfoot, preventing foot and knee valgus. Crutches decrease trunk movement, therefore, decreasing internal knee varus moment. AFOs also reduce excessive
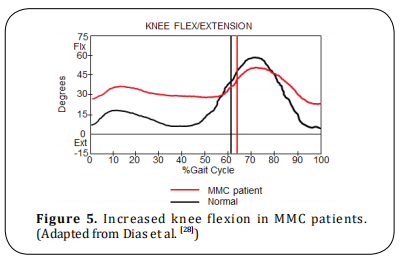
knee flexion in the stance phase as shown in (Figure 8).
Due to the increase of the pelvic tilt and pelvic rotations with the level of the lesion shown by kinematics (Figure 6), a surgery that affects pelvic motion will
make the gait more difficult.
Besides, IGA kinetics have demonstrated that any surgery that decreases the strength of power generation
muscles (i.e. Ileopsoas, Gluteus, Hamstrings), will affect gait. Transfers of the Iliopsoas muscle (Sherrard
procedure) should not be performed as it decreases
the flexor power of the hip. Spinal fusion to the Sacrum in low lumbar and sacral level patients must not be performed.
An overview of current treatments
In this review, we want to show the current state of
disease management, its approach with “in utero”
interventions, and current treatments based on the
knowledge obtained through gait analysis. Existing
treatment protocols depend on the anatomical level
(muscle resources), balance, and bilaterality or not,
present.
Spina bifida is the most common congenital defect,
presenting in a wide range of severity and with poor
postnatal treatment options. The resolution “in utero”
[29-31] have shown beneficial results such as the absence
of a sac over the lesion, an improvement in the functional level, per example: an L3 lesion is significantly
associated with independent ambulation [32,33]. The
decrease in the need for ventriculoperitoneal shunts
remains controversial, as the incidence or not of Chiari
malformation as well [31]. Improved surgical techniques
have controlled a large percentage of obstetric risks
such as premature births and maternal complications
derived from the procedure.
Due to the variety of medical comorbidities involved,
for the evaluation and management of these patients,
the competence of a multidisciplinary team [34], such as
neurosurgery, pediatrics, physiatry, urology, orthopedic surgery, orthotics, physiotherapy, and social work
is necessary for appropriate handling.
The goal of the orthopedic surgeon is to correct deformities and improve function and mobility. This is
where IGA plays an increasingly important role in behaviors, decision-making, and treatment [35]. Decision making about treatment has been made more precise
and with better results based on the scientific method,
so the inclusion of Instrumental Gait Analysis is essential in the patient care process [36].
Gait analysis enabled to have a better understanding of
the gait patterns of each level, allowing to know, which
is the best orthosis or additament, and what deformity
affects gait and currently has a greater influence on
the selection of functional surgical procedures. The
results of the instrumental gait analysis often change
the identification of pathologies and surgical recommendations, e.g. femoral derotation osteotomy, first
metatarsal osteotomy, the release of the plantar fascia,
tibial derotation osteotomy, etc [36].
The magnitude of muscle weakness associated with
the lesion level is the predominant factor that induces
the adapted walking patterns. patients with a low lever
lesion typically walk with AFOs and without external
support. Higher-level typically walks with AFOs and
external support.
The indication of splints on the feet, ankles, and knees
is accepted, as long as an improvement in function is
demonstrated.
In MM3, the use of the AFO stabilizes the ankle and
foot, and its use protects valgus feet and valgus knee.
The AFO substitutes for weak ankle plantar flexor in
the stance phase (improves the abnormal plantar flexion-knee extension couple) and weak ankle dorsiflexors in the swing phase. The stabilization of the ankle
due to AFOs enhances the knee extension in stance (as
shown in Figure 8).
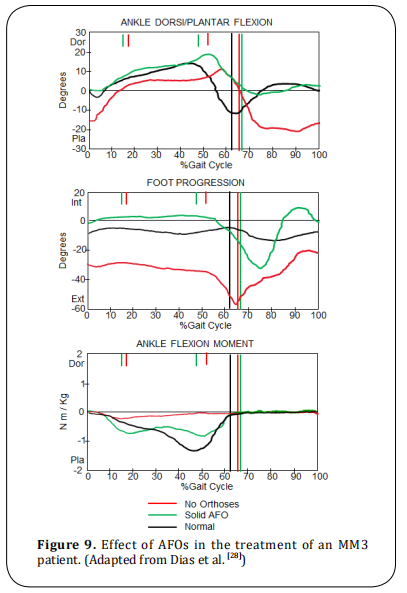
AFOs provide (Figure 9) support to prevent foot drop
in swing, improves the valgus position of the foot in
stance and lever arm of the ankle/foot (resulting in
an improvement of ankle plantar-flexor moment and
power generation in terminal stance/pre-swing). The following paragraphs show the deformities that can affect the function and how they are treated, such as hip
contractures and/or dislocation, knee contractures,
rotational deformities of the femur or tibia, and foot
deformities.
Hip pathologies
It is a consensus to treat hip dislocation in patients
with L5 level or lower and the release of contractures
is limited to being functionally significant.
Hip flexion contracture that produces anterior pelvic
tilt from 20°-60°, or increased hip flexion in stance
which may or may not be associated with increased
knee flexion and decreased hip extension. It could
be treated with the following procedures: hip flexor
lengthening, fascia-lata tensor release, transfer sartorius origin to the anterior inferior iliac spine or free
tendon graft, the proximal release of rectus femoris,
iIiopsoas lengthening above the brim, anterior hip capsulotomy (if necessary). In severe cases after hip flexor
lengthening, proximal femur extension osteotomy.
The final procedure to be applied to the patient will
depend on the surgeon’s criteria.
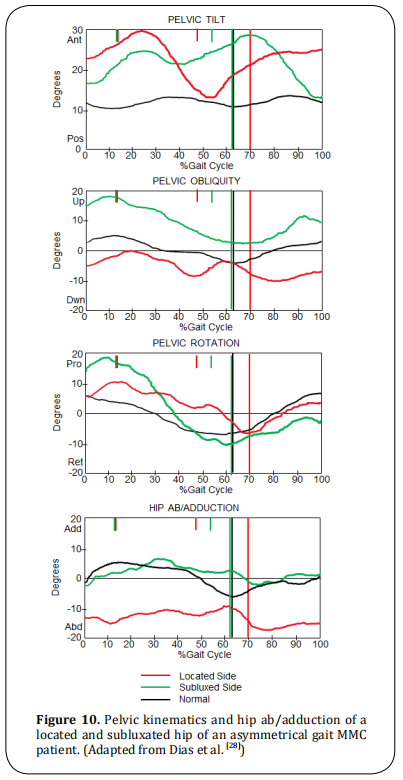
Low lumbar level with unilateral hip subluxation (with hip flexion and adduction contractures)
Those patients walk with AFOs and crutches with any of these characteristics: asymmetrical hip flexion contractures result in asymmetrical pelvic tilt and/or pelvic rotation, asymmetrical hip adduction contracture results in asymmetrical in pelvic obliquity and/ or hip ab/adduction, leg length discrepancy (may also contribute to the gait asymmetry). The treatment may need hip flexor lengthening, adductor myotomy, valgus osteotomy (Shanz) if necessary. Femoral shortening or epiphysiodesis for leg length discrepancy (Figure 10).
Low lumbar level with unilateral hip subluxation (with symmetrical or no contractures)
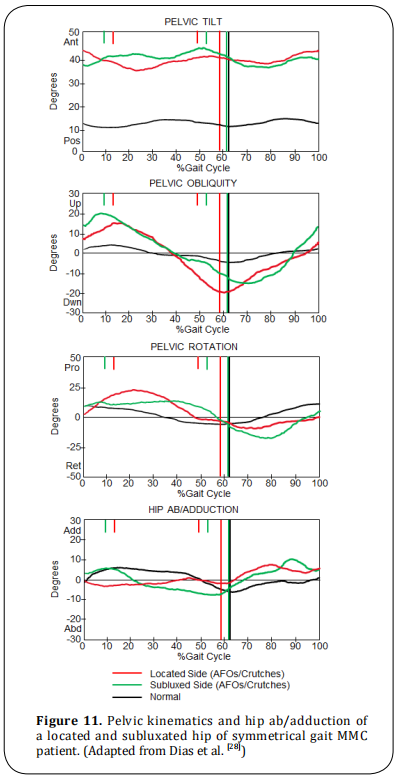
These patients walk with AFOs and crutches (some without it) and with symmetrical anterior pelvic tilt, due to symmetrical hip flexion contractures or absence of contractures (Figure 11). These patients do not need surgical relocation, treat hip flexion contracture if it is above 20 degrees.
Sacral level
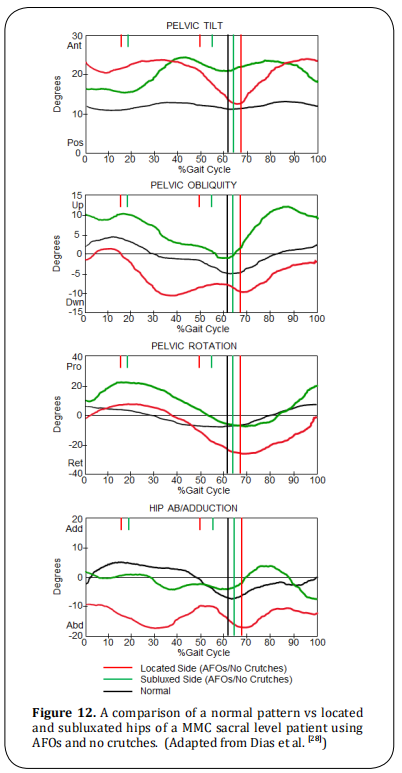
If unilateral hip subluxation and/or dislocation is present, it is important for these patients to rule out a tethered cord. Those patients walk with AFOs and without
crutches and asymmetric gait patterns in terms of the
pelvic obliquity, pelvic rotation, and hip ab/adduction as shown in the following plots (Figure 12).
Depending on the alteration, treatment must include
the relocation of the hip, open reduction, pelvic osteotomy (Shelf, Pemberton, Dega, based on surgeon
criteria), varus derotation osteotomy (VDO), external
oblique transfer or Mustard procedure and treat any of
soft tissue contractures.
Knee pathologies
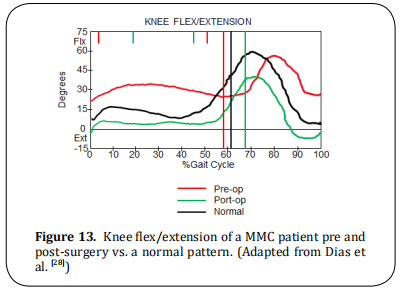
Knee flexion contracture, will augment the tendency for increased knee flexion which results from weak ankle plantar flexor and hip extensor. The IGA graphs show increased stance phase knee flexion (Figure 13). In consequently the corresponding sagittal plane graphs of hip and knee (not displayed in this document), will show hip flexion and ankle dorsiflexion. Treatment: orthoses are of limited benefit when knee flexion is equal to or less than 30 degrees. The surgery will be the lengthening of the hamstrings and in extreme cases distal femoral extension osteotomy.
Rotational deformities of the femur and tibia
Femur
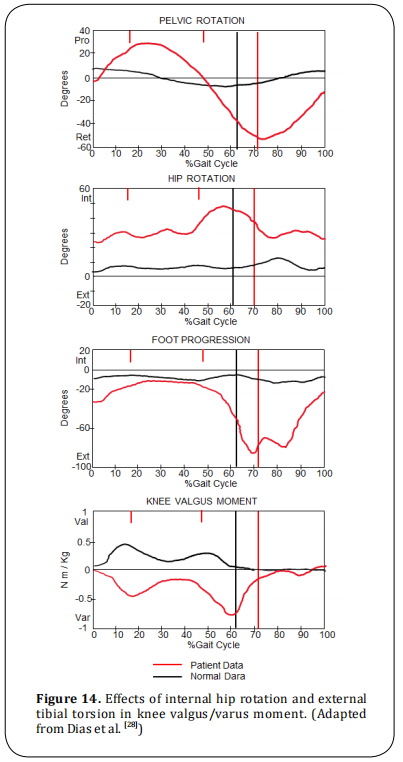
Internal hip rotation may be present, then foot progression angle varies depending upon the degree of external tibial torsion, and kinetics will show internal varus moment at the knee with external tibial torsion (Figure 14). Treatment of such deformities is femoral derotation osteotomy. If there is external tibial torsion, tibia/fibular derotation osteotomy must also be considered.
Tibia
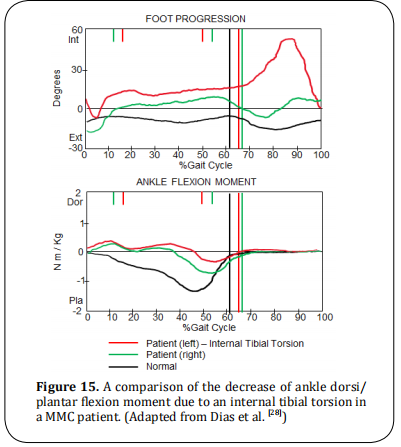
Internal tibial torsion is uncommon in MMC patients.
Internal foot progression angle during the swing
phase, decreases ankle plantar-flexor moment wave at
the terminal stance, as shown in (Figure 15).
The treatment for such cases is the use of orthosis:
AFOs, KAFOs depending on level.
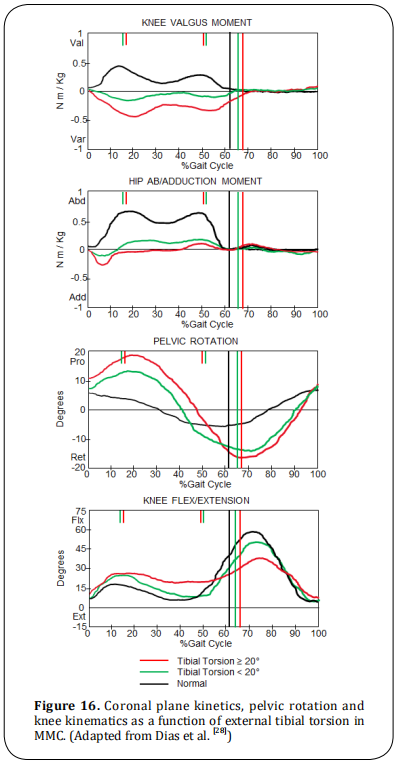
External tibial torsions are common in MMC. The
magnitude of the external tibial torsion affects the kinetics of the coronal plane (Figure 16), which has been
evaluated by several authors [37,38]. Other factors must
be considered because they are consequences of an
external tibial torsion, such as: trunk lean toward in
stance phase, dynamic pelvic rotation internal hip rotation, stance phase knee flexion, and ankle and hindfoot
valgus.
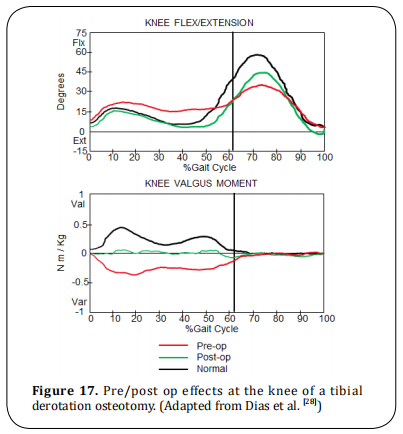
Treatment of external tibial torsion may be either orthotic or surgical. If it is less than 20 degrees then an
AFO/KAFO is recommended. Surgery tIbial/fibular supramalleolar derotation osteotomy should not be done
until 5 or 6 years old (Figure 17).
Foot deformities
Pes valgus is the most common foot deformity, and
consists of hindfoot pronation with supination and
abduction of the forefoot, producing the same effect in
the lower limbs upper segments as the external tibial
torsion (knee varus moment). In addition, abnormal
ankle plantar flexion-knee extension couple due to loss
of lever arm rigidity.
The treatment suggested is anterolateral release secondary to muscle imbalance and progressive deformity, otherwise maintained with UCBL type orthosis until 8 years. After 10 years, an osteotomy of the calcaneus
should be considered and the maintenance of UCBL.
Talipes equinovarus present at birth is also frequent.
Initially treated with serial casting and continuous
splinting until the child is 12 months old, then surgery
if needed. Surgery consists of postero medial lateral
release with tendon excision.
Conclusion
In general, a clinical evaluation alone rarely provides an approach to compromise in detail what the asymmetries and compensatory responses are used during
gait. Instrumental gait analysis should be the standard
of care for children with walking abnormalities secondary to spina bifida or any other pathology that alters movement. The main objective of the gait analysis
is to define the consequences derived from the neural
tube injury in relation to functional activity and future
independence of the patient.
Instrumented gait analysis can also provide clinicians with a better understanding of how neurological impairment affects walking, the compensations or
compensatory responses used, and further define the
functional level of the patient. The current trend is to
minimize dependence on orthosis for ambulation during childhood and optimizing mobility and independence within the expectations and functional level of
the patient.
Decision-making about treatment has been made more
precise and with better results based on the scientific
method, so the inclusion of Instrumental Gait Analysis
is essential in the patient care process.
Declarations
Authors’ contributions
Both authors contributed equally in the development, writing, translation and formatting of this work.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and informed consent
Not applicable.
Consent for publication
Not applicable.
References
1. Menelaus, M. (1980). The Orthopaedic Management of
Spina Bifida Systica.
2. Stein, S. C., Schut, L. (1979). Hydrocephalus in myelomeningocele. Pediatric Neurosurgery, 5(4), 413-419.
3. Mazur, J. M., Stillwell, A. N. N. E., Menelaus, M. A. L. C. O.
L. M. (1986). The significance of spasticity in the upper
and lower limbs in myelomeningocele. The Journal of
bone and joint surgery. British volume, 68(2), 213-217.
4. Mazur, J. M., Menelaus, M. B., Hudson, I., Stillwell, A.
(1986). Hand function in patients with spina bifida cystica. Journal of pediatric orthopedics, 6(4), 442-447.
5. Roach, J. W., Short, B. F., Saltzman, H. M. (2011). Adult
consequences of spina bifida: a cohort study. Clinical Orthopaedics and Related Research®, 469(5), 1246-1252.
6. Swank, M., Dias, L. S. (1994). Walking ability in spina
bifida patients: a model for predicting future ambulatory
status based on sitting balance and motor level. Journal
of pediatric orthopedics, 14(6), 715-718.
7. Dias, L. (2002). Myelomeningocele and intraspinal lipoma. Orthopaedic knowledge update: pediatrics 2nd edn.
American Academy of Orthopaedic Surgeons, 249-259.
8. Swaroop, V. T., Dias, L. (2009). Orthopedic management of spina bifida. Part I: hip, knee, and rotational deformities. Journal of children’s orthopaedics, 3(6), 441-
449.
9. Baghdadi, T. (2016). Surgical management of hip problems in myelomeningocele: a review article. Archives of
Bone and Joint Surgery, 4(3), 197.
10. Swank, M., Dias, L. S. (1994). Walking ability in spina
bifida patients: a model for predicting future ambulatory
status based on sitting balance and motor level. Journal
of pediatric orthopedics, 14(6), 715-718.
11. Battibugli, S., Gryfakis, N., Dias, L., Kelp‐Lenane, C., Figlioli, S., Fitzgerald, E., ... Sullivan, C. (2007). Functional
gait comparison between children with myelomeningocele: shunt versus no shunt. Developmental Medicine
Child Neurology, 49(10), 764-769.
12. Asher, M. A. R. C., Olson, J. O. Y. C. E. (1983). Factors
affecting the ambulatory status of patients with spina
bifida cystica. The Journal of bone and joint surgery.
American volume, 65(3), 350-356.
13. Dunteman, R. C., Vankoski, S. J., Dias, L. S. (2000). Internal derotation osteotomy of the tibia: pre-and postoperative gait analysis in persons with high sacral myelomeningocele. Journal of Pediatric Orthopaedics, 20(5),
623-628.
14. Westcott, M. A., Dynes, M. C., Remer, E. M., Donaldson, J. S., Dias, L. S. (1992). Congenital and acquired orthopedic
abnormalities in patients with myelomeningocele. Radiographics, 12(6), 1155-1173.
15. Moen, T., Gryfakis, N., Dias, L., Lemke, L. (2005).
Crouched gait in myelomeningocele: a comparison
between the degree of knee flexion contracture in the
clinical examination and during gait. Journal of Pediatric
Orthopaedics, 25(5), 657-660.
16. Dias, L. S. (1982). Surgical management of knee contractures in myelomeningocele. Journal of pediatric orthopedics, 2(2), 127-131.
17. Duffy, C. M., Hill, A. E., Cosgrove, A. P., Corry, I. S., Mollan,
R. A. B., Graham, H. K. (1996). Three-dimensional gait
analysis in spina bifida. Journal of Pediatric Orthopaedics, 16(6), 786-791.
18. Dodgin, D. A., De Swart, R. J., Stefko, R. M., Wenger, D. R., Ko, J. Y. (1998). Distal tibial/fibular derotation osteotomy for correction of tibial torsion: review of technique
and results in 63 cases. Journal of Pediatric Orthopaedics, 18(1), 95-101.
19. Farmer, D. L., Thom, E. A., Brock III, J. W., Burrows, P. K.,
Johnson, M. P., Howell, L. J., ... Management of Myelomeningocele Study Investigators. (2018). The Management of Myelomeningocele Study: full cohort 30-month
pediatric outcomes. American journal of obstetrics and
gynecology, 218(2), 256-e1.
20. Vankoski, S. J., Sarwark, J. F., Moore, C., Dias, L. (1995).
Characteristic pelvic, hip, and knee kinematic patterns
in children with lumbosacral myelomeningocele. Gait
Posture, 3(1), 51-57.
21. Dias, L. (2004). Orthopaedic care in spina bifida: past,
present, and future. Developmental medicine and child
neurology, 46(9), 579-579.
22. Fabry, G., Molenaers, G., Desloovere, K., Eyssen, M.
(2000). Gait analysis in myelomeningocele: possibilities
and applications. Journal of pediatric orthopedics. Part B,
9(3), 170-179.
23. Thomson, J. D., Segal, L. S. (2010). Orthopedic management of spina bifida. Developmental disabilities research
reviews, 16(1), 96-103.
24. Õunpuu, S., Thomson, J. D., Davis, R. B., DeLuca, P. A.
(2000). An examination of the knee function during gait
in children with myelomeningocele. Journal of Pediatric
Orthopaedics, 20(5), 629-635.
25. Bartonek, Å., Saraste, H., Eriksson, M., Knutson, L.,
Cresswell, A. G. (2002). Upper body movement during
walking in children with lumbo–sacral myelomeningocele. Gait posture, 15(2), 120-129.
26. Gabrieli, A. P. T., Vankoski, S. J., Dias, L. S., Milani, C.,
Lourenco, A., Laredo Filho, J., Novak, R. (2003). Gait
analysis in low lumbar myelomeningocele patients with
unilateral hip dislocation or subluxation. Journal of Pediatric Orthopaedics, 23(3), 330-334.
27. Gutierrez, E. M., Bartonek, Å., Haglund-Åkerlind, Y.,
Saraste, H. (2005). Kinetics of compensatory gait in persons with myelomeningocele. Gait posture, 21(1), 12-
23.
28. Dias, L., Vankoski, S. J., Kelp-Lenane, C. (2000). Myelomeningocele: orthopedic management and gait analysis.
American Academy of Cerebral Palsy and Developmental
Medicine, Course, 9.
29. Adzick, N. S., Thom, E. A., Spong, C. Y., Brock III, J. W.,
Burrows, P. K., Johnson, M. P., ... Farmer, D. L. (2011). A
randomized trial of prenatal versus postnatal repair of
myelomeningocele. New England Journal of Medicine,
364(11), 993-1004.
30. Tulipan, N., Wellons, J. C., Thom, E. A., Gupta, N., Sutton,
L. N., Burrows, P. K., ... Adzick, N. S. (2015). Prenatal
surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. Journal of Neurosurgery:
Pediatrics, 16(6), 613-620.
31. Johnson, M. P., Sutton, L. N., Rintoul, N., Crombleholme,
T. M., Flake, A. W., Howell, L. J., ... Adzick, N. S. (2003).
Fetal myelomeningocele repair: short-term clinical outcomes. American journal of obstetrics and gynecology,
189(2), 482-487.
32. Danzer, E., Joyeux, L., Flake, A. W., Deprest, J. (2020).
Fetal surgical intervention for myelomeningocele: lessons learned, outcomes, and future implications. Developmental Medicine Child Neurology, 62(4), 417-425.
33. Houtrow, A. J., Thom, E. A., Fletcher, J. M., Burrows, P. K.,
Adzick, N. S., Thomas, N. H., ... Walker, W. O. (2020).
Prenatal repair of myelomeningocele and school-age
functional outcomes. Pediatrics, 145(2).
34. Ntimbani, J., Kelly, A., Lekgwara, P. (2020). Myelomeningocele-A literature review. Interdisciplinary Neurosurgery, 19, 100502.
35. Wren, T. A., Tucker, C. A., Rethlefsen, S. A., Gorton III, G.
E., Õunpuu, S. (2020). Clinical efficacy of instrumented
gait analysis: Systematic review 2020 update. Gait
posture, 80, 274-279.
36. Mueske, N. M., Õunpuu, S., Ryan, D. D., Healy, B. S., Thomson, J., Choi, P., Wren, T. A. (2019). Impact of gait analysis on pathology identification and surgical recommendations in children with spina bifida. Gait posture, 67,
128-132.
37. Shih, Y. C., Chau, M. M., Arendt, E. A., Novacheck, T. F.
(2020). Measuring lower extremity rotational alignment:
a review of methods and case studies of clinical applications. JBJS, 102(4), 343-356.
38. Lim, R., Dias, L., Vankoski, S., Moore, C., Marinello, M.,
Sarwark, J. (1998). Valgus knee stress in lumbosacral
myelomeningocele: a gait-analysis evaluation. Journal of
Pediatric Orthopaedics, 18(4), 428-433.