Open Access | Review
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Open lung biopsy in patients with respiratory failure
*Corresponding author: Dr. Walid Abu Arab
Mailing address: Department of thoracic surgery, Faculty of Medicine and Health Sciences, University of Sherbrooke, 3001, 12th
north avenue, Canada.
Email: walidabuarab@yahoo.com
Received: 24 February 2020 Accepted: 15 March 2020
DOI: 10.31491/CSRC.2020.03.044
Abstract
Open lung biopsy (OLB) is known as the gold standard for the definitive diagnosis of parenchymal lung diseases, whether they are acute or chronic and/or localized or diffused. It has high diagnostic yield over bronchoalveolar lavage and transbronchial needle biopsy. The use of OLB in non-intensive care unit (nonICU) patients is considered a relatively safe technique, but its use in critically ill patients and ICU patients is a subject of argument. Methods: This paper reviews the English literature to evaluate the role of OLB in critically ill patients to determine its safety and outcomes. Twenty-two, original, published articles were found in the literature. Analysis of each study was done regarding indication for OLB, post-OLB management, complications and outcome. In conclusion OLB is a potentially safe procedure that could help to establish a diagnosis in patients with diffuse lung disease and respiratory failure. It may lead to significant changes in therapy but also, it carrries the risk of complications. A large randomized study should be performed to determine the benefits, value, and outcomes of the employment of OLB in critically ill patients with undiagnosed respiratory failure.
Keywords
Open lung biopsy; mechanical ventilation; diffuse lung disease; bronchoalveolar lavage; transbronchial needle biopsy
Introduction
Open lung biopsy (OLB) is considered the gold standard method for definitive diagnosis of parenchymal
lung diseases, whether they are acute or chronic and/
or localized or diffused [1,4]. Precise diagnosis helps to
establish a specific therapy that may be lifesaving [5]. Although there are other different diagnostic methods
that could be used, such as bronchoalveolar lavage (BAL)
and transbronchial needle biopsy (TBB), the OLB is still
considered the best tool to diagnose parenchymal lung
disease [3,6]. A complete history, physical examination,
chest radiography, and cultures will provide a reliable
diagnosis in approximately 30% of patients [7,8]. The diagnostic yield of fiberoptic bronchoscopy investigations
ranges from 38% to 85% according to the classification of histological findings [7,9]. In contrast, the reported diagnostic yield of OLB ranges from 80% to 94% [8,9].
Nevertheless, despite recent advances in surgical techniques with the use of thoracoscopy, OLB is still considered an invasive procedure requiring general anesthesia
and is associated with substantial morbidity and mortality[10-16].
The use of OLB in non-intensive care unit
(ICU) patients is considered a relatively safe technique,
but its use in critically ill patients and ICU patients is
not well-studied and unclearly defined. The use of OLB
is considered the last choice for diagnosis in mechanically ventilated patients and in those for whom empiric
therapy for respiratory failure has been unsuccessful [17-19]. There is considerable controversy regarding the
use of OLB in patients with respiratory failure and those
on mechanical ventilation because of the potential high
morbidity and mortality associated with its use [20,21].
While the role of OLB has become well-established in
the diagnosis of interstitial lung disease [18],its utility
and safety are more controversial in critically ill patients. Proponents of OLB argue that solid diagnosis of
underlying etiology can be helpful in determination of
the best course of treatment [22]. Moreover, the risk of complications from biopsy is low if adequate precautions are undertaken [23]. In contrast, opponents of OLB believe that defining the underlying mechanism of injury is largely academic and will not affect the treatment of those patients because of the lack of specific therapies for underlying etiologies of Adult Respiratory Distress Syndrome (ARDS) and respiratory failure due to infiltrative lung diseases[17].
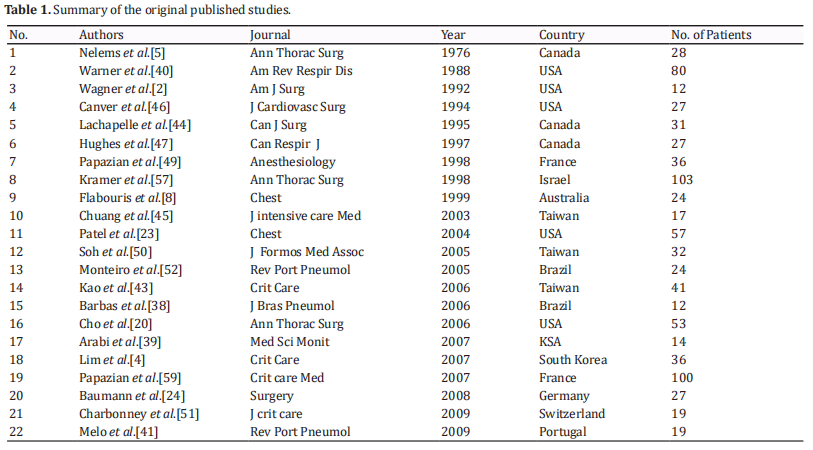
This paper reviews the literature to evaluate the role of OLB in critically ill patients to determine its safety and outcomes. Twenty-two large, original, published articles were found in the literature. Table 1 summarizes the essential data for each study.
Diagnostic instruments in respiratory failure
Different diagnostic instruments are available for diagnosing the causes of respiratory failure of unknown origin associated with pulmonary infiltrates. Clinical, laboratory, and serologic data can help to reduce the number of possible differential diagnoses. Chest x-rays and Computed Tomography of the Chest (CT- Chest) is useful for detecting complications of mechanical ventilation, such as atelectasis or pneumothorax [24]. Additionally, CT chest scans help to determine biopsy location, especially in nodular lesions or non-diffuse lesions [24] However, regarding the underlying pathology, these tests have a limited informative role [25,26].
BAL samples and the use of a bronchoscopic protected specimen brush are reliable for identifying both the quality and quantity of bacterial pneumonia microorganisms present in lung segments [27]. However, positive BAL results for infection may be difficult to interpret. Identification of Candida species in BAL represents a diagnostic difficulty because it is difficult to differentiate colonization from infection [28]. Moreover, identification of Cytomegalovirus in BAL fluid may indicate infection or disease [29].
The value of Trans-bronchial Needel Biopsy (TBB) is
well-established in the diagnosis of pulmonary infiltrates in spontaneously breathing patients [30]. TBB can be performed with acceptable risk in patients on a mechanical ventilator; the risk of pneumothorax can reach up to 19% in ARDS patients [31-35]. The main drawback of TBB is the small size of the specimens, which limits
their use for further microbiologic studies. When BAL
and TBB fail to provide diagnosis in patients with respiratory failure, the clinician must weigh the risk of empiric therapy against that of OLB [24].
OLB and specific diagnoses
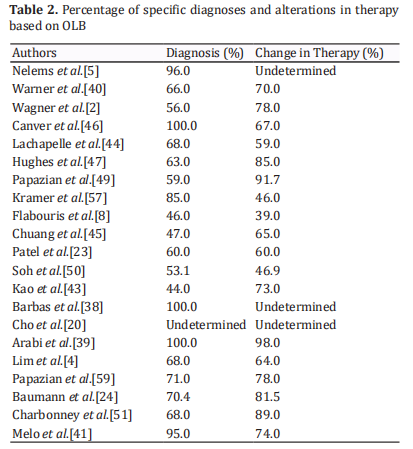
A complete history, thorough physical examination, radiologic studies, sputum cytologic analysis, and cultures can provide a reliable diagnosis in approximately 30% of patients [36]. However, OLB has been found to have a higher specific diagnostic yield, reaching up to 100% [37-39]. In reviewing the literature, it was found that many authors documented that OLB is useful and safe. Moreover, it can
provide a diagnosis that was not previously suspected. This could be of utmost value in the instillation of a new therapy or changing a previously established therapy [23]. Table 2 shows the percentages of specific histological diagnoses obtained after OLB in different studies. In the majority of these studies, more than 50% of patients received a specific diagnosis. Moreover, the specific diagnostic yield was 100% in the studies conducted by Arabi et al.[39] and Barbaras et al.[38] Specific diagnosis could consequently alter the treatment plans and therapy in these patients and hence their outcome. These alterations can include changes in drugs, such as antibiotics and/or corticosteroids. Changes can also include heparinization and the initiation and/or discontinuation of antineoplastic drugs [40]. In a study by Baumann et al. [40], two patients were considered for lung transplantation based on the results of the OLB.
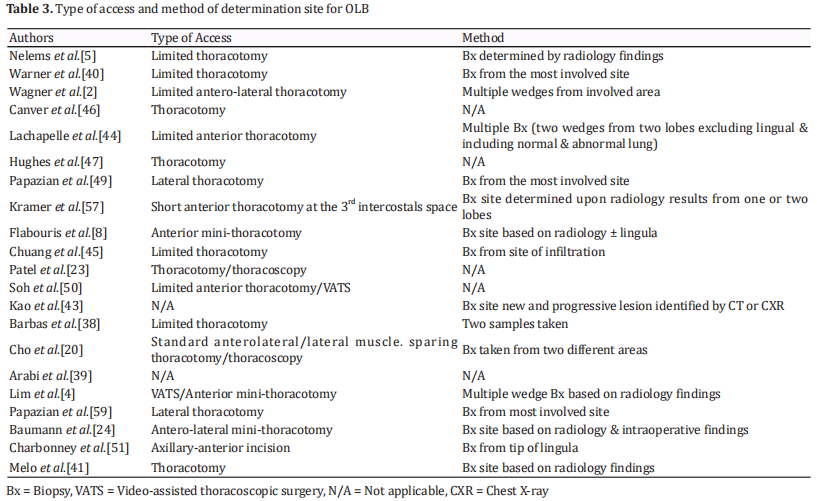
In the 22 studies reviewed, it was found that the specific diagnostic yield following OLB ranged from 44% to 100%. It is difficult to explain this variation in the specific diagnostic yield between different studies. However, it could be related to the size of the sample. Moreover, it could be explained by the variation in classification of the specific diagnosis among the studies. Melo et al. [41] stated that the ideal size of the specimen should measure at least 3cm from the largest point and should be obtained from more than one lobe [41,42]. Thoracotomy is preferred over
video-assisted thoracoscopic biopsy as it is swifter and there is no need to replace the orotracheal tube with a double lumen tube or any need for selective lung ventilation [41]. Furthermore, OLB could be performed in either the operating room or bedside in the ICU by an experienced thoracic surgeon. Kao et al. [43] recommended bedside OLB if FiO2 levels reach 1 with an applied positive end-expiratory pressure (PEEP) of at least 12 cm H2O. Table 3 shows both the method of access and how the site of OLB was determined in each study.
Complications following OLB
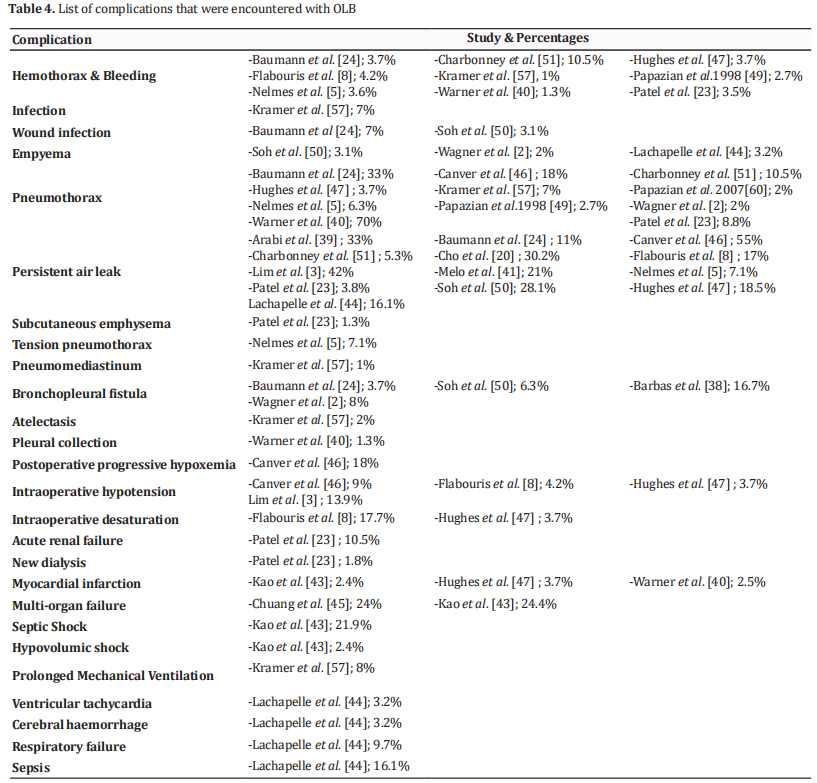
OLB is an invasive surgical procedure, but it is believed to be safe in patients who are not critically ill. Many authors have studied the outcome of OLB in critically ill patients or those who are supported by mechanical ventilation [8,39,44]. However, this procedure can have considerable complications that may result in death [44]. The various complications that have been encountered with OLB are
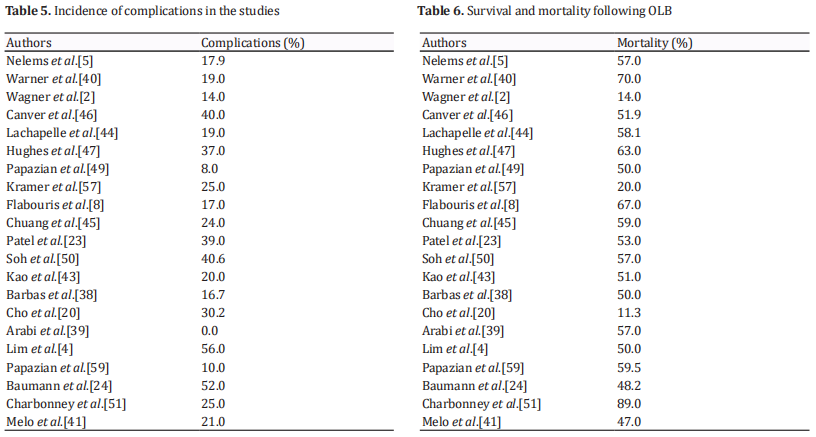
listed in Table 4, and their rates are shown in Table 5. In the reviewed studies, complication rates ranged from 0% to 56%. Arabi et al. [39] reported complications in 0% of cases, which can be explained by the retrospective nature of this study. Also, minor complication may have been encountered but not mentioned, as this study only considered major complications. Melo et al. [41] experienced a high percentage of complications that can be explained by the fact that all patients in this study were under mechanical ventilator support with high PEEP, which predisposes patients to prolonged air leakage.
In general, the difference in complication rates between
studies could be attributed to differences in patient populations and in the various definitions of complications. The most common reported complication in ventilated
patients who underwent OLB was persistent air leak [8,23,45-49]. The incidence of persistent air leak following OLB reached up to 42%. Peak airway pressure (Ppeak) was the only documented factor to predict persistent air leak after OLB. Persistent air leak was found to be reduced by 42% for each 5 cm H2O reduction in Ppeak [41]. Other reported complications included bleeding [8,23,46-48], pneumothorax [8,46,47,49], myocardial infarction [47] intraoperative cardiac arrest [23], acute renal failure [23], hypotension [46,47], bronchopleural fistula, empyema, wound infection [50], and respiratory deterioration [46-48].
Mortality and survival after OLB
Mortality rates ranged between 11.3% and 89.0%. The high mortality rate of 89.0% was reported by Charbonney et al. [51], who did not attribute this to the OLB procedure but rather to the multiple associated organ disorders in those patients and the severity of their organ damage. Many of the studies had a low associated mortality rate, even in patients on mechanical ventilation [4, 8, 23, 38, 39, 43, 46, 52]. Causes of death related to OLB included cardiac arrest [23], hemorrhage, and tension pneumothorax [8].
Some of the studies attributed the deaths to the primary disease or multiple organ failure [39, 41, 51]. In fact, it is difficult to attribute the death of a critically ill patient who may be on mechanical ventilation or have multi-organ failure to a single cause. Death in this category of patients is always multi-factorial. While OLB can be ruled out as directly causing death in critically ill patients, it cannot be excluded as playing an indirect role in these deaths. Table 6 provides the mortality and survival rates following OLB that were documented in the reviewed studies.
Effect of OLB on treatment plan
OLB can potentially result in a specific diagnosis in up to 100% of patients [39]. Table 2 shows the rate of specific diagnosis and changes in therapy in the reviewed studies based on OLB results.
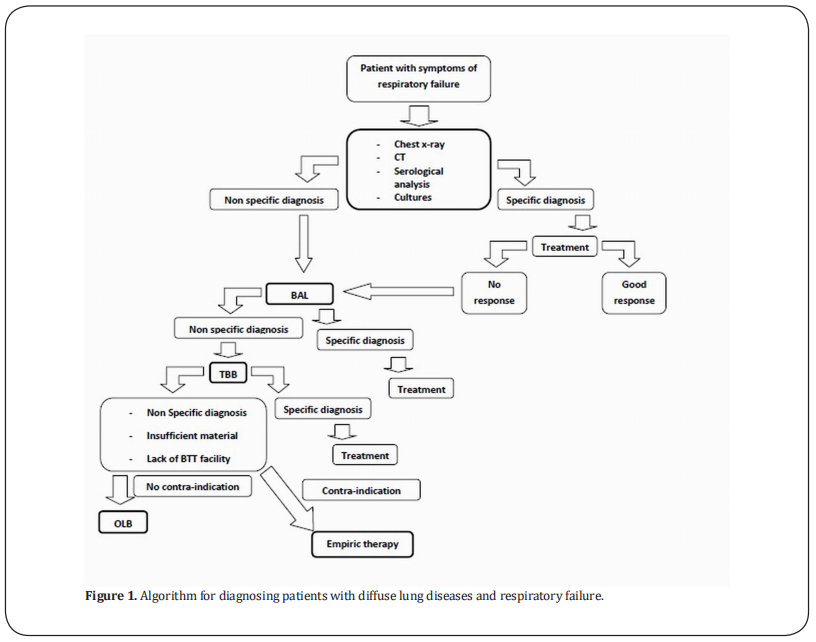
Therapy alteration following OLB ranged up to 75 % [8,39]. The high percentage of therapeutic changes that were made based on the results of biopsy procedures suggests that lung biopsy provides information that is useful to clinicians in decision making and hence in improving patient outcome. Wagner et al. [2] stated uncertainty as to whether the information provided by OLB is sufficiently beneficial to justify its routine use. While some of the previous studies showed improved survival in patients for whom biopsy established a specific diagnosis [53, 54], another failed to demonstrate any difference in mortality [55]. Several studies that included immunocompromised patients showed only a modest impact of OLB on clinical course and no difference in long-term survival [10, 11, 56, 57]. Although Potter et al. [58] stated that OLB-directed therapy may offer no advantage over empiric therapy directed at the most likely pathogens, Charbonney et al. [51] and Papazian et al. [59]found that OLB helped doctors to avoid further futile care in patients with terminal illness.In summary, OLB is of value in establishing a solid diagnosis and hence a clear plan of treatment. While there are some related complications, the most common complications have no or minor effects on outcome. Based on the literature review, we created a probable algorithm (Figure 1) that could be of value for determining if OLB should be used as a last choice to reach a diagnosis in critically ill patients after BAL and BTT trials. It should be noted that delay in diagnosis should be avoided, and these steps should be conducted in rapid sequence in order to reach a correct diagnosis and then to establish a proper treatment plan.
Conclusion
In conclusion, OLB is a potentially safe procedure that could help to establish a diagnosis in patients with diffuse lung disease and respiratory failure. It may lead to significant changes in therapy. Until now, the clinical or biomedical parameters that could predict those at high risk for complications following OLB were unknown. Further randomized clinical trials could be useful to clarify the benefits and drawbacks of OLB in critically ill patients.
Declarations
Authors’ contributions
All authors have contributed equally in the manuscript.
Conflicts of interest
All authors declare that they are bound by confidentiality agreements that prevent them from disclosing their conflicts of interest in this work.
References
1. Krell, W. S. (1988). Pulmonary diagnostic procedures in
the critically ill. Critical care clinics, 4(2), 393-407.
2. Wagner, J. D., Stahler, C., Knox, S., Brinton, M., & Knecht,
B. (1992). Clinical utility of open lung biopsy for
undiagnosed pulmonary infiltrates.The American journal
of surgery, 164(2), 104-107.
3. Walker, W. A., Cole Jr, F. H., Khandekar, A., Mahfood,
S. S., & Watson, D. C. (1989). Does open lung biopsy
affect treatment in patients with diffuse pulmonary
infiltrates?. The Journal of thoracic and cardiovascular
surgery, 97(4), 534-540.
4. Lim, S. Y., Suh, G. Y., Choi, J. C., Koh, W. J., Lim, S. Y., Han,
J., ... & Kwon, O. J. (2007). Usefulness of open lung biopsy
in mechanically ventilated patients with undiagnosed
diffuse pulmonary infiltrates: influence of comorbidities
and organ dysfunction. Critical care, 11(4), R93.
5. Nelems, J. M., Cooper, J. D., Henderson, R. D., Peng, T., &
Phillips, M. J. (1976). Emergency open lung biopsy. The
Annals of thoracic surgery, 22(3), 260-264.
6. Bove, P., Ranger, W., Pursel, S., Glover, J., Bove, K., & Bendick,
P. (1994). Evaluation of outcome following open lung
biopsy. The American surgeon, 60(8), 564-570.
7. Gaensler, E. A., & Carrington, C. B. (1980). Open biopsy
for chronic diffuse infiltrative lung disease: clinical,
roentgenographic, and physiological correlations in 502
patients. The Annals of thoracic surgery, 30(5), 411-426.
8. Flabouris, A., & Myburgh, J. (1999). The utility of
open lung biopsy in patients requiring mechanical
ventilation. Chest, 115(3), 811-817.
9. Utz, J. P., Perrella, M. A., & Rd, R. E. (1991). Lung biopsy.
Adv Intern Med, 37, 337-361.
10. Hiatt, J. R., Gong, H., Mulder, D. G., & Ramming, K. P. (1982).
The value of open lung biopsy in the immunosuppressed
patient. Surgery, 92(2), 285-291.
11. Haverkos, H. W., Dowling, J. N., William, A., Myerowitz,
R. L., Lerberg, D. B., & Hakala, T. R. (1983). Diagnosis of
pneumonitis in immunocompromised patients by open
lung biopsy. Cancer, 52(6), 1093-1097.
12. Prober, C. G., Whyte, H., & Smith, C. R. (1984). Open
lung biopsy in immunocompromised children with
pulmonary infiltrates. American Journal of Diseases of
Children, 138(1), 60-63.
13. Thomas, J. H., Farek, P. E., Hermreck, A. S., & Pierce, G.
E. (1987). Diagnostic value of open lung biopsy in
immunocompromised patients. The American journal of
surgery, 154(6), 692-695.
14. Snyder, C. L., Ramsay, N. K., McGlave, P. B., Ferrell, K. L.,
& Leonard, A. S. (1990). Diagnostic open-lung biopsy
after bone marrow transplantation. Journal of pediatric
surgery, 25(8), 871-877.
15. Early, G. L., Williams, T. E., & Kilman, J. W. (1985). Open
lung biopsy: its effects on therapy in the pediatric
patient. Chest, 87(4), 467-469.
16. Doolin, E. J., Luck, S. R., Sherman, J. O., & Raffensperger,
J. G. (1986). Emergency lung biopsy: Friend or foe of
the immunosuppressed child?. Journal of pediatric
surgery, 21(6), 485-487.
17. Malhotra, A., & Patel, S. (2006). Lung biopsy in ARDS: is it
worth the risk?. Critical Care, 10(4), 160.
18. European, R. S., & American Thoracic Society. (2002).
American Thoracic Society/European Respiratory
Society international multidisciplinary consensus
classification of the idiopathic interstitial pneumonias.
This joint statement of the American Thoracic Society
(ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by
the ERS executive committee, June 2001.American Journal
of Respiratory and Critical Care Medicine, 165(2), 277.
19. Bajwa, E. K., Ayas, N. T., Schulzer, M., Mak, E., Ryu, J. H., &
Malhotra, A. (2005). Interferon-γ1b therapy in idiopathic
pulmonary fibrosis: a metaanalysis. Chest, 128(1), 203-
206.
20. Cho, M. H., Malhotra, A., Donahue, D. M., Wain, J. C., Harris,
R. S., Karmpaliotis, D., & Patel, S. R. (2006). Mechanical
ventilation and air leaks after lung biopsy for acute
respiratory distress syndrome. The Annals of thoracic
surgery, 82(1), 261-266.
21. Meduri, G. U., Headley, A. S., Golden, E., Carson, S. J.,
Umberger, R. A., Kelso, T., & Tolley, E. A. (1998). Effect of
prolonged methylprednisolone therapy in unresolving
acute respiratory distress syndrome: a randomized
controlled trial. Jama, 280(2), 159-165.
22. Esteban, A., Fernández-Segoviano, P., Frutos-Vivar, F.,
Aramburu, J. A., Nájera, L., Ferguson, N. D., ... & Ríos, F. (2004).
Comparison of clinical criteria for the acute respiratory
distress syndrome with autopsy findings. Annals of
internal medicine, 141(6), 440-445.
23. Patel, S. R., Karmpaliotis, D., Ayas, N. T., Mark, E. J., Wain, J.,
Thompson, B. T., & Malhotra, A. (2004). The role of openlung biopsy in ARDS. Chest, 125(1), 197-202.
24. Baumann, H. J., Kluge, S., Balke, L., Yekebas, E., Izbicki,
J. R., Amthor, M., ... & Meyer, A. (2008). Yield and safety
of bedside open lung biopsy in mechanically ventilated
patients with acute lung injury or acute respiratory
distress syndrome. Surgery, 143(3), 426-433.
25. Miller Jr, W. T., Tino, G., & Friedburg, J. S. (1998). Thoracic
CT in the intensive care unit: assessment of clinical
usefulness. Radiology, 209(2), 491-498.
26. Mäurer, J., Kendzia, A., Gerlach, H., Pappert, D., Hierholzer, J.,
Falke, K. J., & Felix, R. (1998). Morphological changes in chest
radiographs of patients with acute respiratory distress
syndrome (ARDS).Intensive care medicine, 24(11), 1152-
1156.
27. Chastre, J., Fagon, J. Y., Bornet-Lecso, M., Calvat, S.,
Dombret, M. C., al Khani, R. H. A. Y. D. A. H., ... & Gibert, C.
(1995). Evaluation of bronchoscopic techniques for the
diagnosis of nosocomial pneumonia. American journal of
respiratory and critical care medicine, 152(1), 231-240.
28. Azoulay, E., Cohen, Y., Zahar, J. R., Garrouste-Orgeas, M.,
Adrie, C., Moine, P., ... & Timsit, J. F. (2004). Practices in nonneutropenic ICU patients with Candida-positive airway
specimens. Intensive care medicine, 30(7), 1384-1389.
29. Tamm, M., Traenkle, P., Solèr, M., Bolliger, C. T., Grilli,
B., Dalquen, P., & Cathomas, G. (2001). Pulmonary
cytomegalovirus infection in immunocompromised
patients. Chest, 119(3), 838-843.
30. Jain, P., Sandur, S., Meli, Y., Arroliga, A. C., Stoller, J. K.,
& Mehta, A. C. (2004). Role of flexible bronchoscopy
in immunocompromised patients with lung infiltrates. Chest, 125(2), 712-722.
31. O'Brien, J. D., Ettinger, N. A., Shevlin, D., & Kollef, M. H. (1997).
Safety and yield of transbronchial biopsy in mechanically
ventilated patients. Critical care medicine, 25(3), 440-
446.
32. Pincus, P. S., Kallenbach, J. M., Hurwitz, M. D., Clinton, C. O.
L. I. N., Feldman, C. H. A. R. L. E. S., Abramowitz, J. A., & Zwi, S.
A. U. L. (1987). Transbronchial biopsy during mechanical
ventilation. Critical care medicine, 15(12), 1136-1139.
33. Papin, T. A., Grum, C. M., & Weg, J. G. (1986). Transbronchial
biopsy during mechanical ventilation. Chest, 89(2), 168-
170.
34. Martin, C., Papazian, L., Payan, M. J., Saux, P., & Gouin, F.
(1995). Pulmonary fibrosis correlates with outcome
in adult respiratory distress syndrome: a study in
mechanically ventilated patients. Chest, 107(1), 196-200.
35. Bulpa, P. A., Dive, A. M., Mertens, L., Delos, M. A., Jamart,
J., Evrard, P. A., & Gonzalez, M. R. (2003). Combined
bronchoalveolar lavage and transbronchial lung biopsy:
safety and yield in ventilated patients. European
Respiratory Journal, 21(3), 489-494.
36. Gaensler, E. A., & Carrington, C. B. (1980). Open biopsy
for chronic diffuse infiltrative lung disease: clinical,
roentgenographic, and physiological correlations in 502
patients. The Annals of thoracic surgery, 30(5), 411-426.
37. Cheson, B. D., Samlowski, W. E., Tang, T. T., &
Spruance, S. L. (1985). Value of open-lung biopsy in
87 immunocompromised patients with pulmonary
infiltrates. Cancer, 55(2), 453-459.
38. Barbas, C. S. V., Capelozzi, V. L., Hoelz, C., Magaldi, R. B.,
Souza, R. D., Sandeville, M. L., ... & Knobel, E. (2006). Impact
of open lung biopsy on refractory acute respiratory
failure. Jornal Brasileiro de Pneumologia, 32(5), 418-423.
39. Arabi, Y., Ahmed, R., Ahmed, Q., Rahman, M. U., & Yamani,
N. (2007). Risks and benefits of open-lung biopsy in
the mechanically ventilated critically ill population:
a cohort study and literature review. Medical Science
Monitor, 13(8), CR365-CR371.
40. Warner, D. O., Warner, M. A., & Divertie, M. B. (1988).
Open lung biopsy in patients with diffuse pulmonary
infiltrates and acute respiratory failure.American Review
of Respiratory Disease, 137(1), 90-94.
41. Melo, N., Figueiredo, S., Morais, A., Moura, C. S., Pinho,
P., Bastos, P., & Oliveira, T. (2009). Open lung biopsy in
patients on mechanical ventilation with suspected diffuse
lung disease.Revista Portuguesa de Pneumologia (English
Edition), 15(4), 597-611.
42. Flint, A., Martinez, F. J., Young, M. L., Whyte, R. I., Toews,
G. B., & Lynch III, J. P. (1995). Influence of sample number
and biopsy site on the histologic diagnosis of diffuse lung
disease.The Annals of thoracic surgery,60(6), 1605-1608.
43. Kao, K. C., Tsai, Y. H., Wu, Y. K., Chen, N. H., Hsieh, M. J.,
Huang, S. F., & Huang, C. C. (2006). Open lung biopsy in
early-stage acute respiratory distress syndrome. Critical
Care, 10(4), R106.
44. Lachapelle KJ, Morin JE. Benefit of open lung biopsy
in patients with respiratory failure. Can J Surg. 1995;
38(4):316-321.
45. Chuang, M. L., Lin, I. F., Tsai, Y. H., Vintch, J. R., & Pang, L.
C. (2003). The utility of open lung biopsy in patients with
diffuse pulmonary infiltrates as related to respiratory
distress, its impact on decision making by urgent
intervention, and the diagnostic accuracy based on the
biopsy location. Journal of intensive care medicine, 18(1),
21-28.
46. Canver, C. C., & Mentzer, J. R. (1994). The role of open lung
biopsy in early and late survival of ventilator-dependent
patients with diffuse idiopathic lung disease. The Journal
of cardiovascular surgery, 35(2), 151-155.
47. Hughes, R., & McGuire, G. (1997). Evaluation of open lung
biopsy in critically ill, ventilator dependent intensive
care unit patients. Canadian Respiratory Journal, 4(5),
246-250.
48. Kornecki, A., & Shemie, S. D. (2001). Open lung biopsy
in children with respiratory failure. Critical care
medicine, 29(6), 1247-1250.
49. Papazian, L., Thomas, P., Bregeon, F., Garbe, L.,
Zandotti, C., Saux, P., ... & Gouin, F. (1998). Open-lung
biopsy in patients with acute respiratory distress
syndrome. Anesthesiology: The Journal of the American
Society of Anesthesiologists, 88(4), 935-944.
50. Soh, L. H., Chian, C. F., Su, W. L., Yan, H. C., Perng, W. C., & Wu,
C. P. (2005). Role of open lung biopsy in patients with diffuse
lung infiltrates and acute respiratory failure.Journal of the
Formosan Medical Association, 104(1), 17-21.
51. Charbonney, E., Robert, J., Pache, J. C., Chevrolet, J. C.,
& Eggimann, P. (2009). Impact of bedside open lung
biopsies on the management of mechanically ventilated
immunocompromised patients with acute respiratory
distress syndrome of unknown etiology.Journal of critical
care, 24(1), 122-128.
52. Monteiro, A. S., Addor, G., Nigri, D. H., & Franco, C. A. D.
B. (2005). Open lung biopsy in patients on mechanical
ventilation and presenting diffuse pulmonary
infiltrate. Jornal Brasileiro de Pneumologia, 31(3), 212-
218.
53. Greenman, R. L., Goodall, P. T., & King, D. (1975). Lung
biopsy in immunocompromised hosts. The American
journal of medicine, 59(4), 488-496.
54. Pennington, J. E., & Feldman, N. T. (1977). Pulmonary
infiltrates and fever in patients with hematologic
malignancy: assessment of transbronchial biopsy. The
American journal of medicine, 62(4), 581-587.
55. Wharton, J. M., COLEMAN, D. L., WOFSY, C. B., LUCE, J. M.,
BLUMENFELD, W., HADLEY, W. K., ... & HOPEWELL, P. C.
(1986). Trimethoprim-sulfamethoxazole or pentamidine
for Pneumocystis carinii pneumonia in the acquired
immunodeficiency syndrome: a prospective randomized trial. Annals of internal medicine, 105(1), 37-44.
56. Hall, T. S., Hutchins, G. M., & Baker, R. R. (1987). A critical
review of the use of open lung biopsy in the management
of the oncologic patient with acute pulmonary
infiltrates. American journal of clinical oncology, 10(3),
249-252.
57. Kramer, M. R., Mintz, B., & Saute, M. (1998). The role
of open lung biopsy in the management and outcome
of patients with diffuse lung disease. The Annals of
thoracic surgery, 65(1), 198-202.
58. Potter, D., Pass, H. I., Brower, S., Macher, A., Browne, M.,
Thaler, M., ... & Pizzo, P. (1985). Prospective randomized
study of open lung biopsy versus empirical antibiotic
therapy for acute pneumonitis in nonneutropenic cancer
patients. The Annals of thoracic surgery, 40(5), 422-428.
59. Papazian, L., Doddoli, C., Chetaille, B., Gernez, Y.,
Thirion, X., Roch, A., ... & Thomas, P. (2007). A
contributive result of open-lung biopsy improves survival
in acute respiratory distress syndrome patients. Critical
care medicine, 35(3), 755-762.