Open Access | Research Article
This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
LncRNA MEG3 promotes glaucomatous retinal ganglion cell apoptosis in acute glaucoma mice via up-regulating miR-106 target gene caspase-8
*Corresponding author: Qiuli Zhang
Affiliated Hospital of Inner Mongolia University for Nationalities,
No. 1742, HuoLinHe Street, Tongliao, Neimenggu 028050, China.
E-mail:qiu_lizhang@126.com
Received: 18 August 2019 Accepted: 19 September 2019
DOI: 10.31491/CSRC.2019.09.002
Abstract
Background: MiR-106b and caspase-8 played a key role in the development of acute glaucoma. Increasing evidence has indicated that long non-coding RNA (lncRNA) maternally expressed gene 3 (MEG3) participated in regulating pathophysiological processes. However, the association among MEG3, miR-106b and caspase-8 remained unclear.
Methods: We employed the mouse model of acute glaucoma and oxygen and glucose deprivation (OGD)/ reoxygenation cellular model for in vivo and in vitro experiments. The miRNA inhibitor and small interfering RNA (siRNA) were transfected into primary retinal ganglion cells (RGCs) for miRNA and lncRNA knockdown. The interaction among MEG3, miR-106b and caspase-8 was assessed by RNA immunoprecipitation, RNA pull down and luciferase reporter assay. The changes in gene expression were assessed by quantitative Real-Time PCR (qRT-PCR) and western blot. Cell apoptosis analysis was performed using flow cytometry.
Results: MEG3 expression was increased in the mouse model of acute glaucoma and OGD-treated RGCs. MEG3 knockdown alleviated RGC apoptosis following OGD. RNA immunoprecipitation and RNA pull down displayed that MEG3 directly targeted miR-106b, and luciferase reporter assay confirmed the interaction between miR- 106b and caspase-8. MEG3 silencing significantly relieved RGC apoptosis via downregulating miR-106b target gene caspase-8.
Conclusion: PMEG3 increased the apoptosis of glaucomatous RGC via miR-106b/caspase-8 axis.
Keywords
acute glaucoma; retinal ganglion cells; MEG3; miR-106b; caspase-8
Introduction
It is generally known that acute glaucoma is one of the
leading causes of permanent vision loss and irreversible
blindness worldwide, which is characterized by a
rapid increase of intraocular pressure (IOP) resulting
from a blockage around drainage canals and consequent
retinal ischemia, leading to progressive damage to retinal
ganglion cells (RGCs)[1,2]. Despite intensive medical
treatment, increasing evidence has suggested that acute
glaucoma continues progressing to blindness in quite
a few patients[3]. Until recently, elevated IOP has been
considered to be a major risk factor for the pathogenesis
of RGC death in acute glaucoma[4]. Nevertheless, the
detailed mechanisms by which elevated IOP ultimately
led to RGC apoptosis were largely unknown.
In the past years, emerging evidence has showed that the
caspase aspartate-specific cysteine protease family are
involved in programmed cell death in eukaryotes[5]. Several
studies have reported that caspase family consists
of at least 14 members in mammalian cells. Caspase-8
is synthesized as a pro-enzyme and comprises a large
N-terminal prodomain as well as a C-terminal catalytic
domain, playing a crucial role in triggering death receptor-
mediated apoptosis[6]. Recently, accumulating evidence
has strongly implied that as an initiator caspase,
caspase-8 has been implicated in acute glaucoma. For
instance, Chi et al found that substantial rise in IOP induces
Toll-like receptor 4 (TLR4)/caspase-8 signaling
pathway activation, thereby leading to retinal ischemic
injury and RGC death[7]. Furthermore, a recent report has
indirectly revealed the apoptotic functions of caspase-8,
demonstrating that high-mobility group box 1 (HMGB1)
promotes the activation of caspase-8 via NF-κB pathway,
resulting in inflammatory response[8].
MicroRNAs (miRNAs) are a class of small single-stranded
(~22-nucleotide-long) non-coding RNAs that play important roles in physiopathologic processes by negatively
regulating target genes. A number of dysregulated
miRNAs have been actively involved in diverse biological
processes. For example, Jamie et al reported that the
miRNA cluster caspase-8~25 promotes the proliferation
of self-renewing neural stem/progenitor cell (NSPC)
and the generation of new neurons under the condition
of self-differentiation[9]. Hari et al have found the significant
downregulation of caspase-8 in glaucomatous
retinae[10]. Nonetheless, the caspase-8-related molecular
mechanisms were not well understood.
MEG3, an lncRNA, acts as a tumor suppressor in various
cancers by influencing the apoptosis and proliferation of
tumor cells, such as neuroblastomas and gliomas[11,12].
Previous reports have shown that MEG3 expression is
positively correlated with the progression of patients
with retinoblastoma and inhibits tumor growth via
Wnt/β-catenin pathway activation[13]. Besides the antineoplastic
effect, other studies also indicated that the
activation of MEG3 triggers ischemic neuronal death[14].
However, little was known about the molecular mechanisms
and biological roles of MEG3 in acute glaucoma.
Therefore, our study was designed to reveal whether
MEG3 affects acute glaucoma progression and ascertain
the potential regulatory mechanism.
Materials and Methods
Mouse model of acute glaucoma
Animal experiments performed in this study were approved by the Animal Ethics Committee of the Affiliated Hospital of Inner Mongolia University for the Nationalities and qualify to the ARVO guidelines of animal use in eye research. Prior to in vivo experiment, a total of 30 adult male C57BL/6 mice obtained from Inner Mongolia University for the Nationalities were anesthetized by an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. The anterior chamber of the right eye was cannulated with a 30-gauge needle connected to a syringe filled with normal saline to maintain an IOP around 120 mmHg for 1h. Retinal ischemia was verified by the whitening of the iris and loss of the red reflex. After withdrawal of the needle, reperfusion occurred as IOP was normalized within 5 min measured by a noncontact tonometer (Nidek Co., Ltd., Aichi, Japan). The contralateral left eye as a control eye carried on sham-operated procedure. All mice were then subjected to 6, 24, 48, or 72 h of reoxygenation before euthanasia. Retinal tissues were collected for the following procedure.
Isolation and culture of primary RGC
Retinal tissues were isolated from enucleated eyeballs of 12-day-old newborn C57BL/6 mice and maintained in calcium/magnesium-free Hank’s balanced salt solution (Life Technologies, Carlsbad, CA, USA) containing 16.5 U/mL of papain (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at room temperature. Primary RGCs were purified from the collected retinal cell suspension using two-step immunopanning (TSI) method as previously described[15] by incubation with rabbit-anti-mouse macrophage antibody (1:50; Fitzgerald Industries International, Concord, MA, USA) for 5 min and goat-anti-rabbit IgG antibody (1:200; Southern Biotechnology Associates, Birmingham, AL, USA) for 30 min at room temperature. All adherent RGCs were harvested by incubation with trypsin solution (Gibco, Carlsbad, CA, USA) and cultured in Dulbecco's modified eagle medium/Ham's F12 (DMEM/F12; Life Technologies) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin (Gibco), and 100 μg/ml streptomycin (Gibco) at 37°C in humidified 5% CO2 and 95% air.
Oxygen and glucose deprivation (OGD) cellular model
Primary RGCs were seeded on poly-L-ornithine and laminin precoated coverslips in 24-well plate with 2.5×105 cells per well and incubated at 37°C in humidified 5% CO2 and 95% air. Twenty-four hours after seeding, cells were washed twice with phosphate-buffered saline (PBS), cultured in glucose-free Roswell Park Memorial Institute (RPMI) 1640 medium containing L-glutamine and incubated in 5% CO2/95% N2 in an anaerobic chamber at 37°C for 4h. Subsequently, cells were then grown in DMEM/F12 containing glucose and returned to a normoxic environment (5% CO2 and 95% air) for another 12h at 37°C. In addition, RGCs exposed to normal culture media in a normoxic incubator were used as controls. OGD treated cells and controls were collected to qRTPCR and western blotting analysis for MEG3, caspase-8 and caspase-8 expression.
RNA interference
To investigate the biological role of MEG3 in cellular ischemia/reperfusion (I/R) injury, primary RGCs were randomly divided into 4 groups: control, OGD, OGD+siRNA control (si-Ctrl) and OGD+siRNA-MEG3 (si-MEG3). Briefly, cells were cultured in 96-well plates at 1×104 cells/well overnight, transfected with si-MEG3 or si-Ctrl using X-tremeGENE siRNA Transfection Reagent (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions and exposed to OGD treatment for 4h after 24h transfection. The sequence of si- MEG3 was shown in the Table S1. The mRNA expression of MEG3 was determined by qRT-PCR and cell apoptosis analysis was performed after 12h reoxygenation.
RNA immunoprecipitation
DIANA tools (http://carolina.imis.athena-innovation. gr/) were used to predict the potential interaction of MEG3 and caspase-8. RIP assay was performed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. RGCs at 80% density (approximately 1.0 × 107 cells) were washed with cold PBS and lysed with RIP lysis buffer at 4°C for 30min. Cell extracts were incubated with protein A/G sepharose beads conjugated to anti-Ago2 antibody (Millipore) or normal IgG at 4 °C and washed with lysis buffer for five times. Immunoprecipitated RNAs and total RNA from the whole cell lysates (input controls) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for western blotting or extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol for qRT-PCR analysis.
RNA pull-down
The interaction between MEG3 and caspase-8 was further examined by RNA pull-down using a Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. Protein extracts from RGCs (approximately 1.0 × 107 cells) were mixed with 50 pmol of biotinylateds MEG3 (or its negative control LOC) and incubated with 50μL of streptavidin magnetic beads 4°C for 1h. The associated RNA-protein complex was isolated using Biotin Elution Buffer and boiled in SDS buffer for 10 min. The retrieved protein was detected using western blot analysis for Ago2 protein levels, while caspase-8 mRNA levels were measured by qRT-PCR.
Luciferase reporter assay
Targetscan online bioinformatics software (http:// www.targetscan.org) was used to identify the underlying binding sites of caspase-8 and caspase-8. To verify the interaction between them, luciferase reporter assay was performed in RGCs. The caspase-8 recombinant plasmids containg the wild type binding site of caspase-8 (caspase-8-WT) or mutated binding site of caspase-8 (caspase-8-Mut) were constructed, and then respectively co-transfected with caspase-8 mimic or inhibitor or their corresponding negative controls into RGCs using Lipofectamine 2000 (Invitrogen, USA). Cells were harvested 24h post-transfection and were incubated with passive lysis buffer at room temperature for 10 minutes. Luciferase activity was measured using the Dual Luciferase Assay kit (Promega, Madison, WI, USA) following the manufacturer’s instructions.
Cell transfection
To further explore the molecular mechanism and biological function of MEG3 in OGD-induced RGCs ischemic injury, primary RGCs were then randomized to 6 groups as follows: control, OGD, OGD+si-Ctrl, OGD+si-MEG3, OGD+ si-MEG3+NC and OGD+si-MEG3+caspase-8 inhibitor. Before transfection, cells were seeded in 6-well plates at a density of 4×105 cells/ml for one day. When cell confluence reached more than 70%, the miRNA inhibitor and small interfering RNA (siRNA) as well as negative controls (NC or si-Ctrl) purchased from GenePharma (Shanghai, China) were transfected into cells using a genefectine transfection reagent (Sigma-Aldrich). The sequences of caspase-8 mimic and inhibitor were shown in the Table S2. After 24h transfection, cells were subjected to OGD/reoxygenation treatment followed by the subsequent experiments including MEG3, caspase-8 and caspase-8 expression and cell apoptosis.
QRT-PCR analysis
Total RNA was extracted from retinal tissues and RGCs using Trizol reagent and reverse-transcribed to cDNA using a PrimeScript RT Reagent Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. The relative mRNA expression levels of MEG3, caspase-8 and caspase-8 were normalized to GAPDH, U6 and GAPDH snRNA expression, respectively, determined using SYBR Premix Ex Taq (TaKaRa) on an ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) and calculated by the 2−ΔΔCt method. The primer sequences used for qRT-PCR were shown in the Table S2.
Western blot
For analysis of caspase-8 protein expression in retinal samples and RGCs, total protein was extracted using Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China), centrifuged with 14,000 rpm for 15 min at 4°C. Protein extracts and Prestained Protein Marker (Beyotime, China) were run on 10% SDS, transferred onto PVDF membranes and blocked with Tris buffered saline tween (TBST) containing 5% skim milk at room temperature for 2h. Blots were probed with rabbit anti-mouse caspase-8 polyclonal antibody (1:1000; Cell Signaling Technology, Boston, MA, USA) or β-actin mouse monoclonal antibody (1:1000; Beyotime, China) at 4°C overnight, incubated with horseradish-peroxidase (HRP)-coupled goat anti-rabbit IgG (1:2000; Abcam, Cambridge, UK) at room temperature for 1~2h and visualized on a Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Hercules, CA, USA) using an ECL Plus Western Blotting Substrate (Thermo Scientific, Shanghai, China).
Cell apoptosis assay
Cell apoptosis was analyzed by flow cytometry using Annexin V-FITC apoptosis detection kit (BD Biosciences; San Jose, CA, USA) according to the manufacturer’s protocols. Cells following transfection and OGD/reoxygenation treatment were collected, washed with cold PBS and stained with binding buffer containing Annexin V-FITC and propidine iodide (PI) at 4°C under darkness for 15 min. Finally, cells were recorded using flow cytometry (Beckman Coulter, Fullerton, CA, USA).
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL, USA). Significant differences between groups were analyzed using two-sided Student’s t test, and P<0.05 was considered to be statistically significant.
Results
MEG3 and caspase-8 was upregulated while caspase-8 was downregulated in mouse model of acute glaucoma and OGD-treated RGCs
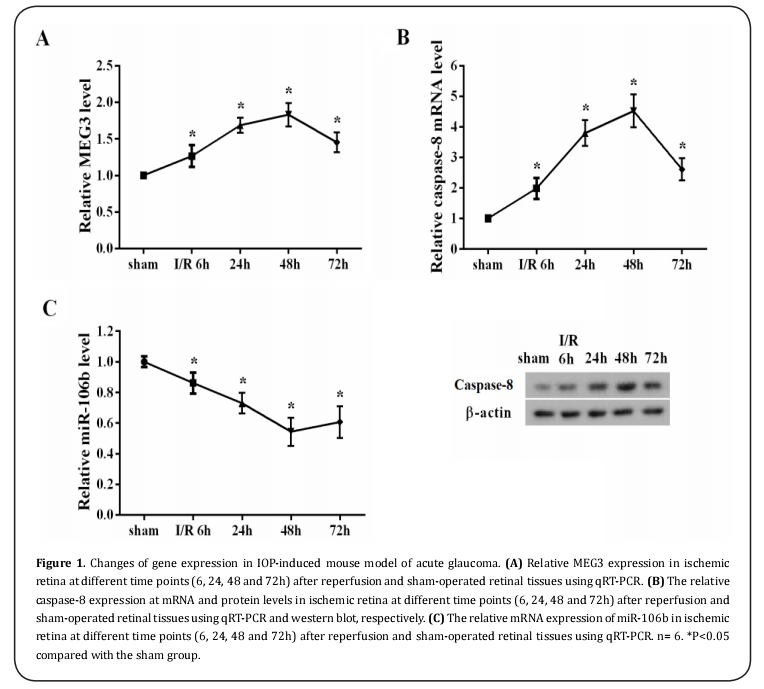
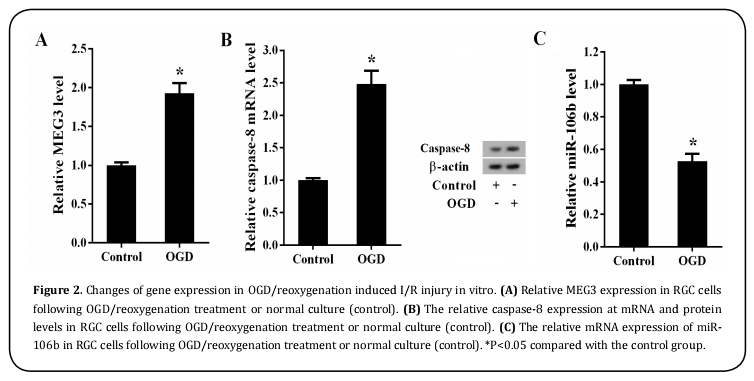
To investigate the underlying role of MEG3 in acute glaucoma, we examined MEG3 as well as caspase-8 and caspase-8 expression in 6 paired high IOP-induced ischemic retinal tissues with different reperfusion time points (6, 24, 48 and 72h) and sham-operated contralateral tissues by qRT-PCR and western blot. Our data revealed that the expression of MEG3 (Figure 1A, P<0.05) and caspase-8 (Figure 1B, P<0.05) were elevated while caspase-8 expression (Figure 1C, P<0.05) was decreased in ischemic retina at different time points after reperfusion relative to the sham-operated controls. Conformably, higher levels of MEG3 (Figure 2A, P<0.05) and caspase-8 (Figure 2B, P<0.05) were observed, whereas, caspase-8 was significantly downregulated (Figure 2C, P<0.05) in OGD/reoxygenation treated primary RGCs as compared with the normal cultured controls. Taken together, these data indicated that MEG3, caspase-8 and caspase-8 might be involved in the development of acute glaucoma.
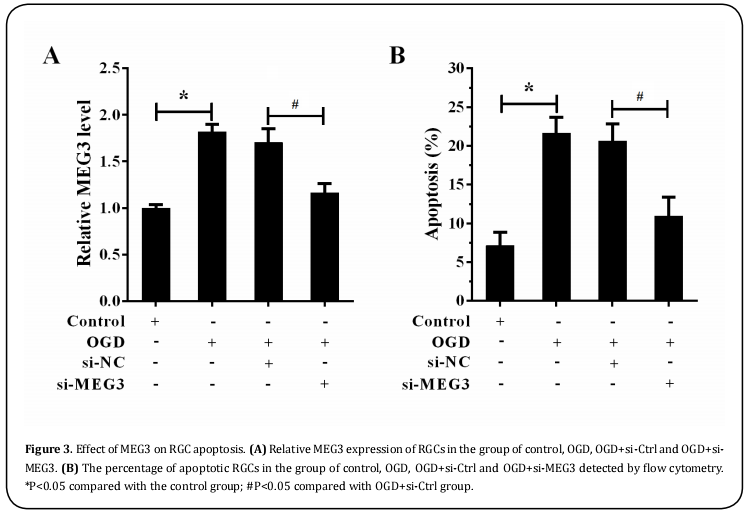
MEG3 knockdown suppressed OGD-induced RGC apoptosis
To evaluate the biological functions of MEG3, the MEG3 expression levels and apoptosis rate of RGCs following OGD and/or MEG3 knockdown with siRNA transfection were analyzed by qRT-PCR and flow cytometry. Following OGD, the mRNA expression of MEG3 (Figure 3A, P<0.05) and the percentage of apoptotic RGCs (Figure 3B, P<0.05) were markedly enhanced as compared with those of the normal control group. However, the knockdown of MEG3 expression dramatically reduced the MEG3 mRNA expression levels (Figure 3A, P<0.05) and the apoptosis rate of RGCs (Figure 3B, P<0.05) following OGD treatment. These findings demonstrated that MEG3 inhibition reduced the apoptotic rate of RGCs in vitro.
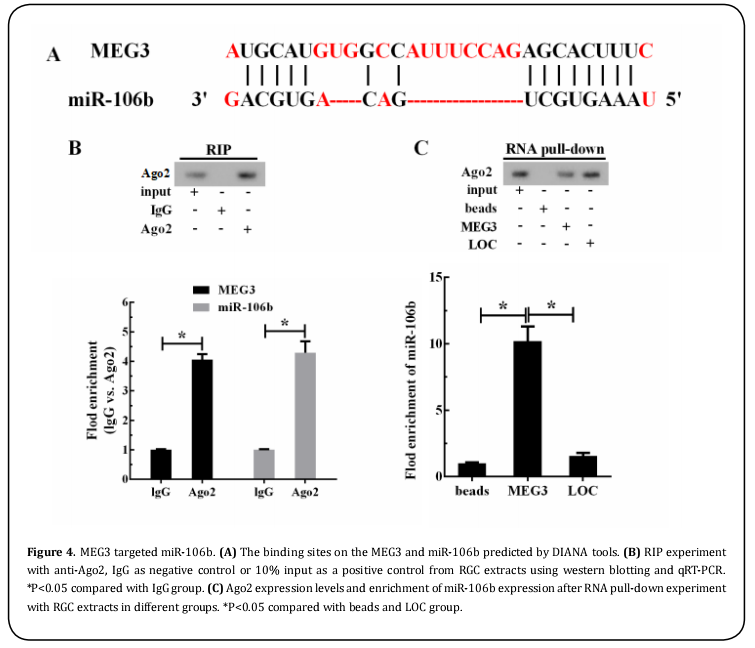
MEG3 targeted caspase-8
MEG3 was reported to play important roles in post-transcriptional regulation in various cancers[16]. However, the specific downstream regulators involved in the abnormal expression of MEG3 in acute glaucoma still remained unknown. Previously, our study has found that MEG3 harbored one putative binding site for caspase-8 predicted by the online DIANA tools (Figure 4A). The RNA immunoprecipitation and RNA pull-down assay were applied to confirm the potential binding protein. As shown in Figure 4B, MEG3 and caspase-8 enrichment was observed in Ago2-RNA precipitates, while less enrichment was found in IgG precipitates (P<0.05). Furthermore, RNA pull down assay revealed that the expression levels of caspase-8 in MEG3 pulled down pellet was higher than those of beads and loading control (Figure 4C, P<0.05). Together, these results demonstrated that MEG3 targets caspase-8.mained unknown. Previously, our study has found that MEG3 harbored one putative binding site for caspase-8 predicted by the online DIANA tools (Figure 4A). The RNA immunoprecipitation and RNA pull-down assay were applied to confirm the potential binding protein. As shown in Figure 4B, MEG3 and caspase-8 enrichment was observed in Ago2-RNA precipitates, while less enrichment was found in IgG precipitates (P<0.05). Furthermore, RNA pull down assay revealed that the expression levels of caspase-8 in MEG3 pulled down pellet was higher than those of beads and loading control (Figure 4C, P<0.05). Together, these results demonstrated that MEG3 targets caspase-8.
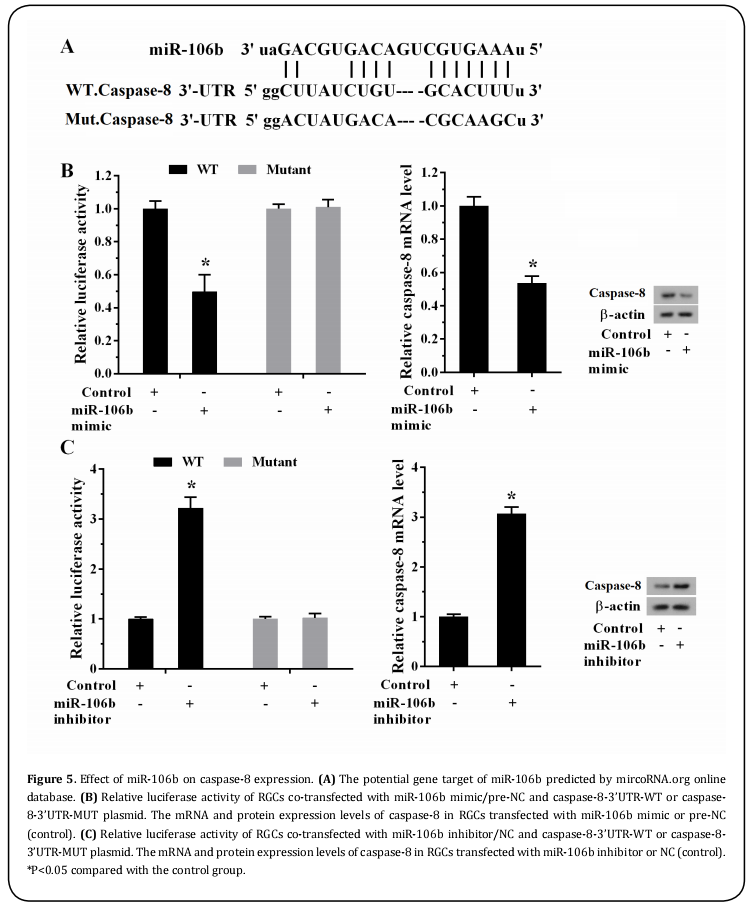
Caspase-8 negatively regulated caspase-8
It was well known that caspase-8 was a carcinogenic miRNA, in contrast, caspase-8 acted as a tumor suppressor in cancers[17,18]. To explore whether caspase-8 was a direct target of caspase-8, luciferase reporter assay was performed, since Targetscan software (http:// www.targetscan.org) has predicted the interaction between caspase-8 and caspase-8 (Figure 5A). The results revealed that the caspase-8 mimic decreased the luciferase activity (Figure 5B, P<0.05), while the caspase-8 inhibitor elevated the luciferase activity (Figure 5C, P<0.05) in caspase-8-WT co-transfected system, conversely, this caspase-8-MUT scarcely responded to neither caspase-8 mimic nor caspase-8 inhibitor. In addition, caspase-8 overexpression led to a decrease in the expression of caspase-8 at mRNA and protein levels (Figure 5B, P<0.05), on the contrary, caspase-8 inhibitor transfection reversed this trend (Figure 5C, P<0.05), suggesting that the direct binding existed between caspase-8 and caspase-8.
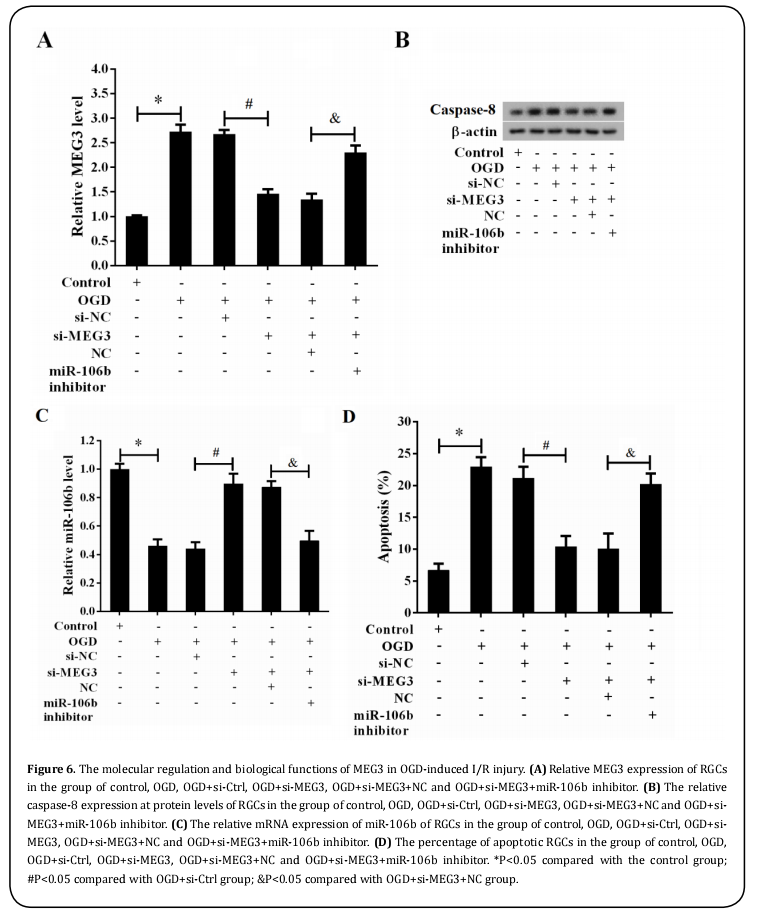
MEG3 exacerbated RGC apoptosis through caspase-8/ caspase-8 axis
We further gained insights into the molecular mechanism by which MEG3 knockdown inhibited RGC apoptosis. As expected, OGD treatment upregulated the expression of MEG3 (Figure 6A, P<0.05) and caspase-8 protein (Figure 6B, P<0.05), downregulated the mRNA expression of caspase-8 (Figure 6C, P<0.05) and increased RGC apoptosis rate (Figure 6D, P<0.05), respectively. Nevertheless, MEG3 knockdown resulted in a marked reduction in the expression of MEG3 (Figure 6A, P<0.05) and caspase-8 (Figure 6B, P<0.05) as well as the percentage of apoptotic RGCs (Figure 6D, P<0.05), but caused an elevated expression of caspase-8 (Figure 6C, P<0.05), however, co-transfection of si-MEG3 and caspase-8 inhibitor led to an opposite effect. Conjointly, our results manifested that MEG3 deteriorated cell apoptosis of RGCs through regulating caspase-8/caspase-8 axis in acute glaucoma.
Discussion
Acute glaucoma is a momentously sight-threatening
cause of non-reversible blindness worldwide featured
with a sudden and extensive IOP increase, which in turn
led to RGC apoptosis[19]. Increasing number of studies
have demonstrated the fact that MEG3 is aberrantly expressed
in the pathogenesis and development of some
tumors and functions as a novel tumor suppressor[20,21].
Hence, we speculated that MEG3 might also play an
underlying role in the development of acute glaucoma.
In this study, we found that MEG3 was upregulated in
increased IOP-induced ischemic retinae following the
mouse model of acute glaucoma as compared with the
sham controls, which was consistent with a previous
study showing that MEG3 is expressed with higher levels
following ischemia in adult mice[14]. Besides, it has
recently been shown that the abnormal expression of
caspase-8 and caspase-8 are observed in retinal ischemic
injury[7,10], suggesting that they were the vital regulators
in occurrence and progression of acute glaucoma. In our
study, we also found a marked increase and decrease in
the expression of caspase-8 and caspase-8, respectively,
in glaucomatous retinae. In the further in vitro experiments,
we discovered that OGD treatment increased
MEG3 expression and caspase-8 mRNA and protein expression
while downregulated caspase-8 mRNA expression
in addition to facilitating RGC apoptosis.
To investigate the biological roles of MEG3 in acute glaucoma,
primary RGCs were transfected with si-MEG3 or
si-Ctrl following OGD/ reoxygenation treatment. Our
current study showed that the knockdown of MEG3
significantly resulted in a decrease of the percentage of
apoptotic RGCs following OGD treatment in vitro, manifesting
that MEG3 might exert pro-apoptotic effect in
glaucomatous RGCs. The aforementioned evidence has
elucidated that caspase-8 as well as caspase-8 also have
important roles in RGC apoptosis. Recently, evidence
is emerging that lncRNAs are involved in regulation of
downstream target miRNAs[22]. Therefore, investigations
regarding the interaction between lncRNAs and miRNAs
can deepen our understanding of the mechanisms underlying
acute glaucoma. Therefore, we further explored
the possible molecular mechanisms of MEG3 action in
RGCs. Interestingly, The RIP and RNA pull-down assay
both confirmed that MEG3 might be directly bind with
caspase-8 as expected. Our study further examined the
interaction between caspase-8 and caspase-8 owing
to existence of underlying binding sites of caspase-8
and caspase-8 predicted by Targetscan bioinformatics
software. Luciferase reporter assay illustrated that
caspase-8 was a downstream target gene of caspase-8.
What’s more, the overexpression of caspase-8 suppressed
caspase-8 expression at mRNA and protein levels,
while caspase-8 knockdown upregulated caspase-8
in RGCs. Since MEG3 downregulated caspase-8 by direct
targeting in vitro, we assumed that MEG3 might regulate
caspase-8 through caspase-8.
To detect the correlation between MEG3 and caspase-8
in RGC apoptosis regulation, siRNA and miRNA inhibitor
have been transfected into RGCs to knock down MEG3
and caspase-8, respectively. We confirmed that MEG3
and caspase-8 were upregulated, whereas, caspase-8
expression was inhibited by both MEG3 and caspase-8
knockdown after OGD treatment. In addition, the apoptosis
of RGCs was promoted and the protein level of
caspase-8 was up-regulated. These data indicated that MEG3 promoted RGC apoptosis following ischemia/reperfusion
(I/R) injury induced by OGD/reoxygenation via
negatively regulating caspase-8, which in turn directly
targeted caspase-8.
There are still two limitations in the current study. First,
although we have detected the expression of MEG3,
caspase-8 and caspase-8 in the mouse model of glaucoma
and OGD/reoxygenation induced cell model, we have
no data about their expressions in the retinas of patients
with primary open-angle glaucoma (POAG). Second, although
we have demonstrated that MEG3 promoted
the apoptosis of OGD/ reoxygenation-induced RGC by
regulating caspase-8/caspase-8 pathway in vitro, the
function of MEG3 in acute glaucoma have not be verified
in vivo. Therefore, in the future, we will perform more
in-depth study to improve the two shortfalls and make
our study more clinically significant.
In summary, the present study authenticated for the first
time that the interaction might exist among the lncRNA
MEG3, caspase-8 and caspase-8 in increased IOP-induced
acute glaucoma. MEG3 exacerbated ischemic RGC
apoptosis via directly regulating caspase-8/caspase-8
axis. Our research would deepen the understanding of
the pathogenesis of acute glaucoma and provide a novel
insight into seeking for the treatment strategy of it.
Competing Interests
The authors declare no conflict of interest.
References
1. Wan, P., Su, W., Zhang, Y., Li, Z., Deng, C., and Zhuo, Y. (2017)
Trimetazidine protects retinal ganglion cells from acute
glaucoma via the Nrf2/Ho-1 pathway. Clinical Science
131, CS20171182
2. Ha, Y., Liu, H., Xu, Z., Yokota, H., Narayanan, S. P., Lemtalsi,
T., et al. (2015) Endoplasmic reticulum stress-regulated
CXCR3 pathway mediates inflammation and neuronal
injury in acute glaucoma. Cell death & disease 6, e1900
3. Quek, D. T., Koh, V. T., Tan, G. S., Perera, S. A., Wong, T. T., and
Aung, T. (2011) Blindness and long-term progression of
visual field defects in chinese patients with primary angleclosure
glaucoma. American Journal of Ophthalmology
152, 463-469
4. Ernawati, T., Suhendro, G., Sudiana, I. K., Putra, S. T.,
Harjanto, J. M., Sunarjo, Turchan, A., et al. (2016) Hypoxic
Preconditioning Improved Neuroprotective Effect of
Bone Marrow-Mesenchymal Stem Cells Transplantation
in Acute Glaucoma Models. Journal of Biomedical Science
& Engineering 09, 245-257
5. Kang, T. B., Benmoshe, T., Varfolomeev, E. E., Pewznerjung,
Y., Yogev, N., Jurewicz, A., et al. (2004) Caspase-8 serves
both apoptotic and nonapoptotic roles. Journal of
Immunology 173, 2976-2984
6. Zhang, Z. W., Li, H., Chen, S. S., Li, Y., Cui, Z. Y., and Ma, J.
(2017) MicroRNA-122 regulates caspase-8 and promotes
the apoptosis of mouse cardiomyocytes. Brazilian Journal
of Medical & Biological Research 50, e5760
7. Chi, W., Li, F., Chen, H., Wang, Y., Zhu, Y., Yang, X., et al. (2014)
Caspase-8 promotes NLRP1/NLRP3 inflammasome
activation and IL-1β production in acute glaucoma.
Proceedings of the National Academy of Sciences of the
United States of America 111, 11181-11186
8. Wei, C., Chen, H., Li, F., Zhu, Y., Wei, Y., and Zhuo, Y. (2015)
HMGB1 promotes the activation of NLRP3 and caspase-8
inflammasomes via NF-κB pathway in acute glaucoma.
Journal of neuroinflammation 12, 137
9. Brett, J. O., Renault, V. M., Rafalski, V. A., Webb, A. E., and
Brunet, A. (2011) The microRNA cluster caspase-8~25
regulates adult neural stem/progenitor cell proliferation
and neuronal differentiation. Aging 3, 108-124
10. Jayaram, H., Cepurna, W. O., Johnson, E. C., and Morrison,
J. C. (2015) MicroRNA Expression in the Glaucomatous
Retina. Investigative Ophthalmology & Visual Science 56,
7971
11. Tang, W., Dong, K., Li, K., Dong, R., and Zheng, S. (2016)
MEG3, HCN3 and linc01105 influence the proliferation
and apoptosis of neuroblastoma cells via the HIF-1α and
p53 pathways. Scientific reports 6, 36268
12. Wang, X., Zhou, L., and Liu, C. (2017) Expressions of
LncRNA MEG3 and Prognosis in Gliomas. Liaoning Journal
of Traditional Chinese Medicine
13. Gao, Y., and Lu, X. (2016) Decreased expression of MEG3
contributes to retinoblastoma progression and affects
retinoblastoma cell growth by regulating the activity of
Wnt/β-catenin pathway. Tumour Biology 37, 1461-1469
14. Yan, H., Yuan, J., Gao, L., Rao, J., and Hu, J. (2016) Long
noncoding RNA MEG3 activation of p53 mediates ischemic
neuronal death in stroke. Neuroscience 337, 191-199
15. Hong, S., Iizuka, Y., Chan, Y. K., and Gong, J. S. (2012)
Isolation of primary mouse retinal ganglion cells using
immunopanning-magnetic separation. Molecular Vision
18, 2922
16. Zhuo, H., Tang, J., Lin, Z., Jiang, R., Zhang, X., Ji, J., et al. (2016)
The aberrant expression of MEG3 regulated by UHRF1
predicts the prognosis of hepatocellular carcinoma. Mol
Carcinog 55, 209-219
17. Zheng, L., Zhang, Y., Liu, Y., Zhou, M., Lu, Y., Yuan, L., et
al. (2015) Caspase-8 induces cell radioresistance via the
PTEN/PI3K/AKT pathways and p21 in colorectal cancer.
Journal of Translational Medicine 13, 252
18. Pu, X., Storr, S. J., Zhang, Y., Rakha, E. A., Green, A. R., Ellis,
I. O., and Martin, S. G. (2017) Caspase-3 and caspase-8
expression in breast cancer: caspase-3 is associated with
survival. Apoptosis 22, 357-368
19. Su, W., Li, Z., Jia, Y., Zhu, Y., Cai, W., Wan, P., ... & Zhuo,
Y. (2017). microRNA-21a-5p/PDCD4 axis regulates
mesenchymal stem cell-induced neuroprotection in acute
glaucoma. Journal of molecular cell biology, 9(4), 289-301.
20. Zhou, Y., Zhang, X., & Klibanski, A. (2012). MEG3
noncoding RNA: a tumor suppressor. Journal of molecular
endocrinology, 48(3), R45-R53
21. Hu, D., Su, C., Jiang, M., Shen, Y., Shi, A., Zhao, F., et al. (2016)
Fenofibrate inhibited pancreatic cancer cells proliferation
via activation of p53 mediated by upregulation of
LncRNA MEG3. Biochemical & Biophysical Research
Communications 471, 290-295
22. Tang, Y., Jin, X., Xiang, Y., Chen, Y., Shen, C. X., Zhang, Y. C., et
al. (2015) The lncRNA MALAT1 protects the endothelium
against ox‐LDL‐induced dysfunction via upregulating the
expression of the miR‐22‐3p target genes CXCR2 and AKT.
FEBS letters 589, 3189-3196